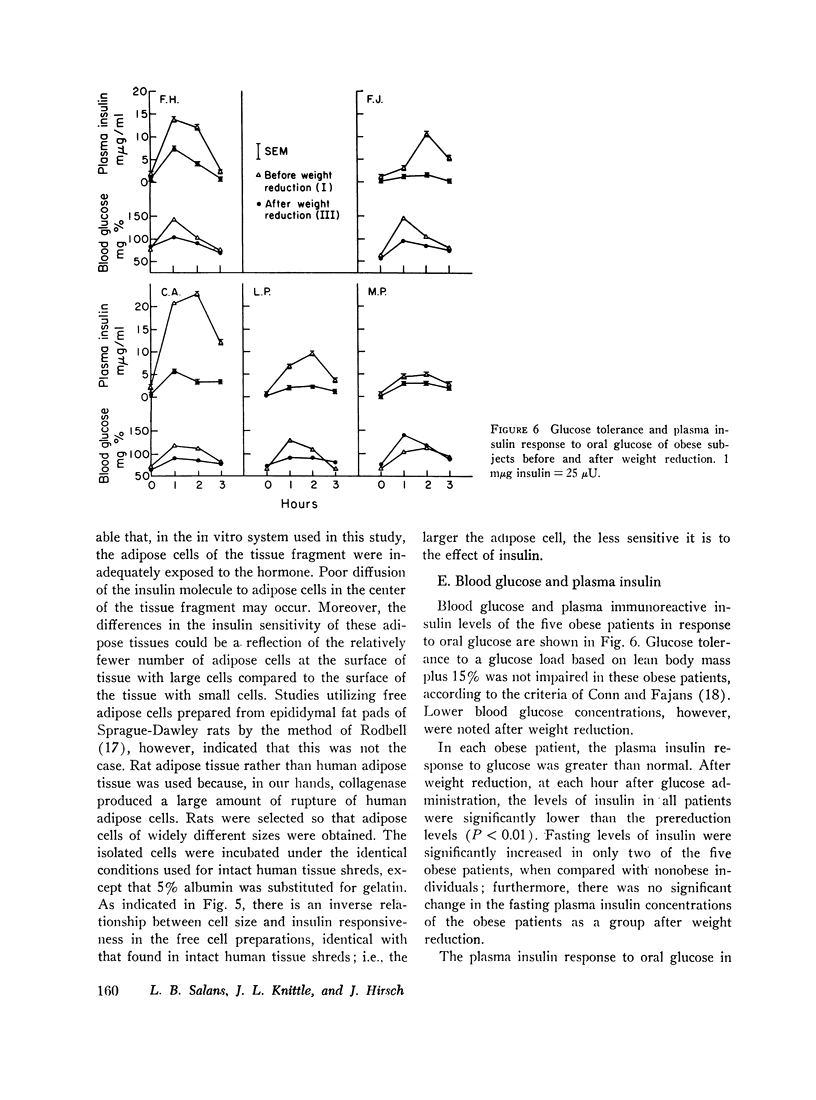
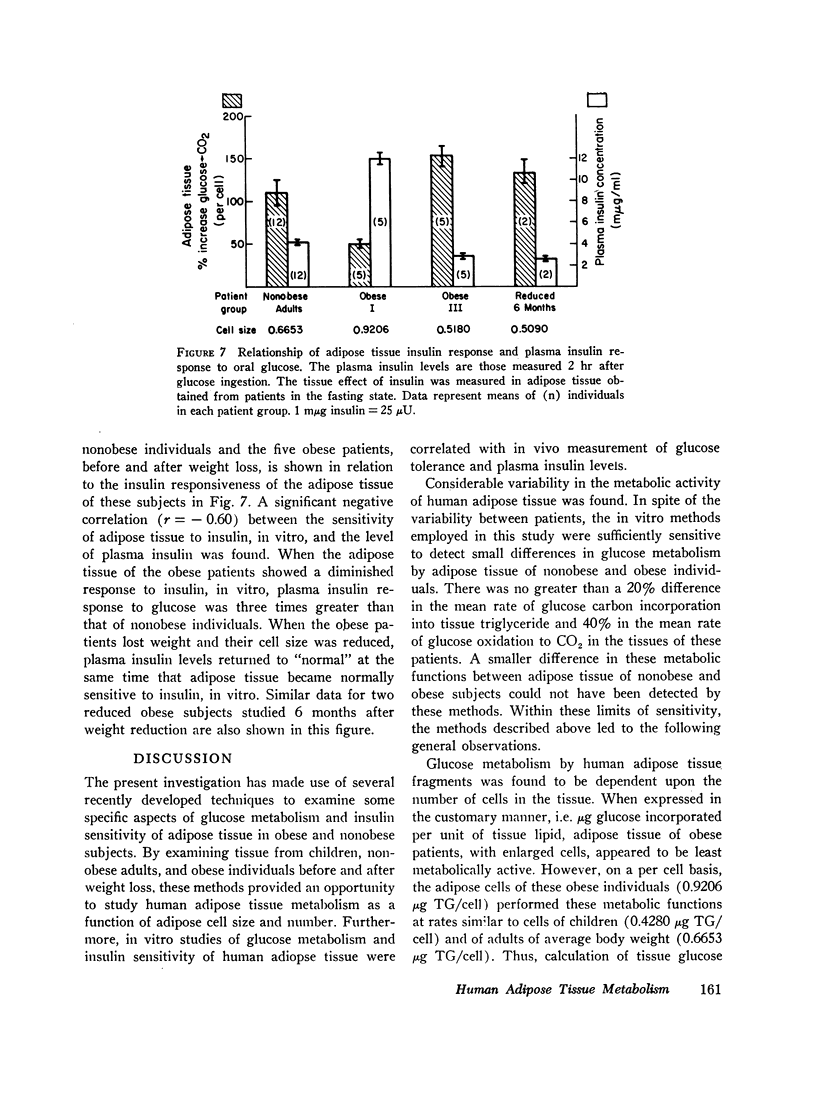
Abstract
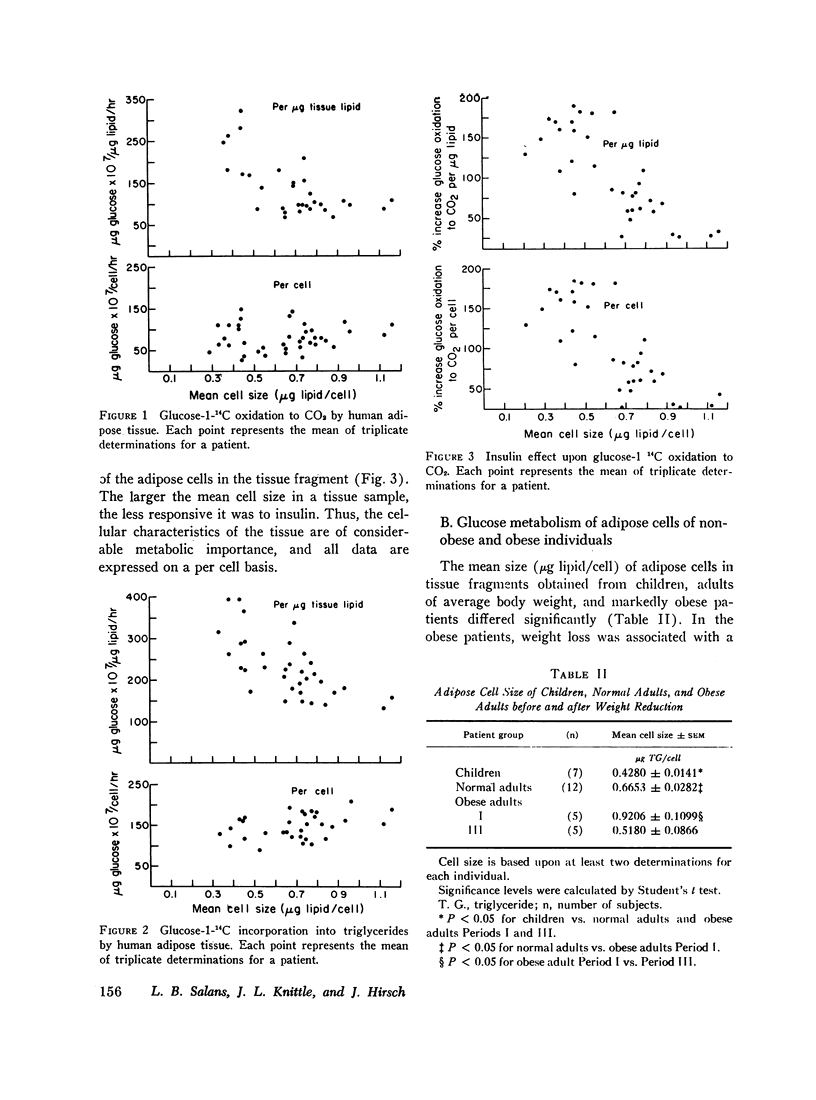
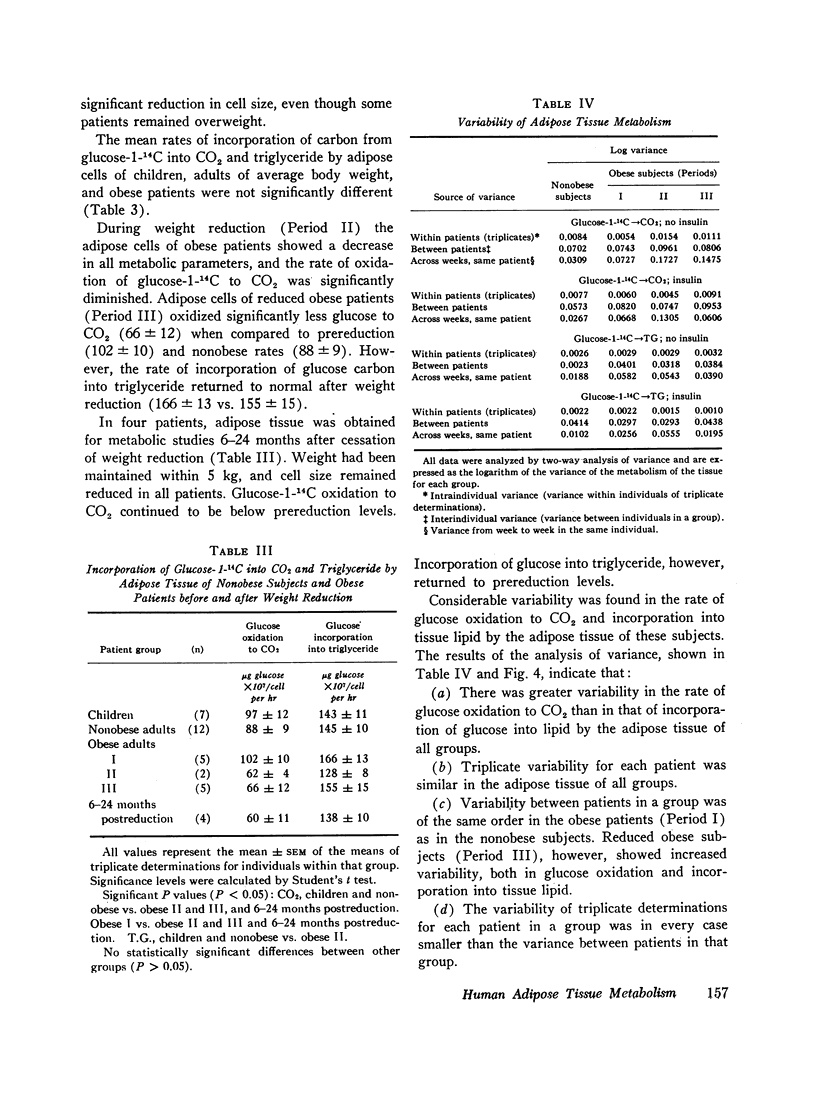
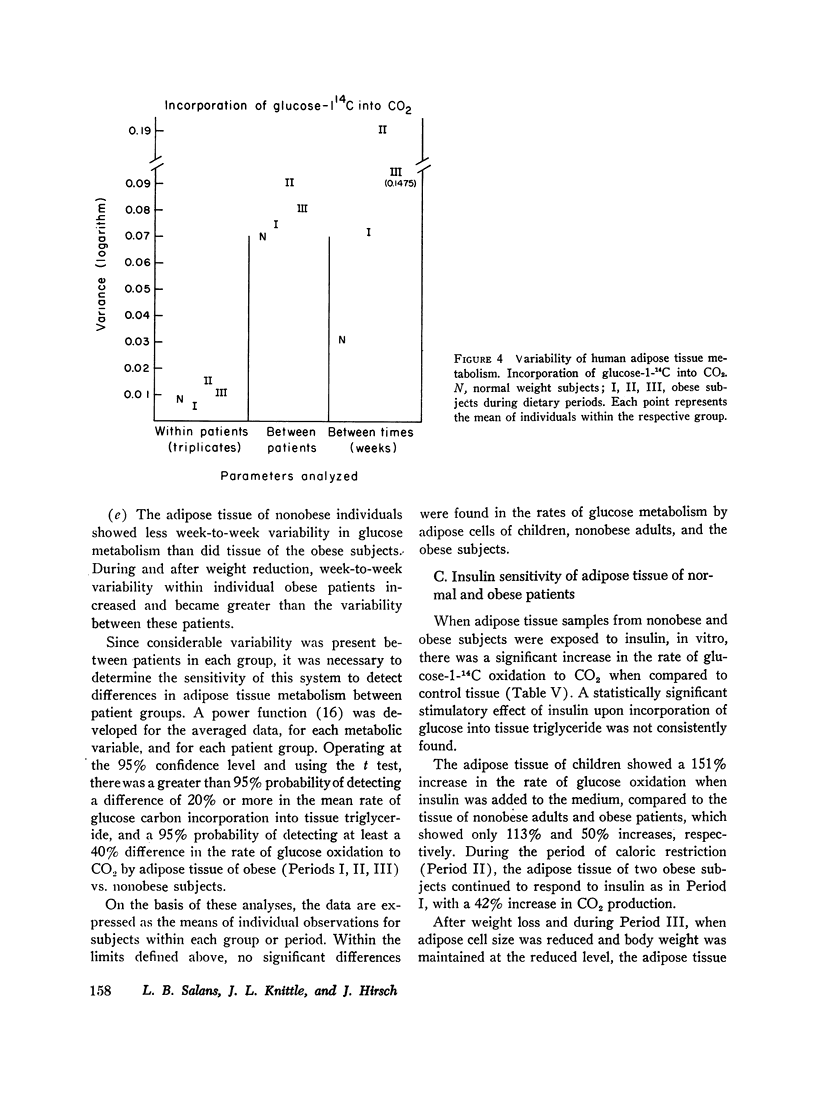
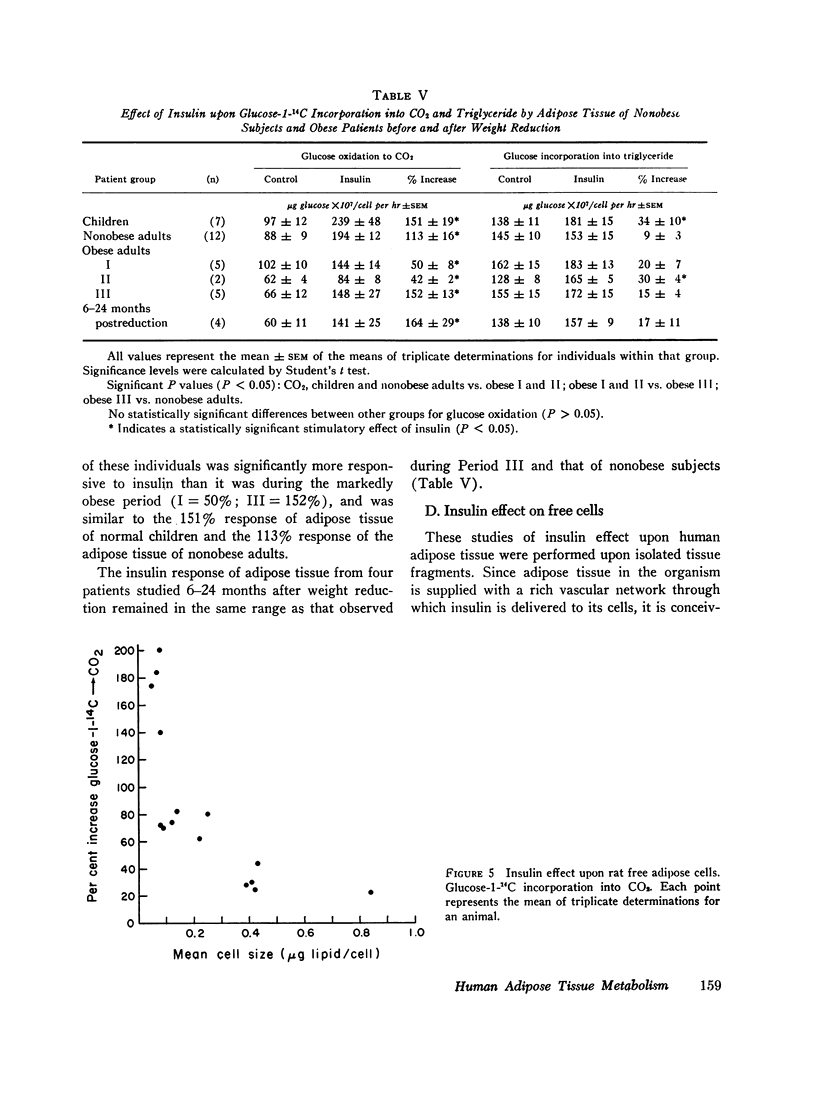
Glucose metabolism and insulin sensitivity of isolated human adipose tissue was studied as a function of adipose cell size and number. Glucose metabolism by these tissues was closely related to the number of cells in the fragment, irrespective of cell size. Adipose cells of obese individuals metabolized glucose to carbon dioxide and triglyceride at rates similar to adipose cells of nonobese subjects. In contrast, insulin responsiveness of adipose tissue was dependent upon adipose cell size. The larger its adipose cells the less insulin sensitive was the tissue. Thus, adipose tissue of obese subjects, with enlarged cells, showed a diminished response to insulin. After weight loss and reduction in adipose cell size, insulin sensitivity of the adipose tissue of obese patients was restored to normal. When adipose tissue of obese individuals showed impaired responsiveness to insulin, their plasma insulin levels, after oral glucose, were elevated. Weight loss and reduction in adipose cell size restored plasma insulin concentration to normal, concomitant with the return of normal tissue insulin sensitivity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALP H., RECANT L. EFFECT OF THE INSULIN-INHIBITORY ALBUMIN FRACTION FROM NORMAL AND DIABETIC SUBJECTS ON ADIPOSE TISSUE. Metabolism. 1964 Jul;13:609–619. doi: 10.1016/0026-0495(64)90069-1. [DOI] [PubMed] [Google Scholar]
- ANTONIADES H. N., BOUGAS J. A., PYLE H. M. Studies on the state of insulin in blood. Examination of splenic, portal and peripheral blood serum of diabetic and nondiabetic subjects for "free" insulin and insulin complexes. N Engl J Med. 1962 Aug 2;267:218–222. doi: 10.1056/NEJM196208022670502. [DOI] [PubMed] [Google Scholar]
- BECK P., KOUMANS J. H., WINTERLING C. A., STEIN M. F., DAUGHADAY W. H., KIPNIS D. M. STUDIES OF INSULIN AND GROWTH HORMONE SECRETION IN HUMAN OBESITY. J Lab Clin Med. 1964 Oct;64:654–667. [PubMed] [Google Scholar]
- BOSHELL B. R., BARRETT J. C., WILENSKY A. S., PATTON T. B. INSULIN RESISTANCE. RESPONSE TO INSULIN FROM VARIOUS ANIMAL SOURCES, INCLUDING HUMAN. Diabetes. 1964 Mar-Apr;13:144–152. doi: 10.2337/diab.13.2.144. [DOI] [PubMed] [Google Scholar]
- BUTTERFIELD W. J., HANLEY T., WHICHELOW M. J. PERIPHERAL METABOLISM OF GLUCOSE AND FREE FATTY ACIDS DURING ORAL GLUCOSE TOLERANCE TESTS. Metabolism. 1965 Aug;14:851–866. doi: 10.1016/0026-0495(65)90122-8. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Martinsson A. The composition of human subcutaneous adipose tissue in relation to its morphology. Acta Med Scand. 1966 Apr;179(4):475–481. doi: 10.1111/j.0954-6820.1966.tb05485.x. [DOI] [PubMed] [Google Scholar]
- CHRISTOPHE J., JEANRENAUD B., MAYER J., RENOLD A. E. Metabolism in vitro of adipose tissue in obese-hyperglycemic and goldthioglucose-treated mice. I. Metabolism of glucose. J Biol Chem. 1961 Mar;236:642–647. [PubMed] [Google Scholar]
- CONN J. W., FAJANS S. S. The prediabetic state. A concept of dynamic resistance to a genetic diabetogenic influence. Am J Med. 1961 Dec;31:839–850. doi: 10.1016/0002-9343(61)90023-7. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo M., Rudman D. Species differences in glucose metabolism and insulin responsiveness of adipose tissue. Am J Physiol. 1966 Apr;210(4):721–727. doi: 10.1152/ajplegacy.1966.210.4.721. [DOI] [PubMed] [Google Scholar]
- FROESCH E. R., BUERGI H., RAMSEIER E. B., BALLY P., LABHART A. ANTIBODY-SUPPRESSIBLE AND NONSUPPRESSIBLE INSULIN-LIKE ACTIVITIES IN HUMAN SERUM AND THEIR PHYSIOLOGIC SIGNIFICANCE. AN INSULIN ASSAY WITH ADIPOSE TISSUE OF INCREASED PRECISION AND SPECIFICITY. J Clin Invest. 1963 Nov;42:1816–1834. doi: 10.1172/JCI104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., KARAM J. H., PAVLATOS F. C., FORSHAM P. H. Reduction by phenformin of excessive insulin levels after glucose loading in obese and diabetic subjects. Metabolism. 1963 Apr;12:278–286. [PubMed] [Google Scholar]
- HELLMAN B., WESTMAN S. PALMITATE UTILIZATION IN OBESE-HYPERGLYCEMIC MICE. IN VITRO STUDIES OF EPIDIDYMAL ADIPOSE TISSUE AND LIVER. Acta Physiol Scand. 1964 May-Jun;61:65–72. doi: 10.1111/j.1748-1716.1964.tb02943.x. [DOI] [PubMed] [Google Scholar]
- HIRSCH J., FARQUHAR J. W., AHRENS E. H., Jr, PETERSON M. L., STOFFEL W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am J Clin Nutr. 1960 Jul-Aug;8:499–511. doi: 10.1093/ajcn/8.4.499. [DOI] [PubMed] [Google Scholar]
- HIRSCH J., GOLDRICK R. B. SERIAL STUDIES ON THE METABOLISM OF HUMAN ADIPOSE TISSUE. I. LIPOGENESIS AND FREE FATTY ACID UPTAKE AND RELEASE IN SMALL ASPIRATED SAMPLES OF SUBCUTANEOUS FAT. J Clin Invest. 1964 Sep;43:1776–1792. doi: 10.1172/JCI105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Role of cyclic-3',5'-amp in the response of adipose tissue to insulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):757–763. doi: 10.1073/pnas.56.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAM J. H., GRODSKY G. M., FORSHAM P. H. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963 May-Jun;12:197–204. doi: 10.2337/diab.12.3.197. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Boshell B. R., DiPlacido J., Roddam R. F. Insulin secretion in obesity. N Engl J Med. 1967 Feb 9;276(6):314–319. doi: 10.1056/NEJM196702092760603. [DOI] [PubMed] [Google Scholar]
- LESSER G. T., KUMAR I., STEELE J. M. CHANGES IN BODY COMPOSITION WITH AGE. Ann N Y Acad Sci. 1963 Sep 26;110:578–588. doi: 10.1111/j.1749-6632.1963.tb15781.x. [DOI] [PubMed] [Google Scholar]
- LOWY C., BLANSHARD G., PHEAR D. Antagonism of insulin by albumin. Lancet. 1961 Apr 15;1(7181):802–804. doi: 10.1016/s0140-6736(61)90121-0. [DOI] [PubMed] [Google Scholar]
- Owen J. A., Jr, Lindsay R. W., Gaskin J. H., Hollifield G. Response of human adipose tissue to endogenous serum insulin-like activity in vitro. Metabolism. 1967 Jan;16(1):47–56. doi: 10.1016/0026-0495(67)90158-8. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Evidence for adaptive hyperinsulinism. Lancet. 1961 Sep 23;2(7204):690–692. doi: 10.1016/s0140-6736(61)92838-0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rudman D., Garcia L. A., DiGirolamo M., Shank P. W. Cleavage of bovine insulin by rat adipose tissue. Endocrinology. 1966 Jan;78(1):169–185. doi: 10.1210/endo-78-1-169. [DOI] [PubMed] [Google Scholar]
- SAMAAN N., FRASER R., DEMPSTER W. J. THE "TYPICAL" AND "ATYPICAL" FORMS OF SERUM INSULIN. Diabetes. 1963 Jul-Aug;12:339–348. doi: 10.2337/diab.12.4.339. [DOI] [PubMed] [Google Scholar]
- Tucker J., Trethewey J., Stewart G. A., Neville R. W., Hanley T. Insulin sensitivity and carbohydrate metabolism of rats with hypothalamic obesity. J Endocrinol. 1965 Nov;33(3):437–446. doi: 10.1677/joe.0.0330437. [DOI] [PubMed] [Google Scholar]
- VALLANCE-OWEN J., DENNES E., CAMPBELL P. N. Insulin antagonism in plasma of diabetic patients and normal subjects. Lancet. 1958 Aug 16;2(7042):336–338. doi: 10.1016/s0140-6736(58)90257-5. [DOI] [PubMed] [Google Scholar]


