Abstract
Several pathways function to remove aberrant mRNA in eukaryotic cells; however, the exact mechanisms underlying the restriction of aberrant mRNA transcription are poorly understood. In this study, we found that MORPHEUS’ MOLECULE1 (MOM1) is a key component of this regulatory machinery. The Arabidopsis (Arabidopsis thaliana) mom1-44 mutation was identified by luciferase imaging in transgenic plants harboring a cauliflower mosaic virus 35S promoter-LUCIFERASE transgene lacking the 3′-untranslated region. In the mom1-44 mutant, transcriptional read-though occurred in genes with an aberrant RNA structure. Analysis of an RNA-dependent RNA polymerase2 mom1 double mutant revealed that the RNA-directed DNA methylation pathway is not involved in this regulatory process. Moreover, the prevention of aberrant mRNA transcriptional read-through by MOM1 is gene locus and transgene copy number independent.
Transcription is tightly controlled in eukaryotic cells. Most mRNA regulatory elements are located in the 5′- and 3′-untranslated regions (UTRs) of genes rather than in the coding region. The 5′-UTR functions mainly in the regulation of translation, whereas the 3′-UTR plays important roles in nuclear export, cytoplasmic localization, translation efficiency, and mRNA stability (Proudfoot et al., 2002). The highly conserved sequences in 3′-UTR are recognized by a poly(A) complex that promotes transcript cleavage at the poly(A) site and polyadenylation (Proudfoot et al., 2002; Moore and Proudfoot, 2009), which in turn directs RNA polymerase II-mediated termination (Whitelaw and Proudfoot, 1986). Recent studies have shown that transcriptional termination enhances pre-mRNA processing and protein expression (West and Proudfoot, 2009), whereas transcripts bearing an aberrant or missing 3′-UTR are often improperly terminated and unpolyadenylated, leading to degradation via RNA-dependent RNA polymerase6 (RDR6)-mediated posttranscriptional gene silencing (Herr et al., 2006; Luo and Chen, 2007). Several RNA surveillance pathways, including nonsense-mediated mRNA decay, exist for the removal of aberrant RNAs (Behm-Ansmant et al., 2007; Chang et al., 2007; Isken and Maquat, 2007; Kim et al., 2009); however, it is not fully understood how aberrant transcription and/or transcriptional read-through is controlled during the transcription initiation/elongation stage.
MORPHEUS’ MOLECULE1 (MOM1) mediates transcriptional gene silencing (TGS) in a methylation-independent manner (Amedeo et al., 2000). The reactivation of targets with an aberrant RNA structure, including transcriptionally silent information (TSI) and 5S rRNA genes, has been shown in plants lacking MOM1 (Steimer et al., 2000; Habu et al., 2006). Recent studies suggest that MOM1 and RNA polymerase V mediate TGS together. MOM1 functions in small interfering RNA (siRNA) accumulation and is genetically linked to RNA-directed DNA methylation (RdDM; Yokthongwattana et al., 2010); MOM1 may help transduce RdDM signals to repress histone modification in the core region (Numa et al., 2010). In this study, we found that MOM1 plays a role in preventing aberrant RNA transcriptional read-through in Arabidopsis (Arabidopsis thaliana) plants.
RESULTS
Identification of a Mutant That Expresses a Cauliflower Mosaic Virus 35S Promoter-LUCIFERASE Transgene Lacking the 3′-UTR
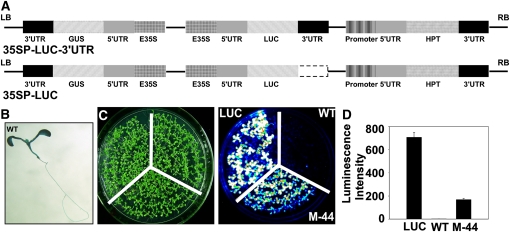
To study the machinery responsible for preventing aberrant RNA transcriptional read-through, we constructed two chimeric genes: the coding sequence (CDS) of firefly LUCIFERASE (LUC) under the control of the cauliflower mosaic virus (CaMV) 35S promoter (35SP) in the presence (35SP-LUC-3′UTR) or absence (35SP-LUC) of a CaMV 35S 3′-UTR fragment. The resulting chimeras were cloned into pCAMBIA1301 containing GUS as a reporter gene driven by the CaMV 35SP (Fig. 1A). Thus, the only difference between the resulting plasmids was the presence or absence of the 3′-UTR fragment (Fig. 1A). The plasmids were transformed into the Columbia (Col-0) background, and positive plants were selected based on hygromycin resistance. Two types of stable transgenic lines, LUC (35SP-LUC-3′UTR) and the wild type (35SP-LUC), were thus produced. In contrast to the LUC plants, of which 90% were LUC luminance positive, no LUC was detected in more than 80% of the wild-type plants. However, GUS activity was detected in the wild-type seedlings by histologic analysis (Fig. 1B).
Figure 1.
Identification of the M-44 mutant. A, Schematic diagram of the LUC constructs in the binary vector pCAMBIA1301. Both T-DNA regions contain the complete GUS and HPT genes driven by the CaMV 35SP and the 35SP-controlled LUC CDS with (35SP-LUC-3′UTR) or without (35SP-LUC) the 3′-UTR. E35S, CaMV 35SP; 3′-UTR, 35S terminator; LB, left border; RB, right border. B, GUS staining of a wild-type (WT) seedling. C, Luminescence images showing LUC expression in M-44 mutant, wild-type (35SP-LUC), and LUC (35SP-LUC-3′UTR) transgenic plants. D, Luminescence intensity indicating LUC expression in C. One representative experiment of three replicates is shown. Error bars represent sd (n = 20).
One LUC-negative wild-type line carrying a single copy of the 35SP-LUC transgene was mutagenized with ethyl methanesulfonate and screened for LUC activity using a high-throughput luminescence imaging system. A mutant designated M-44 was obtained (Fig. 1, C and D). Backcrossing of the M-44 mutant with wild-type plants produced F1 plants, all of which exhibited a wild-type phenotype. The ratio of luminescent to nonluminescent plants in the F2 generation was 1:3, indicating that the M-44 phenotype was controlled by a recessive mutation in a single nuclear gene.
Map-Based Cloning of MOM1
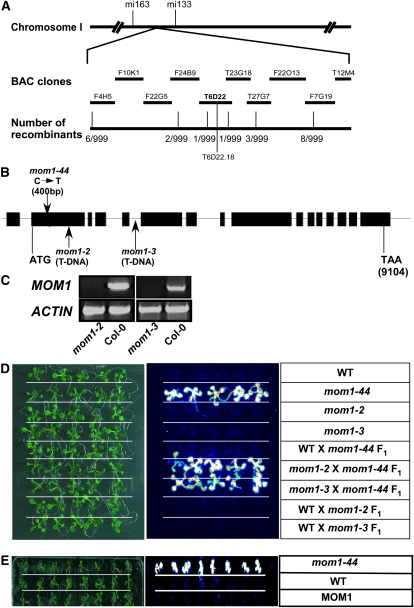
To identify the gene affected in M-44, we crossed the M-44 mutant (Col-0) with wild-type Landsberg erecta (Ler) plants and allowed the F1 progeny to self. F2 seedlings with LUC activity were then selected. Using simple sequence-length polymorphism markers, we mapped the M-44 locus to the upper arm of chromosome I between F24B9 and T23G18. We then sequenced all of the CDSs in the region. A single nucleotide substitution (C to T) located 400 bp downstream of the MOM1 (At1g08060) translation initiation codon was identified that introduced a premature stop codon in place of Arg-134, leading to early truncation of the MOM1 protein (Fig. 2, A and B). Thus, we renamed our mutant mom1-44.
Figure 2.
Positional cloning and confirmation of MOM1. A, Genetic mapping delimited M-44 to bacterial artificial chromosome clone (BAC) T6D22. B, Structure of MOM1 and position of the M-44/mom1-44 mutation. Boxes, exons; lines, introns. The mom1-44 mutation and T-DNA insertions in mom1-2 and mom1-3 are indicated by arrows. The M-44 mutation is located 400 bp downstream of the MOM1 transcription start site. C, Expression of MOM1 in Col-0, mom1-2, and mom1-3 as shown by RT-PCR. ACTIN was used as a control. D, M-44/mom1-44 is allelic to mom1. M-44/mom1-44 was crossed to wild-type (WT), mom1-2, or mom1-3 plants. LUC imaging was done using 7-d-old F1 wild-type or mom1 mutant seedlings. E, Complementation of mom1-44 as shown by LUC imaging. [See online article for color version of this figure.]
To determine whether the LUC phenotype of mom1-44 was due to the absence of MOM1, two mom1 mutant lines (SAIL_610_G01/mom1-2 and SALK_141293/mom1-3) with a T-DNA insertion in MOM1 were obtained from the Arabidopsis Biological Resource Center (ABRC; Alonso et al., 2003). The T-DNA insertions in exon 2 and intron 5 of MOM1 were confirmed by PCR using a MOM1-specific primer and T-DNA left border primers. The absence of a full-length MOM1 transcript in the mom1-2 and mom1-3 mutants was confirmed by reverse transcription (RT)-PCR analysis (Fig. 2C). These mutants were then crossed to wild-type or mom1-44 plants, and the F1 seedlings were subjected to luminescence imaging. Introduction of the mom1 mutation (mom1-2 or mom1-3) into wild-type or mom1-44 plants had no significant effect on the level of LUC activity (Fig. 2D), suggesting that M-44/mom1-44 is allelic to mom1-2 and mom1-3. The MOM1 CDS under the control of the native MOM1 promoter (2.09 kb) in the presence of a MOM1 3′-UTR fragment (1.5 kb) was generated, and the resulting construct rescued the LUC activity of the mom1-44 mutant to the level seen in wild-type plants (Fig. 2E).
Reduced LUC Transcriptional Activity in Wild-Type Plants Lacking the 3′-UTR and Phenotypic Rescue by the mom1 Mutation
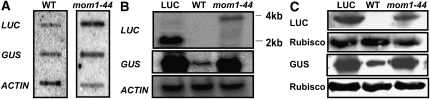
To determine whether the transcriptional activity of LUC in the wild-type plants was altered, we performed nuclear run-on assays. LUC pre-mRNA was detected at a level similar to that of GUS in the wild-type plants; in comparison, the mom1-44 mutation increased the transcriptional activity of both GUS and LUC (Fig. 3A).
Figure 3.
LUC and GUS expression in wild-type (WT) and mom1-44 plants. A, Nuclear run-on assays of LUC and GUS. ACTIN was used as a control. B, Transcript levels and sizes of LUC and GUS. ACTIN was used as a control. C, Protein expression of LUC and GUS in wild-type and mom1-44 seedlings. Rubisco was used as a control.
We next examined the accumulation of LUC and GUS mRNA and protein by northern and western blotting. LUC mRNA and LUC protein were not detected in the wild-type plants, whereas GUS transcripts and protein were identified in the same assays, although the levels were significantly reduced compared with those seen in mom1-44 (Fig. 3, B and C). These results are consistent with the observation of a GUS but not a LUC signal in the wild-type seedlings, and they suggest that without the 3′-UTR, although LUC can be transcribed at a low level, it is quickly degraded in wild-type plants. Surprisingly, a 4-kb LUC transcript was produced in the mom1-44 plants, which is longer than its regular size (2 kb) in the LUC plants (Fig. 3B). The 4-kb LUC transcript was translated into a normal-sized and functional LUC protein (Fig. 3C), resulting in the recovery of LUC activity in the mom1-44 seedlings. These results suggest that MOM1 prevents the formation of aberrant mRNAs rather than functioning in their degradation. MOM1 mediates TGS through a dynamic process (Tariq et al., 2002) that may cause the suppression of LUC and GUS transcription in wild-type plants.
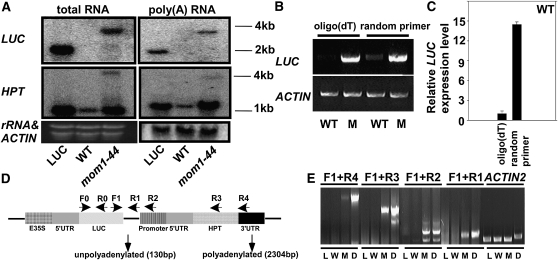
The LUC Transcript Was Unpolyadenylated in Wild-Type Plants But Polyadenylated in mom1-44 Due to the 3′-UTR of HPT
In the mom1 mutant, a number of endogenous targets have been found to produce aberrantly long transcripts (Steimer et al., 2000; Habu et al., 2006; Yokthongwattana et al., 2010), but how these long transcripts are generated is unknown. To determine how the extended LUC transcript was formed in mom1-44 plants, we first examined the polyadenylation status of LUC in wild-type and mom1-44 plants by RNA gel blotting using total and poly(A)+ RNA extracted from LUC, wild-type, and mom1-44 plants. In both types of RNA, an extended LUC transcript was detected in mom1-44, whereas the regular LUC transcript was detected in LUC; neither transcript was detected in the wild-type samples (Fig. 4A). Consistent with our northern-blotting results, cDNA reverse transcribed from the wild-type and mom1-44 RNA samples using oligo(dT) or random primers revealed the presence of LUC mRNA in mom1-44 from both cDNA pools. LUC was weakly detected in cDNA produced from the total RNA of the wild-type plants using random primers, but only if the number of PCR cycles was increased (Fig. 4B). The relative amount of LUC in the cDNA pool generated by RT using random primers was about 14 times that generated from the poly(A)+ RNA pool (Fig. 4C). Our results suggest that LUC mRNA was largely unpolyadenylated in the wild-type plants and that the unpolyadenylated mRNA was unstable and not translated. In contrast, the extended LUC transcript existed mainly in the polyadenylated state in the mom1-44 mutant. Northern-blot analysis using hygromycin phosphotransferase (HPT) as a probe revealed the presence of two HPT transcripts in mom1-44 (Fig. 4A). The lower, more intense band, which corresponds to HPT, was also observed in the LUC and wild-type plants, while the upper band, which was similar in size to the 4-kb LUC transcript, was detected in mom1-44. The HPT gene was located immediately behind LUC in the 35SP-LUC plasmid (Fig. 4D); thus, the calculated length from the translation start codon of LUC to the translation stop codon of HPT was about 4 kb. This indicates that the extended LUC transcript in mom1-44 was due to transcriptional read-through and that LUC was terminated by the HPT 3′-UTR. To confirm this hypothesis, RT-PCR analyses were performed using a forward primer specific for the LUC CDS (F) with reverse primers located between LUC and HPT (R1), in the HPT promoter (R2), in HPT (R3), or in the HPT 3′-UTR (R4) using oligo(dT) reverse-transcribed cDNA (Fig. 4D). Products were produced using all four primer combinations only from mom1-44 cDNA, and they were similar in size to the product amplified from mom1-44 genomic DNA (Fig. 4E). To rule out DNA contamination of the cDNAs, intron 1 of ACTIN2 was amplified from the same samples. As shown in Figure 4E, ACTIN2 cDNA was amplified from all of the samples, whereas a genomic ACTIN2 fragment containing the intron was amplified only from mom1-44 DNA. Our data indicate that the recovery of LUC activity in the wild-type plants by mutations in mom1 was due to stable read-through of the LUC transcript.
Figure 4.
LUC transcript status in wild-type (WT) and mom1-44 plants. A, Expression analysis of LUC and HPT in LUC, wild-type, and mom1-44 plants by northern blotting using poly(A)+ (right) or total (left) RNA. B, Expression analysis of LUC in wild-type and mom1-44 plants by RT-PCR using total RNA reverse transcribed using oligo(dT) (left) or random (right) primers. Primers used were F0 and R0. ACTIN was used as a loading control. C, Relative LUC expression in wild-type plants as shown by real-time PCR using total RNA reverse transcribed using oligo(dT) (left) or random (right) primers. Primers used were F0 and R0. ACTIN was used as an internal control. D, Structure of the 35SP-LUC and 35SP-HPT-3′UTR clusters. The positions of the primers used are indicated by arrows. Two forms of LUC mRNA were observed in the mom1-44 mutant (the positions are indicated by arrows): an unpolyadenylated transcript located 130 bp downstream of the LUC translation stop codon and a polyadenylated transcript produced using the HPT 3′-UTR located 2,304 bp downstream of the LUC translation stop codon. E, LUC transcriptional read-through was detected in mom1-44. The 4-kb LUC transcript was polyadenylated and terminated using the HPT 3′-UTR. The cDNAs were reverse transcribed from LUC plants (L), wild-type plants (W), and mom1-44 (M) plants. D indicates genomic DNA from mom1-44.
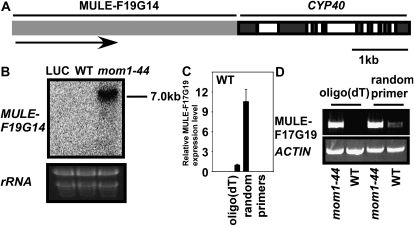
To further assess the termination status of LUC mRNA in wild-type and mom1-44 plants, we carried out a 3′-RACE assay. Following the addition of a 3′-RNA linker, we detected fewer unpolyadenylated LUC transcripts in the mom1-44 mutant, which terminated 130 bp downstream of the LUC stop codon (Fig. 4D). Although we successfully amplified LUC (Fig. 4B), we were unable to obtain a LUC RACE product in the wild-type case. A possible explanation for this is that LUC mRNA was rare and unstable in the wild-type plants; in addition, the efficiency of addition of the 3′-RNA linker may have been low. We also found that the LUC transcript was terminated at the 3′-UTR of HPT, corresponding to the 4-kb LUC transcript detected in mom1-44 (Fig. 4E). Our results demonstrate that deletion of the 3′-UTR led to the production of improperly terminated, unpolyadenylated LUC mRNA in wild-type plants. Mutations in MOM1 caused transcriptional read-through resulting in extended (4-kb) LUC transcripts terminated by the HPT 3′-UTR. The HPT gene within this cluster served as part of the 3′-UTR and had little effect on LUC translation. Consistent with this observation, a 7-kb-long transcript of MULE-F19G14, a mutator-like element with an abnormal RNA structure, was only detected in mom1-44 terminated by the 3′-UTR of downstream gene CYCLOPHILIN40 (CYP40; Fig. 5). It is well known that elements in the 3′-UTR direct the addition of a poly(A) tail (Colgan and Manley, 1997; Wahle and Rüegsegger, 1999; Zhao et al., 1999), which plays an important role in the termination of transcription (Proudfoot, 1989). The polyadenylation of mRNAs is critical both for export into the cytoplasm and translation (Eckner et al., 1991; Huang and Carmichael, 1996, 2001). The 4-kb LUC mRNA in mom1-44 was structurally complete and therefore was more stable and translatable.
Figure 5.
Endogens MULE-F19G14 transcript status in wild-type (WT) and mom1-44 plants. A, Scheme of the chromosomal region containing MULE-F19G14 and CYP40. The gray box represents MULE-F19G14; dark and open boxes represent exons and introns of CYP40, respectively. B, A 7-kb-long transcript of MULE-F19G14 was only detected in mom1-44 by northern-blotting assay. rRNA was used as a loading control. C, Relative MULE-F19G14 expression in wild-type plants as shown by real-time PCR using total RNA reverse transcribed using oligo(dT) (left) or random (right) primers. ACTIN was used as an internal control. D, Expression analysis of MULE-F19G14 in wild-type and mom1-44 plants by RT-PCR using total RNA reverse transcribed using oligo(dT) (left) or random (right) primers.
MOM1 Regulates CaMV 35SP-Directed Small RNA Production Independent of Its Inhibitory Function in Transcriptional Read-Through
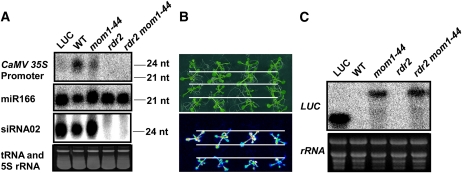
GUS and HPT expression was reduced in the wild-type plants compared to that in mom1-44 (Figs. 3B and 4A). Notably, the GUS, HPT, and LUC genes in 35SP-LUC were all under the control of the 35SP. It is possible that the presence of multiple 35SPs led to the production of siRNAs, which in turn repressed gene expression in the wild-type plants. This is in agreement with the notion that MOM1 helps mediate siRNA accumulation at the core regions of RdDM (Yokthongwattana et al., 2010). To confirm whether 35SP-directed siRNA was generated with a role in the transcription of aberrant mRNA in wild-type plants, we tested for the accumulation of siRNA in mom1-44 and wild-type plants. As shown in Figure 6A, a 35SP-directed siRNA was indeed generated in the wild-type plants and at a reduced level in the mom1-44 mutant. These data may explain why the transcription of LUC, GUS, and HPT was reduced in the wild-type plants compared with mom1-44. We also monitored the accumulation of other endogenous small RNAs: the microRNA miR166 and RDR2-dependent siRNA02 (Axtell and Bartel, 2005). The expression levels of these small RNAs were similar between the wild-type and mom1-44 mutant plants (Fig. 6A), suggesting that MOM1 is not universally involved in small RNA generation; rather, it helps regulate the expression of specific siRNAs. This hypothesis is consistent with previous findings (Elmayan et al., 2005; Vaillant et al., 2006; Yokthongwattana et al., 2010). RDR2 is a key component of the RdDM pathway (Matzke et al., 2009). We crossed wild-type or mom1-44 plants with rdr2 plants to generate 35SP-LUC/rdr2 and 35SP-LUC/rdr2 mom1-44 mutant plants and tested whether RDR2 regulates 35SP-directed siRNA generation and aberrant LUC transcription. In the rdr2 and rdr2 mom1-44 mutants, no 35SP-directed siRNA was detected (Fig. 6A), indicating that 35SP-directed siRNA was generated by the RDR2 pathway in wild-type and mom1-44 plants. We next assessed the level of LUC activity in the 35SP-LUC/rdr2 and 35SP-LUC/rdr2 mom1-44 plants. As shown in Figure 6B, the loss of RDR2 function did not affect LUC activity in either the wild-type or mom1-44 plants. This observation was confirmed by an RNA gel-blot assay (Fig. 6C). Taken together, the siRNA generated due to the presence of multiple CaMV 35SPs had no effect on the aberrant RNA transcriptional read-through.
Figure 6.
35SP-directed siRNA production in wild-type (WT) and mom1-44 plants is not related to LUC transcriptional read-through. A, Detection of CaMV 35SP-directed siRNA, miR166, and siRNA02 in the indicated genotypes. An ethidium bromide-stained gel corresponding to tRNA and 5S rRNA was used as a loading control. The positions of the size markers are indicated (24 or 21 nucleotides [nt]). B, LUC luminescence in mom1-44, rdr2, and rdr2 mom1-44 mutant plants. The plants shown (from top to bottom) are as follows: wild type, mom1-44, rdr2, and rdr2 mom1-44. C, Expression of LUC in LUC, wild-type, mom1-44, rdr2, and rdr2 mom1-44 plants. Ethidium bromide-stained rRNA was used as a loading control. [See online article for color version of this figure.]
The Transcriptional Read-Through of Genes with an Aberrant 3′-UTR Occurs Genome Wide in mom1 Plants
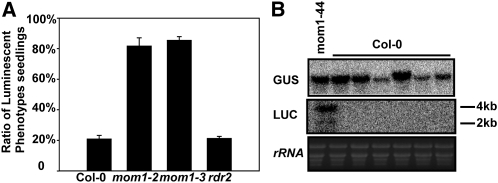
It was previously shown that MOM1 mediates TGS mainly in intermediate heterochromatin (Habu et al., 2006). To test whether the regulation of aberrant RNA transcriptional read-through by MOM1 is a universal event that is independent of gene position and copy number, we transformed the 35SP-LUC or 35SP-LUC-3′UTR plasmid into Col-0, mom1-2, mom1-3, and rdr2 plants. More than 100 independent T1 hygromycin-resistant plants from each transformation were subjected to LUC luminescence imaging. As shown in Figure 7A, 20.9%, 81.7%, 85.4%, and 21.2% of the 35SP-LUC transgenic plants produced in the Col-0, mom1-2, mom1-3, and rdr2 backgrounds were LUC positive, respectively. However, in all four backgrounds, about 90% of the transgenic plants harboring 35SP-LUC-3′UTR were LUC positive. These results indicate that the prevention of aberrant RNA transcription by MOM1 is independent of gene position, gene copy number, and RDR2.
Figure 7.
LUC transcriptional read-through in mom1 is independent of the insertion position, copy number, and RDR2. A, Luminescence intensity (LUC expression) in various genetic backgrounds. Error bars represent sd (n = 20). B, Expression of 4-kb LUC and GUS transcripts in LUC-negative 35S-LUC transgenic seedlings (six independent lines) produced in the Col-0 background. rRNA was used as a loading control.
We randomly selected LUC-negative seedlings in the Col-0 background to examine the relationship between LUC and GUS expression by RNA gel-blot analysis. LUC expression was undetectable in the Col-0 background but was rescued in the mom1-44 mutant by generating a 4-kb stable LUC-HPT transcript, and its expression level was not correlated with that of GUS in the same plant (Fig. 7B). These results suggest that the regulation of transcriptional read-through (LUC) and TGS (LUC, GUS, and HPT) by MOM1 are independent events.
DISCUSSION
In eukaryotic cells, the 3′-UTR plays important roles in transcription and translation. Polyadenylation is directed by the 3′-UTR and contributes to the termination of transcription (Proudfoot et al., 2002; Moore and Proudfoot, 2009; West and Proudfoot, 2009). Lack of the 3′-UTR results in the production of an improperly terminated transcript that is rapidly degraded by the cell’s quality control system through such methods as nonsense-mediated mRNA decay (Kim et al., 2009). However, the molecular mechanisms that serve to prevent aberrant RNA transcription are not well understood. In this study, we found that MOM1 is a key player in the repression of aberrant RNA transcriptional read-through.
MOM1 Plays an Important Role in the Prevention of Transcriptional Read-Through
The regulation of transcription read-through is not well understood. Many factors, such as cis/trans-acting regulators and TGS regulators, could be involved in this process. Read-through transcription is found in at least 2% to 5% of human genes and 1% of plant genes (Münk et al., 2008; Xing et al., 2010).
Eukaryotic genomes contain large numbers of transposons, and the regulation of their transcription is important to maintain genome integrity. Most of the up-regulated targets in mom1 mutant plants are located in regions of intermediate heterochromatin (Numa et al., 2010; Yokthongwattana et al., 2010). A number of endogenous targets of MOM1 exist in an extended form in which the transcript is longer than the expected size, including TSI (Steimer et al., 2000), MULE-F19G14 (Habu et al., 2006), and 5S genes (Vaillant et al., 2006). In the mom1 mutant, TSI exists in two forms: as a long polyadenylated transcript and as a short unpolyadenylated transcript. The MULE-F19G14 transcript is inverted relative to its original direction, and the antisense transcript is terminated by the downstream gene CYP40. A long transcript (210 bp) is generated at 5S rDNA loci that is longer than its regular size of 120 bp (Vaillant et al., 2006). However, the molecular mechanism involved in generating these extended transcripts is not understood. In this study, we found that the absence of the 3′-UTR of LUC led to the production of a transcript that was unpolyadenylated and degraded in wild-type plants; in contrast, in mom1, the corresponding transcript was polyadenylated and stable and was found to be 4 kb in length due to the use of the HPT 3′-UTR. TSI, MULE-F19G14, and 5S genes all contain aberrant RNA structures and form extended, stable transcripts in mom1.
Regulation of Transcriptional Read-Through by MOM1 Is Independent of the RdDM Pathway
MOM1 regulates TGS in a DNA methylation-independent manner (Amedeo et al., 2000). In the mom1 mutant, histone modification of the coding regions of MOM1 targets is intact (Habu et al., 2006; Vaillant et al., 2006), and nuclear organization appears to be unaltered (Probst et al., 2003). A recent study showed that RNA polymerase V interacts genetically with MOM1 in the control of gene silencing and that MOM1 contributes to the accumulation of small RNAs at promoter regions (Yokthongwattana et al., 2010). The transcriptional activation of various genes in the mom1 mutant is associated with a reduction in dimethylated histone H3 Lys-9 in the promoter region, and MOM1 transduces RdDM signals to repress histone modification (Numa et al., 2010). We detected small RNAs generated from the CaMV 35SP. The siRNA produced did not affect LUC expression or transcriptional read-through, because in the rdr2 mutant the LUC gene was still silenced, whereas in the rdr2 mom1 double mutant we were able to detect a 4-kb LUC transcript when the siRNA was removed. The ratio of luminescent to nonluminescent plants among the 35SP-LUC transgenic lines produced in a mom1-2 or mom1-3 background was greater than that in the Col-0 and rdr2 backgrounds (Fig. 7A). These data indicate that the function of MOM1 in the repression of transcriptional read-through differs from its function in the regulation of TGS, and this regulation is independent of the gene locus and transgene copy number and instead is related to the structural integrity of the gene. Twenty percent of the 35SP-LUC transgenic lines in Col-0 contained the 4-kb-long transcript and were LUC positive. This may indicate that preventing transcriptional read-through is regulated by multiple genes, and MOM1 is one of them.
It has been known that aberrant transcripts trigger RDR6-mediated RNA silencing (Luo and Chen, 2007). MOM1 maintains the transcriptionally inactive status of TGS targets; however, this activity does not correlate with releasing the aberrant transcript (Amedeo et al., 2000). Our results only imply that the effect of MOM1 on transcriptional read-through is independent of the RdDM pathway. However, we cannot rule out the possibility that, for example, MOM1 may control the TGS of one or more genes such as RDR6, which in turn can promote transcriptional read-through.
MATERIALS AND METHODS
Plant Materials and Mutant Isolation
For LUC imaging, 1-week-old Arabidopsis (Arabidopsis thaliana) seedlings grown on Murashige and Skoog agar plates under constant light at 21°C ± 1°C were used. Plants were grown in soil in growth chambers at 70% relative humidity with 16 h of light at 22°C and 8 h of dark at 18°C per day.
To construct the 35SP-LUC and 35SP-LUC-3′UTR plasmids, we extracted the CaMV 35SP-LUC coding region 3′-UTR with HindIII and EcoRI and the CaMV 35SP-LUC coding region with HindIII and SacI from pZH01 LUC+ and subcloned them into the binary vector pCAMBIA1301 at the corresponding sites. Arabidopsis (Col-0) expressing the 35SP-LUC (3′-UTR deletion) and 35S-LUC-3′UTR reporter genes are referred to as wild-type and LUC plants, respectively, in this study. The wild-type plants were mutagenized with ethyl methanesulfonate. Mutants were identified by luminescence imaging and GUS staining. Two T-DNA insertion lines, SAIL_610_G01 (mom1-2) and SALK_141293 (mom1-3), were obtained from the ABRC, and the insertion site in each was confirmed by PCR using MOM1- and left border-specific primers.
Positional Cloning
For map-based cloning, homozygous mom1-44 mutants produced in the Col-0 background were crossed to Ler plants and the F1 progeny were allowed to self. The level of LUC activity in the F2 plants was determined by luminescence imaging to select homozygous mom1-44 plants. A total of 999 homozygous mom1-44 mutant plants were selected for mapping with molecular markers that are polymorphic between Ler and Col-0. Genetic mapping was performed as described previously (Guo et al., 2001). Using simple sequence-length polymorphism markers, mom1-44 was first mapped to chromosome 1 between mi163 and mi133. Markers F22G5, T27G7, F24B9, and T23G18 were then used to pinpoint the mom1-44 mutation to bacterial artificial chromosome clone T6D22. To identify the mutation in mom1-44, candidate genes from mom1-44 mutant plants were sequenced.
RNA Analysis
Total RNA was isolated from 2-week-old wild-type or mutant seedlings using RNAVzol (Vigorous), and poly(A)+ RNA prepared using an Oligotex mRNA Kit (Qiagen) according to the manufacturer’s protocol was used for RNA gel-blot or RT-PCR analysis.
Small RNAs were enriched by precipitation with 5% polyethylene glycol (Mr =8,000) and 0.5 m NaCl, separated on a 15% polyacrylamide-7 m urea gel in 1× Tris-borate/EDTA, and transferred to a Hybond N+ Nucleic Acid Transfer Membrane (Amersham). The [32P]35SP probe was labeled using a Random Primer Kit (Takara), while the [32P]miR166 and [32P]siRNA02 probes were labeled using T4 Polynucleotide Kinase (New England Biolabs).
The primers used for RNA analysis are described in Supplemental Table S1.
Western Blotting
Total protein was extracted from 2-week-old wild-type and mom1-44 mutant seedlings. Equal amounts of total protein were separated by 12% (w/v) SDS-PAGE and blotted onto a polyvinylidene difluoride membrane (Millipore). The blots were then probed with primary anti-LUC (Sigma-Aldrich; L2164) or anti-GUS (made by National Institute of Biological Sciences; http://www.nibs.ac.cn/antibody/zxjs) antibodies; chemiluminescence was detected using x-ray film.
Run-On Assay
We blotted 1 μg of LUC, GUS, or ACTIN cDNA on a Hybridization Nitrocellulose Filter (Millipore) using a Bio-Dot SF Blotting Apparatus (Bio-Rad). Nuclei were isolated from 2-week-old seedlings. Nuclear run-on assays were then carried out as described previously (Folta and Kaufman, 2006). The primers used for probe amplification are described in Supplemental Table S2.
3′-RACE
3′-RACE was performed using a Clontech cDNA Amplification Kit according to the manufacturer’s protocol. A 3′ linker was added using T4 RNA Ligase 2, Truncated (New England Biolabs). The primers used were 5′-CCCGCTTTCCAGTCGGGAAACCTGTC-3′ (LUC-GSP) and 5′-CCCACTATCCTTCGCAAGACCTTCCTC-3′ (nested primer LUC-NGSP); the sequence of the 3′ linker was 5′-CGACGTAAAGCAGAAGACGGCATACGA-3′.
Complementation
A 2-kb ACTIN2 promoter fragment spanning the transcription start site was cloned into the binary vector pCAMBIA2300 at the BstXI-NcoI sites to drive NPTII expression. A 2-kb MOM1 promoter fragment upstream of the transcription start site followed by the MOM1 CDS and a 1.5-kb DNA fragment downstream of the MOM1 transcription stop site was assembled in series by cloning into the SalI-BamHI, BamHI-KpnI, and KpnI-EcoRI sites in pCAMBIA2300, respectively. The primers used for complementation are provided in Supplemental Table S3.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Primer list for northern blot and PCR.
Supplemental Table S2. Primer list for run-on assays.
Supplemental Table S3. Primer list for the MOM1 gene.
Supplementary Material
Acknowledgments
We thank Drs. Bing Zhu, Yijun Qi, and Xialu Li at the National Institute of Biological Sciences for helpful advice and support during this project. We thank the ABRC (The Ohio State University) for the T-DNA insertion lines.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Amedeo P, Habu Y, Afsar K, Mittelsten Scheid O, Paszkowski J. (2000) Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 405: 203–206 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Bartel DP. (2005) Antiquity of microRNAs and their targets in land plants. Plant Cell 17: 1658–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm-Ansmant I, Kashima I, Rehwinkel J, Saulière J, Wittkopp N, Izaurralde E. (2007) mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett 581: 2845–2853 [DOI] [PubMed] [Google Scholar]
- Chang YF, Imam JS, Wilkinson MF. (2007) The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76: 51–74 [DOI] [PubMed] [Google Scholar]
- Colgan DF, Manley JL. (1997) Mechanism and regulation of mRNA polyadenylation. Genes Dev 11: 2755–2766 [DOI] [PubMed] [Google Scholar]
- Eckner R, Ellmeier W, Birnstiel ML. (1991) Mature mRNA 3′ end formation stimulates RNA export from the nucleus. EMBO J 10: 3513–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmayan T, Proux F, Vaucheret H. (2005) Arabidopsis RPA2: a genetic link among transcriptional gene silencing, DNA repair, and DNA replication. Curr Biol 15: 1919–1925 [DOI] [PubMed] [Google Scholar]
- Folta KM, Kaufman LS. (2006) Isolation of Arabidopsis nuclei and measurement of gene transcription rates using nuclear run-on assays. Nat Protoc 1: 3094–3100 [DOI] [PubMed] [Google Scholar]
- Guo Y, Halfter U, Ishitani M, Zhu JK. (2001) Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13: 1383–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu Y, Mathieu O, Tariq M, Probst AV, Smathajitt C, Zhu T, Paszkowski J. (2006) Epigenetic regulation of transcription in intermediate heterochromatin. EMBO Rep 7: 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr AJ, Molnàr A, Jones A, Baulcombe DC. (2006) Defective RNA processing enhances RNA silencing and influences flowering of Arabidopsis. Proc Natl Acad Sci USA 103: 14994–15001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Carmichael GG. (1996) Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol Cell Biol 16: 1534–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Carmichael GG. (2001) Nucleocytoplasmic mRNA transport. Results Probl Cell Differ 34: 139–155 [DOI] [PubMed] [Google Scholar]
- Isken O, Maquat LE. (2007) Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21: 1833–1856 [DOI] [PubMed] [Google Scholar]
- Kim SH, Koroleva OA, Lewandowska D, Pendle AF, Clark GP, Simpson CG, Shaw PJ, Brown JW. (2009) Aberrant mRNA transcripts and the nonsense-mediated decay proteins UPF2 and UPF3 are enriched in the Arabidopsis nucleolus. Plant Cell 21: 2045–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Chen Z. (2007) Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell 19: 943–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ. (2009) RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21: 367–376 [DOI] [PubMed] [Google Scholar]
- Moore MJ, Proudfoot NJ. (2009) Pre-mRNA processing reaches back to transcription and ahead to translation. Cell 136: 688–700 [DOI] [PubMed] [Google Scholar]
- Münk C, Beck T, Zielonka J, Hotz-Wagenblatt A, Chareza S, Battenberg M, Thielebein J, Cichutek K, Bravo IG, O’Brien SJ, et al. (2008) Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome Biol 9: R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa H, Kim JM, Matsui A, Kurihara Y, Morosawa T, Ishida J, Mochizuki Y, Kimura H, Shinozaki K, Toyoda T, et al. (2010) Transduction of RNA-directed DNA methylation signals to repressive histone marks in Arabidopsis thaliana. EMBO J 29: 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst AV, Fransz PF, Paszkowski J, Mittelsten Scheid O. (2003) Two means of transcriptional reactivation within heterochromatin. Plant J 33: 743–749 [DOI] [PubMed] [Google Scholar]
- Proudfoot NJ. (1989) How RNA polymerase II terminates transcription in higher eukaryotes. Trends Biochem Sci 14: 105–110 [DOI] [PubMed] [Google Scholar]
- Proudfoot NJ, Furger A, Dye MJ. (2002) Integrating mRNA processing with transcription. Cell 108: 501–512 [DOI] [PubMed] [Google Scholar]
- Steimer A, Amedeo P, Afsar K, Fransz P, Mittelsten Scheid O, Paszkowski J. (2000) Endogenous targets of transcriptional gene silencing in Arabidopsis. Plant Cell 12: 1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq M, Habu Y, Paszkowski J. (2002) Depletion of MOM1 in non-dividing cells of Arabidopsis plants releases transcriptional gene silencing. EMBO Rep 3: 951–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant I, Schubert I, Tourmente S, Mathieu O. (2006) MOM1 mediates DNA-methylation-independent silencing of repetitive sequences in Arabidopsis. EMBO Rep 7: 1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E, Rüegsegger U. (1999) 3′-end processing of pre-mRNA in eukaryotes. FEMS Microbiol Rev 23: 277–295 [DOI] [PubMed] [Google Scholar]
- West S, Proudfoot NJ. (2009) Transcriptional termination enhances protein expression in human cells. Mol Cell 33: 354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E, Proudfoot N. (1986) Alpha-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3′ end processing in the human alpha 2 globin gene. EMBO J 5: 2915–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing A, Moon BP, Mills KM, Falco SC, Li Z. (2010) Revealing frequent alternative polyadenylation and widespread low-level transcription read-through of novel plant transcription terminators. Plant Biotechnol J 8: 772–782 [DOI] [PubMed] [Google Scholar]
- Yokthongwattana C, Bucher E, Caikovski M, Vaillant I, Nicolet J, Scheid OM, Paszkowski J. (2010) MOM1 and Pol-IV/V interactions regulate the intensity and specificity of transcriptional gene silencing. EMBO J 29: 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Hyman L, Moore C. (1999) Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol Mol Biol Rev 63: 405–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.