Abstract
The regioselectivity of Diels-Alder cycloadditions of indole arynes (indolynes) at all three benzenoid positions was examined. Cycloadditions with the 4,5-and 5,6-indolynes, derived via metal-halogen exchange from the corresponding o-dibromo indoles, showed essentially no selectivity with 2-t-butylfuran. In contrast, the 6,7-indolyne displayed virtually complete preference for the more sterically congested cycloadduct. This same cycloadduct undergoes a facile acid-catalyzed rearrangement to afford the annulated enone, or alternatively, undergoes hydrolysis and oxidation in the presence of air to give the indolobenzoquinone. The 5,6-difluoroindoles show anomolous behavior and give either 5-fluoro-6,7-indolynes with n-BuLi in ether, or 5,6-indolynes with n-BuLi in toluene. We have also demonstrated that benzenoid indolynes can be easily and conveniently generated by the fluoride-induced decomposition of o-trimethylsilyl triflates.
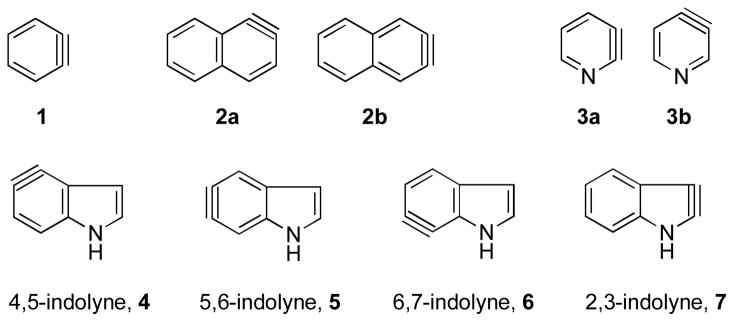
Unlike the familiar benzenoid arynes such as benzyne 1,1 the naphthalynes (2a and 2b),2 and the pyridynes (3a and 3b) (Figure 1),3 all of which have been known for at least 40 years, the tetrad of arynes 4–7 derived from the ubiquitous indole aromatic nucleus was until recently unknown.4–5
Figure 1.
Benzenoid aryne systems.
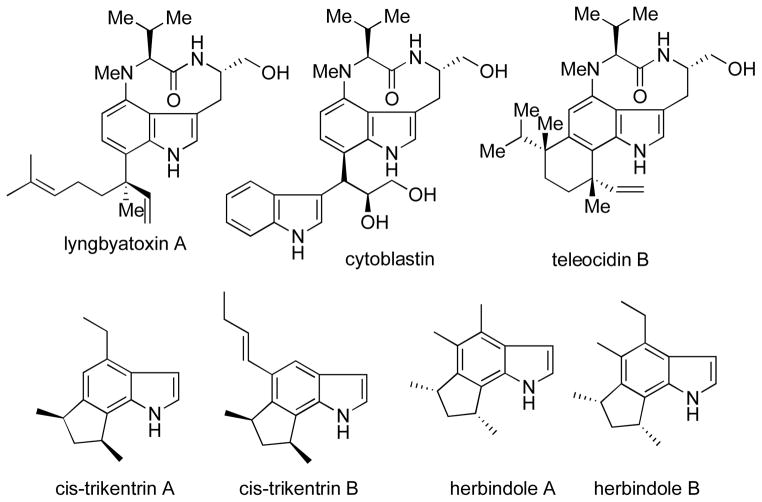
The absence of these arynes from the literature was surprising given the potential utility of these reactive intermediates for an attractive entry into the indole alkaloid class of complex natural products such as the trikentrins,6 herbindoles,6a,c teleocidins,7 cytoblastin,8 and lyngbyatoxin9 (Figure 2).
Figure 2.
Indole-containing complex natural products.
We recently reported the existence of and first Diels-Alder cycloaddition examples with all three indole arynes derived from the benzenoid part of the indole nucleus.4 We are now pleased to follow that communication with this report on the regiochemical behavior of these cycloadditions.10
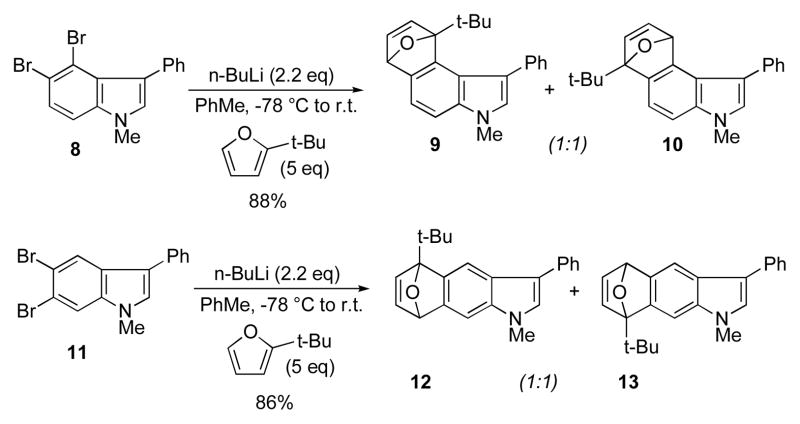
The 4,5-, 5,6-, and 6,7-indolynes were generated from the corresponding o-dibromoindoles in the manner previously described.4 Treatment of 8 with 2.2 eq of n-BuLi in either ether or toluene11 at −78 °C in the presence of an excess of 2-t-butylfuran was found to give an approximate 1:1 mixture of regioisomers 9 and 10 (Scheme 1).12 In a similar fashion, the generation of the 5,6-indolyne from 11 and trapping in the same manner also afforded a 1:1 mixture of regioisomers 12 and 13.
Scheme 1.
Regioselectivity of 4,5- and 5,6-indolyne cycloadducts.
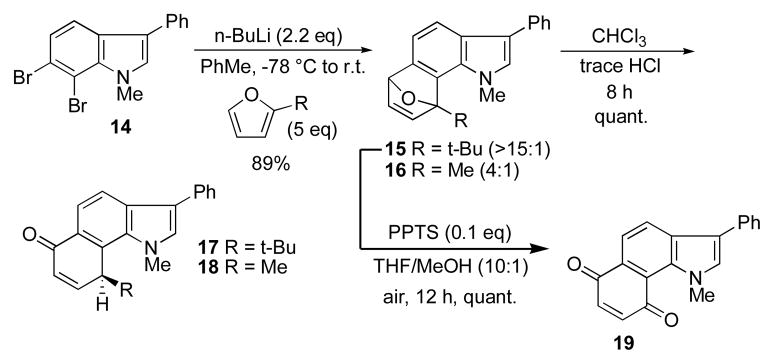
By stark contrast, the 6,7-indolyne derived from 14 gave a virtually completely regioselective cycloadduct 15 (Scheme 2). The structure was unequivocally confirmed via NOESY and COSY analysis. The formation of the more sterically crowded product can be rationalized in terms of a previously observed polarization phenomenon that has been invoked for substituted benzynes in which fluorine acts as powerful regiochemical director.13–15 The effect correlates with electronegativity values and is reduced with methoxy substiutents14 and finally reversed with electron-donating groups such as methyl.15 The powerful polarizing effect of the pyrrole unit is apparent even with 2-methylfuran which exhibits a 4:1 preference for the more sterically congested product 16.
Scheme 2.
Regioselectivity of 6,7-indolyne cycloadducts.
The stable initial cycloadducts 15–16 can be made to undergo rearrangement upon prolonged exposure to a trace of acid in chloroform to give the annulated enones 17–18. Aromatization is precluded in this case which would otherwise force the bulky t-butyl group and even smaller methyl group into an unavoidable steric conflict with the planar N-methyl substituent on the pyrrole. Interestingly, hydrolysis of the cycloadduct 15 in the presence of air resulted in loss of the t-butyl group and concommitant oxidation to give the indoloquinone 19. This rearrangement was only observed with the t-butyl-substituted arynes.
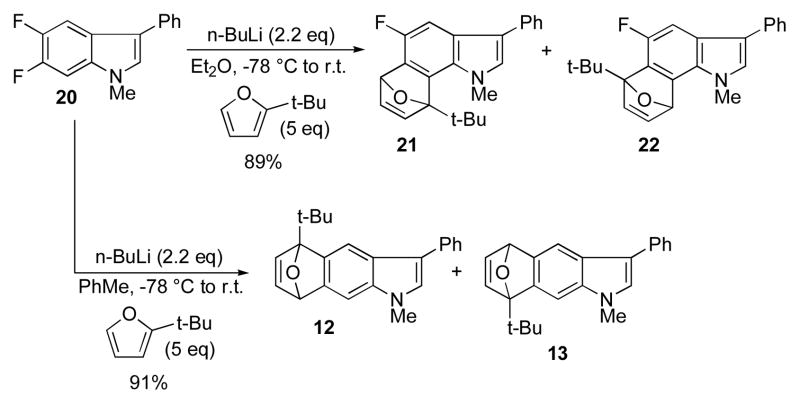
The 5,6-difluoro indole 20 displays anomalous behavior (Scheme 3). Treatment of this compound with 4 eq of t-butyllithium (but not n-BuLi) resulted in exclusive deprotonation at the C7 position, followed by dehydrohalogenation to give the 5-fluoro-6,7-aryne which was trapped as a nearly equimolar mixture of regioisomers 21 and 22. In this case, the fluorine presumably competes effectively with the polarizing properties of the pyrrole unit and therefore displays no selectivity. As observed by Coe,11 by changing solvents from ether to toluene, the acid-base reaction manifold is almost completely suppresed in favor of metal-halogen exchange, and the 5,6-indolyne is generated instead which as noted above affords an 1:1 mixture of the same cycloadducts 12 and 13.
Scheme 3.
Regioselectivity of 5,6-difluoroindole-derived indolynes.
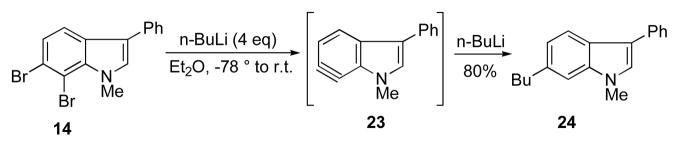
In the absence of any diene, the putative 6,7-indolyne 23 undergoes cine substitution with excess n-butyllithium to give the 6-butyl indole 24 (Scheme 4). This finding, which is reminiscent of typical substituted benzyne behavior,16 is a potentially useful method for installing substition at this postion. This operation is usually difficult to acccomplish in indoles with other non-aryne strategies. So far the reaction selectivity in this reaction manifold is only seen with the 6,7-indolynes.
Scheme 4.
Cine substitution in 6,7-indolynes.
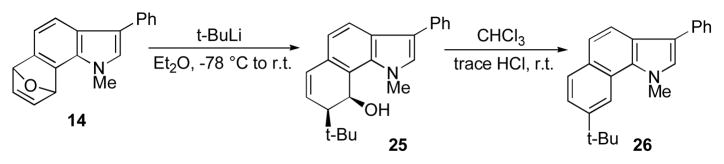
These 6,7-indolyne-derived cycloaddducts can also be opened in a highly regio-selective and exo-selective manner by exposure to alkyllithium reagents in ether. We first noticed this behavior with t-butyllithium and the unsubstituted furan cycloadduct 25 from the 6,7-indolyne 14.4 In this case, acid-promoted elimination of occurs preferentially to give the aromatic annulated product 26 (Scheme 5).
Scheme 5.
Exo- and regioselective ring-opening and aromatization in 6,7-indolyne furan cycloadducts.
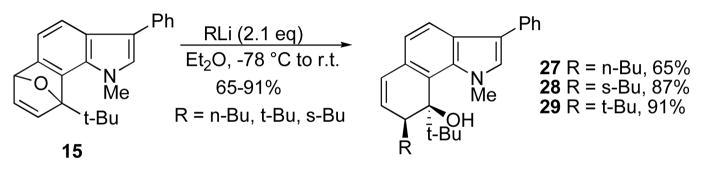
However in the present substituted example, elimination is precluded due to steric congestion with the N-methyl substituent (Scheme 6). Symmetrical furan cycloadducts have been opened under metal-catalyzed conditions with carbon, oxygen, and nitrogen nucleophiles.17 We have found that catalysis is unnecessary with our cycloadducts, at least with carbon-based nucleophiles.
Scheme 6.
Regio- and exo-selective ring-opening of 6,7-indolyne furan cycloadducts.
Thus, simply recommitting the cycloadduct to ether and treatment with n-Bu-, s-Bu-, or t-BuLi gives exclusively the ring-opened products 27–29.
These results with the 6,7-indolyne cycloadducts have clear implications for the production of libraries based on these heretofore unknown chemotypes. The 6,7-furan cycloadducts thus represent a potentially versatile platform for the synthesis of novel 6,7-annulated indole libraries of either type 17–18 or 27–29. Additionally, the olefin handle in either type of structure provides a further point of diversification and the opportunity to generate stereochemically dense annulated indole arynes.
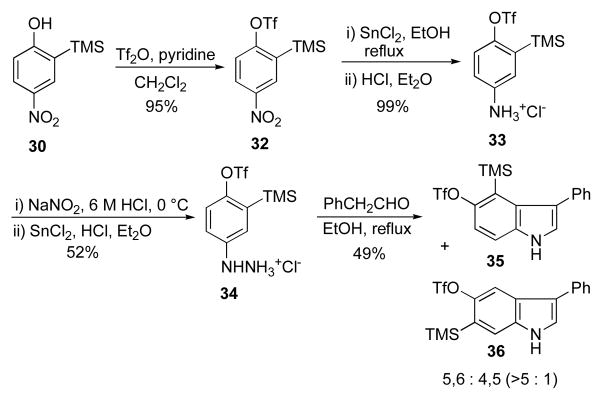
Finally, in an effort to find an alternate route to the indole arynes that does not rely exclusively on metal-halogen exchange protocols, we developed a Fischer indole synthesis to the 4,5-, 5,6-, and 6,7-o-trimethylsilyl triflates (Scheme 7). Treatment of 2-trimethylsilylphenol18 with Cu(NO3)2 (0.55 eq) in acetic acid at 0 °C for 3 h afforded an equimolar mixture of 4-nitro-2-(trimethylsilyl)phenol 30 and 2-nitro-6-(trimethylsilyl)phenol 31.19 The nitrophenol 30 was triflated (95%) and converted to its hydrazine hydrochloride salt 34 in 51% overall yield. Reaction of 34 under Fischer conditions (EtOH, reflux, 4 h) with phenylacetaldehyde was remarkably regioselective albeit moderately yielding, giving a greater than 5-to-1 ratio of the 5,6- and 4,5-indole products 36 and 35, respectively. This result stands in contrast to the corresponding dibromides or dichlorides4 which under the same conditions resulted in a nearly 1-to-1 mixture of isomers. By carrying 2-nitro-6-(trimethylsilyl)phenol 31 through the same scheme, the corresponding 6-(trimethylsilyl)-1H-indol-7-yl triflate 37 is also available by this method in 32% overall yield.
Scheme 7.
Fischer indole synthesis of 4,5- and 5,6-o-trimethylsilyl triflate indolyne precursors.
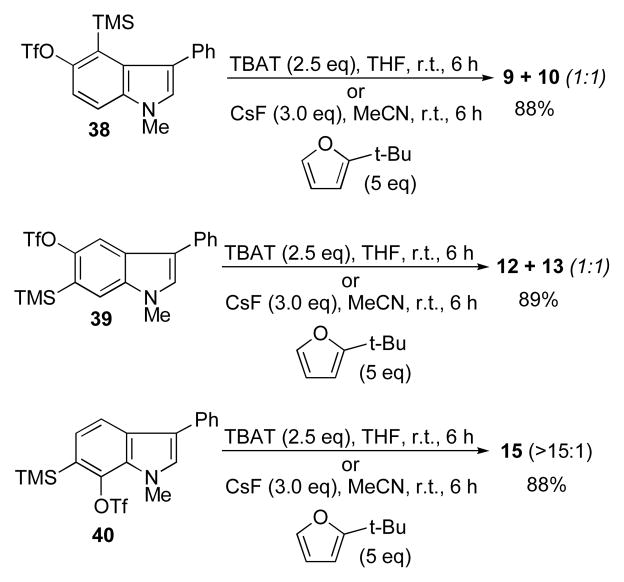
The compounds 35, 36, and 37 each give, after N-methylation, their respective 5,6-, 4,5, and 6,7-indolyne cycloadducts by treatment with TBAT20 (2.5 eq) in THF, or alternatively, CsF (3.0 eq) in MeCN, for several hours at room temperature in the presence of 2-t-butylfuran (Scheme 8). The same ratio of isomeric cycloadducts as shown previously were obtained.
Scheme 8.
4,5-, 5,6-, and 6,7-indolynes derived from o-silyltriflates.
In conclusion, we have shown that while 4,5- and 5,6-indolynes show virtually no regioselectivity in Diels-Alder cycladditions, the 6,7-indolyne by contrast is highly regioselective, presumably due to the polarization influence of the pyrrole ring. Adducts derived from this flexible system can undergo acid-catalyzed rearrangement to give stable annulated enones, hydrolysis in air to give benzoquinones, or react with alkyllithiums to afford exo-and regioselective ring-opened products. We are presently adapting this versatile methodology for use in library development as well as the the total synthesis of indole alkaloid natural products. The results of these efforts will be reported as developments warrant.
Acknowledgments
We acknowledge support of this work by the National Institutes of Health, Grant R01 GM069711. Additional support of this work was provided by the NIH (Grant P50 GM069663 via the University of Kansas Chemical Methodologies and Library Development Center of Excellence (KU-CMLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts JD, Simmons HE, Jr, Carlsmith LA, Vaughn CW. J Am Chem Soc. 1953;75:3290–3291.Roberts JD, Semenow DA, Simmons HE, Jr, Carlsmith LA. J Am Chem Soc. 1956;78:601–611.For a review of arynes in general, see: Hoffmann RW. Dehydrobenzene and Cycloalkynes. Academic; New York: 1967.
- 2.(a) Bunnett JF, Brotherton TK. J Org Chem. 1958;23:904–906. [Google Scholar]; (b) Huisgen R, Zirngibl L. Chem Ber. 1958;91:1438–1452. [Google Scholar]
- 3.(a) Kauffmann T, Boettcher FP. Angew Chem. 1961;73:65–66. [Google Scholar]; (b) Martens RJ, den Hertog HJ. Tetrahedron Lett. 1962:643–645. [Google Scholar]
- 4.Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org Lett. 2007;9:4135–4137. doi: 10.1021/ol701595n. [DOI] [PubMed] [Google Scholar]
- 5.For attempts to generate the elusive 2,3-indolyne, see: Conway SC, Gribble GW. Heterocycles. 1992;34:2095–2108.Gribble GW, Conway SC. Synth Commun. 1992;22:2129–2141.
- 6.(a) Jackson SK, Kerr MA. J Org Chem. 2007;72:1405–1411. doi: 10.1021/jo062350v. [DOI] [PubMed] [Google Scholar]; (b) Huntley RJ, Funk RL. Org Lett. 2006;8:3403–3406. doi: 10.1021/ol061259a. [DOI] [PubMed] [Google Scholar]; (c) Jackson SK, Banfield SC, Kerr MA. Org Lett. 2005;7:1215–1218. doi: 10.1021/ol047498k. [DOI] [PubMed] [Google Scholar]; (d) MacLeod JK, Monahan LC. Tetrahedron Lett. 1988;29:391–392. [Google Scholar]
- 7.(a) Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1987;38:3671–3674. [Google Scholar]; (b) Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1986;27:6245–6248. [Google Scholar]; (c) Nakatsuka S, Masuda T, Asano O, Terame T, Goto T. Tetrahedron Lett. 1986;27:4327–4330. [Google Scholar]
- 8.Moreno OA, Kishi Y. J Am Chem Soc. 1996;118:8180–8181. [Google Scholar]
- 9.Muratake H, Natsume M. Tetrahedron Lett. 1987;28:2265–2268. [Google Scholar]
- 10.Presented at the 236th American Chemical Society National Meeting; Philadelphia, PA. August 17–21; 2008. (ORGN 679) [Google Scholar]
- 11.Coe JW, Wirtz MC, Bashore CG, Candler J. Org Lett. 2004;6:1589–1592. doi: 10.1021/ol049655l. [DOI] [PubMed] [Google Scholar]
- 12.All new compounds reported herein exhibited satisfactory 1H NMR, 13C NMR, COSY and NOESY NMR, mass spectral, and elemental analysis data consistent with their structures.
- 13.Gribble GW, Keavy DJ, Branz SE, Kelly WJ, Pals MA. Tetrahedron Lett. 1988;29:6227–6230. [Google Scholar]
- 14.Giles RGF, Sargent MV, Sianipar H. J Chem Soc, PT1. 1991:1571–1579. [Google Scholar]
- 15.Newman MS, Kannan R. J Org Chem. 1976;41:3356–3359. [Google Scholar]
- 16.Bunnett JF, Zahler RE. Chem Rev. 1951;49:273–412. [Google Scholar]
- 17.Lautens M, Fagnou K, Hiebert S. Acc Chem Res. 2003;36:48–58. doi: 10.1021/ar010112a. [DOI] [PubMed] [Google Scholar]
- 18.(a) Himeshima Y, Sonoda T, Kobayashi H. Chem Lett. 1983:1211–1214. [Google Scholar]; (b) Wilbur DS, Stone WE, Anderson KW. J Org Chem. 1983;48:1542–1544. [Google Scholar]
- 19.Anuradha V, Srinivas PV, Aparna P, Rao JM. Tetrahedron Lett. 2006;47:4933–4935. [Google Scholar]
- 20.Pilcher AS, DeShong P. J Org Chem. 1996;61:6901–6905. doi: 10.1021/jo960922d. [DOI] [PubMed] [Google Scholar]