Abstract
Drought is a major limiting factor for crop production. To identify critical genes for drought resistance in rice (Oryza sativa), we screened T-DNA mutants and identified a drought-hypersensitive mutant, dsm2. The mutant phenotype was caused by a T-DNA insertion in a gene encoding a putative β-carotene hydroxylase (BCH). BCH is predicted for the biosynthesis of zeaxanthin, a carotenoid precursor of abscisic acid (ABA). The amounts of zeaxanthin and ABA were significantly reduced in two allelic dsm2 mutants after drought stress compared with the wild type. Under drought stress conditions, the mutant leaves lost water faster than the wild type and the photosynthesis rate, biomass, and grain yield were significantly reduced, whereas malondialdehyde level and stomata aperture were increased in the mutant. The mutant is also hypersensitive to oxidative stresses. The mutant had significantly lower maximal efficiency of photosystem II photochemistry and nonphotochemical quenching capacity than the wild type, indicating photoinhibition in photosystem II and decreased capacity for eliminating excess energy by thermal dissipation. Overexpression of DSM2 in rice resulted in significantly increased resistance to drought and oxidative stresses and increases of the xanthophylls and nonphotochemical quenching. Some stress-related ABA-responsive genes were up-regulated in the overexpression line. DSM2 is a chloroplast protein, and the response of DSM2 to environmental stimuli is distinctive from the other two BCH members in rice. We conclude that the DSM2 gene significantly contributes to control of the xanthophyll cycle and ABA synthesis, both of which play critical roles in the establishment of drought resistance in rice.
Abiotic stresses such as drought, salinity, and adverse temperatures are major limiting factors for plant growth and reproduction. To respond to environmental cues, plants have evolved a variety of biochemical and physiological mechanisms to adapt to adverse conditions during their growth and development (Boyer, 1982). Abscisic acid (ABA) has been recognized as a stress hormone that coordinates the complex networks of stress responses. Under drought or salt stress conditions, plant endogenous ABA level can rise to about 40-fold, triggering the closure of stomata and accumulating reactive oxygen species (ROS), dehydrins, and late embryogenesis abundant proteins for osmotic adjustment (Verslues et al., 2006). The endogenous ABA level is determined by ABA biosynthesis, catabolism, and release of ABA from ABA-Glc conjugates (Nambara and Marion-Poll, 2005; Lee et al., 2006). Therefore, identification of all the components affecting active ABA content is essential for a complete understanding of the action of the hormone.
Numerous ABA biosynthetic genes have been identified through mutant analysis, such as maize (Zea mays) viviparous mutants vp2, vp5, vp7, vp9, vp14, w3, y3, and y9 (Schwartz et al., 1997; Hable et al., 1998; Singh et al., 2003); rice (Oryza sativa) preharvest-sprouting mutants psh1, psh2, psh3, and psh4 (Fang et al., 2008); sunflower (Helianthus annuus) nondormant mutant nd-1 (Conti et al., 2004); Arabidopsis (Arabidopsis thaliana) ABA- and nonphotochemical quenching (NPQ)-deficient mutants aba1, aba2, aba3, aba4, npq1, npq2, b1, b2, and nced3 (Havaux et al., 2000; Xiong et al., 2001; Tian et al., 2003; Barrero et al., 2005; Kim and DellaPenna, 2006; North et al., 2007); and tomato (Solanum lycopersicum) white-flower mutant wf (Galpaz et al., 2006; Supplemental Fig. S1). The mutants unable to biosynthesize carotenoid precursors for endogenous ABA synthesis often produced preharvest-sprouting seeds and wilted or white leaves (Gubler et al., 2005; Nambara and Marion-Poll, 2005; Finch-Savage and Leubner-Metzger, 2006).
ABA biosynthesis initiates with the synthesis of a C5 building block, isopentenyl pyrophosphate, and its isomer dimethylallyl pyrophosphate through a plastid methylerythritol phosphate pathway (Eisenreich et al., 2001; Hunter, 2007). The three isopentenyl pyrophosphate molecules are then added to dimethylallyl pyrophosphate by geranylgeranyl diphosphate synthase to produce C20 geranylgeranyl diphosphate. Two geranylgeranyl diphosphates are condensed by a committing enzyme, phytoene synthase, to produce colorless C40 carotenoid phytoene, which is then desaturated and isomerized into red-colored lycopene by phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), and Z-ISO and CRTISO isomerases in plants (Isaacson et al., 2002; Park et al., 2002). Subsequently, several cyclization and hydroxylation reactions take place to yield α-carotene and β-carotene (Li et al., 1996; Hable et al., 1998; Park et al., 2002; Miki and Shimamoto, 2004; Fang et al., 2008). Heme-type cytochrome P450-type CYP97 and non-heme-type β-carotene hydroxylase (BCH) are primarily responsible for the hydroxylation of α-carotene and β-carotene to produce lutein and zeaxanthin, respectively. Zeaxanthin, an important component of the xanthophyll cycle, is epoxidated by zeaxanthin epoxidase to produce violaxanthin, and this reaction can be reversed by violaxanthin deepoxidase to increase the xanthophyll cycle for plants to adapt to high-light stress (Johnson et al., 2008). Neoxanthin synthase converts violaxanthin into neoxanthin (North et al., 2007). In chloroplast, 9-cis-epoxycarotenoid dioxygenase (NCED) cleaves violaxanthin and neoxanthin to produce xanthoxin, the direct substrate for ABA synthesis via ABA aldehyde (Schwartz et al., 1997, 2003; Xiong and Zhu, 2003). Increasing evidence suggest that the endogenous ABA level is fine-tuned by differential regulation of the multiple steps of ABA biosynthesis (Seo and Koshiba, 2002; Nambara and Marion-Poll, 2005; Destefano-Beltrán et al., 2006; Thompson et al., 2007; Rodríguez-Gacio et al., 2009; Supplemental Fig. S1).
The xanthophyll cycle (light-dependent reversible conversion between violaxanthin and zeaxanthin) is involved in photoprotection in PSII by regulating the nonradiative dissipation of excess absorbed light energy as heat (Gilmore et al., 1994). Mutants with defects in the xanthophyll cycle exhibit a weak photoprotective ability and produce ROS such as hydrogen peroxide (H2O2) when the absorption of light energy exceeds that consumed for photosynthesis (Niyogi, 1999). Under dehydration stress, electrons at a high energy state can easily form ROS, which are toxic to proteins, DNA, and lipids (Mittler, 2002; Apel and Hirt, 2004). However, plants have evolved a variety of biochemical and physiological mechanisms to scavenge ROS, thus maintaining a balance between ROS production and scavenging (Mittler et al., 2004).
An association between the xanthophyll cycle and stress tolerance has been reported in plants. In Arabidopsis, overexpression of a bacterial BCH gene caused a specific 2-fold increase in the size of the xanthophyll cycle and enhanced photooxidative tolerance (Davison et al., 2002). Constitutive overexpression of a bacterial BCH gene, crtZ, in tobacco (Nicotiana tabacum) led to increased zeaxanthin synthesis and enhanced UV light tolerance (Götz et al., 2002). In Arabidopsis, zeaxanthin synthesis can be catalyzed by both heme-type CYP97 hydroxylases LUT1 and LUT5 and non-heme-type hydroxylases BCH1 and BCH2, and these two types exhibit some overlapping activities (Tian et al., 2003, 2004; Kim and DellaPenna, 2006). In contrast to the intensive molecular and genetic studies of BCH in Arabidopsis, the counterpart in economically important crops such as rice has not been identified.
In this study, we characterized the rice drought-sensitive mutant dsm2, impaired in the gene DSM2 encoding a BCH. Our results demonstrate that DSM2 acts as a putative enzyme catalyzing the biosynthesis of zeaxanthin, one of the precursors of ABA that participates in the process of NPQ. Decreases of NPQ, maximal efficiency of PSII photochemistry (Fv/Fm), xanthophylls, and ABA in the dsm2 mutant suggest that the drought hypersensitivity of dsm2 is due to the combination of impairments in the xanthophyll cycle and ABA synthesis under drought stress conditions. DSM2 overexpression lines, possessing high Fv/Fm and NPQ, showed significantly improved drought resistance at both seedling and reproductive stages. Furthermore, our results imply that DSM2 may be the major member of the BCH family in rice for controlling zeaxanthin synthesis in response to dehydration stresses.
RESULTS
Identification of the Drought-Hypersensitive Mutant dsm2 in Rice
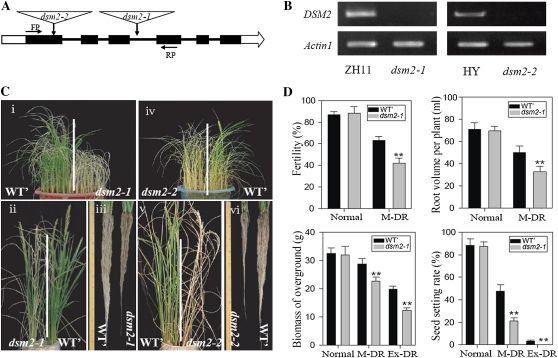
To identify critical genes required for drought resistance in rice, we screened T-DNA insertion mutants in the background of japonica Zhonghua11 (ZH11) rice, selected from the Rice Mutant Database (Wu et al., 2003; Zhang et al., 2006), for drought resistance under field conditions. One of the drought-hypersensitive mutants showing drought sensitivity at both the seedling and panicle development stages, designated as dsm2-1, was further characterized in this study. Flanking sequence analysis indicated that a gene (LOC_Os03g03370) encoding a putative BCH (named DSM2/OsBCH1) belonging to the BCH family was interrupted (Fig. 1A). This gene was also named OsHYD3 (Vallabhaneni et al., 2009). We collected an allelic mutant of the OsBCH1 gene (Hwayoung background), named dsm2-2, from the POSTECH Rice T-DNA Insertion Sequence Database (An et al., 2003; Jeong et al., 2006). The T-DNA insertion sites of dsm2-1 and dsm2-2 are located in the third intron and the first exon, respectively. Transcript analysis suggested that the expression of DSM2 was abolished in the two allelic dsm2 mutants (Fig. 1B). Under normal conditions, the homozygous mutants showed no obvious phenotypic change compared with the wild-type genotype segregated from the heterozygous mutant (Supplemental Fig. S2, A and C). To verify the drought-sensitive phenotype, mutant and wild-type plants at the four-leaf stage grown in sandy soil were subjected to drought stress. Under the moderate stress condition, the dsm2 mutant lines wilted faster than the wild type. After severe drought stress treatment followed by rewatering, almost all the mutant plants died, whereas wild-type plants had a significantly higher survival rate (Fig. 1C). Cosegregation analysis also suggested that the drought sensitivity was due to the T-DNA insertion in the OsBCH1 gene (data not shown). The dsm2-1 mutant was also more sensitive than the wild type to salt stress (Supplemental Fig. S3), but no significant difference was observed under cold or heat shock stress (data not shown).
Figure 1.
Identification of dsm2 T-DNA insertion mutants and its drought sensitivity. A, Schematic diagram of DSM2 gene structure and two alleles of the T-DNA insertion mutants, dsm2-1 and dsm2-2. FP, Forward primer for RT-PCR; RP, reverse primer for RT-PCR. Exons and introns are indicated by black boxes and black lines, respectively. B, RT-PCR analysis of DSM2 expression level in ZH11, dsm2-1, Hwayoung, and dsm2-2. Actin1 was used as an internal control. C, Drought-sensitive phenotype of the two allelic mutants at the four-leaf stage (i, iv) and the heading stage (ii, v). The photographs were taken after severe drought stress followed by recovery (see “Materials and Methods”). Root was also photographed after the stress at the heading stage (iii, vi). WT’, Wild-type genotype segregated from the heterozygous dsm2 mutant. D, Evaluation of the drought sensitivity of the mutants in terms of spikelet fertility, root volume, seed-setting rate, and biomass under moderate drought stress (M-DR) and/or extreme drought (Ex-DR) at the heading stage. Values are means ± sd (n = 4). ** P < 0.01 (t test).
Drought sensitivity of the dsm2-1 mutant was also tested at the reproductive stage by growing the mutant and the wild type in a paddy field facilitated with a removable rain-off shelter and in polyvinyl chloride (PVC) tubes filled with sandy soil, as described previously (Hu et al., 2006; Yue et al., 2006). During the course of drought stress development, dsm2-1 showed wilting earlier than the wild type (Supplemental Fig. S2F). After moderate drought stress in the PVC tubes, the total grain yield of dsm2-1 was reduced by about 52% compared with the wild type, and the pollen fertility of dsm2-1 (23%) was also significantly lower than that of the wild type (58%; Fig. 1D). The root depth and volume of dsm2-1 were significantly reduced compared with the wild type (Fig. 1D), and contents of chlorophyll and Pro in dsm2-1 were also reduced prominently (Supplemental Fig. S2I). After severe drought stress applied at the panicle development stage in the field, the spikelet fertility, root volume, grain yield, and biomass were markedly decreased in the mutant compared with the wild type (Fig. 1D), and the photosynthesis rate was significantly lower in the dsm2-1 mutant than in the wild type (Supplemental Fig. S2I). These results suggest that the reduced yield and biomass of dsm2-1 may be partially due to the reduced root growth, pollen fertility, and photosynthesis rate under drought stress.
Overexpression of DSM2 Significantly Improves Drought Resistance
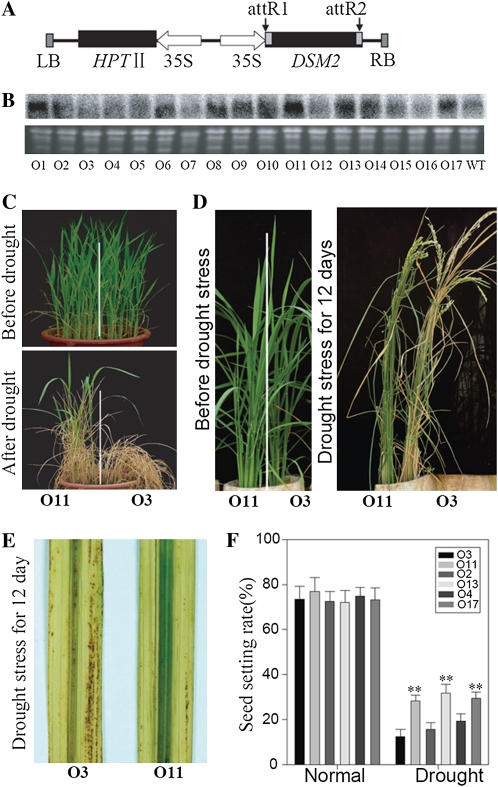
The drought-hypersensitive phenotype of the dsm2 mutant suggested that DSM2 is essential for drought resistance in rice. To test whether DSM2 overexpression has a significant effect on improving drought resistance, the full-length cDNA of DSM2 under the control of the cauliflower mosaic virus 35S promoter (Fig. 2A) was transformed into japonica rice ZH11. Northern-blot analysis of all 17 independent transgenic lines identified five independent transgenic lines showing overexpression of the DSM2 gene (Fig. 2B), and the result was confirmed by real-time PCR (Supplemental Fig. S4A). Three positive transgenic lines (O11, O13, O17) and three negative transgenic lines (O2, O3, O4) were tested for drought resistance at the four-leaf stage in barrels and at the reproductive stage in PVC tubes. After severe drought stress (no watering for 1 week), the overexpression lines had a significantly higher survival rate (approximately 74%) than the negative control (completely died; Fig. 2, C and D; Supplemental Fig. S4D). After drought treatments at the reproductive stage, the overexpression lines had more green leaves and higher spikelet fertility than negative transgenic lines (Supplemental Fig. S4, B and C). The overexpression lines had less oxidative damage on the leaves and higher seed-setting rates than the negative control (Fig. 2, E and F). These results suggest that overexpression of DSM2 has a significant effect on the improvement of drought resistance in rice.
Figure 2.
Improved drought resistance of DSM2-overexpressing transgenic rice. A, Schematic diagram of the overexpression construct. LB, Left border; RB, right border. B, Northern-blot analysis of transgenic plants using DSM2-specific cDNA as a probe. C, Appearance of one negative (O3) and one positive (O11) transgenic line at the seedling stage before and after drought stress. D, Appearance of the transgenic lines in PVC tubes after severe drought stress at the reproductive stage. E, Diaminobenzidine staining of drought-stressed leaves of the O3 and O11 transgenic lines at the reproductive stage. F, Spikelet fertility (seed-setting rate) of three negative (O2, O3, O4) and three positive (O11, O13, O17) transgenic lines after severe drought stress at the reproductive stage. Values are means ± sd (n = 12). ** P < 0.01 (t test).
Expression Profiles of DSM2
As dsm2 showed hypersensitivity to drought stress, we examined the expression level of DSM2 under drought as well as other treatments, including cold, heat, salt, light, UV light, and ABA, at the seedling stage (Fig. 3A). The DSM2 transcript level was induced (7- to 9-fold) by drought and salt treatments and slightly induced by ABA. However, DSM2 was not induced by the other treatments (and was actually slightly suppressed by cold stress). These results seem to be consistent with the role of dsm2 phenotypes in stress responses and imply that DSM2 may function mainly in drought and salt stress responses.
Figure 3.
Expression profile of DSM2 under normal and stress conditions. A, Expression level of DSM2 under stress treatment and in various tissues or organs examined by quantitative real-time PCR. The treatments include cold (4°C), heat shock (42°C), salt (200 mm NaCl), ABA (200 μm), UV light, or drought stress at the four-leaf stage. re24h, Recovery for 1 d; re2d, recovery for 2 d. Sixteen tissues or organs checked are as follows: 1, culm; 2, node; 3, sheath; 4, three-leaf-stage shoot; 5, hull; 6, seed; 7, secondary branching of inflorescence; 8, anther; 9, calli induction stage; 10, calli screening stage; 11, calli differentiation stage; 12, young shoot; 13, young root; 14, flag leaf in the morning; 15, flag leaf in the evening; 16, pulvinus. B, Diagram of the OsDSM2:GFP construct and expression pattern of GFP driven by the DSM2 promoter in transgenic rice plants (ZH11 background): i, calli; ii, endosperm and embryo; iii, plumule and radicle, 48 h after emergence in the dark; iv, ligule, auricle, pulvinus, and sheath; v, anther and filaments; vi, spikelet; vii, root, four-tiller stage; viii, vascular tissue in leaves, four-tiller stage; ix, flag leaves before drought; x, flag leaves under drought conditions for 2 d. LB, Left border; RB, right border.
To investigate the tempospatial expression pattern of DSM2, the GFP reporter gene under the control of the DSM2 promoter was transformed into ZH11 rice. A strong GFP signal was observed in the stamen, plumule, hull, pistil, mature leaf, and root, and a weak GFP signal was detected in calli, young shoot and root, and endosperm (Fig. 3B), suggesting an organ/tissue-dependent differential expression pattern of DSM2 in rice. When transgenic plants were drought stressed to leaf rolling, strong induction of GFP was observed in leaves, especially in vascular bundles (Fig. 3B).
Functional Analysis of DSM2 Featuring a BCH
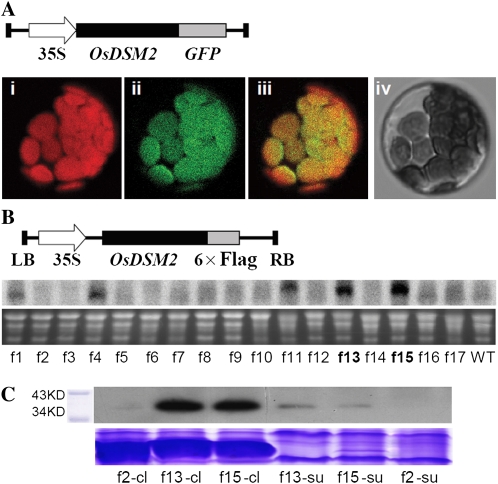
DSM2 is predicted to encode a putative BCH of 285 amino acids with a predicted molecular mass of 31.46 kD. We tried to examine the enzymatic activity of DSM2 in vitro by expressing the DSM2-GST in Escherichia coli, but the recombinant protein was mainly in the insoluble extract, which prevented the enzymatic assay. It has been suggested that carotenoid synthesis occurs in chloroplast (Bick and Lange, 2003). To determine the subcellular localization of DSM2, the open reading frame of DSM2 was fused in frame to the GFP reporter gene under the control of the 35S promoter and transformed into Arabidopsis mesophyll protoplasts. The fluorescence was observed only in the chloroplast (Fig. 4A). To confirm the chloroplast localization of DSM2 in plants, the open reading frame of DSM2 fused with the FLAG tag under the control of the maize ubiquitin promoter was transformed into ZH11 rice (Fig. 4B). The chloroplast proteins of positive and negative transgenic plants were extracted for western blot with antibody against the FLAG tag, and the result showed that the DSM2-FLAG protein existed exclusively in chloroplast protein (Fig. 4C).
Figure 4.
Subcellular localization of DSM2. A, Diagram of the OsDSM2:GFP construct and subcellular localization of the DSM2-GFP fusion protein. i, Chloroplast autofluorescence; ii, localization of the OsDSM2-GFP fusion protein; iii, merged image of i and ii; iv, image of the cell in bright field. B, Northern-blot analysis of the OsDSM2-Flag-overexpressed plants. LB, Left border; RB, right border; WT, wild type. C, Two positive transgenic lines (f13, f15) and one negative line (f2) are used for western-blot analysis using anti-FLAG antibody. cl, Chloroplast extract; su, supernatant of chloroplast extract.
To further clarify the function of DSM2, we checked the composition of carotenoids and ABA in the DSM2 overexpression and dsm2-1 mutant plants. Under normal conditions, the level of xanthophyll, a product of BCH, was significantly reduced (59% of the wild type) in the mutant (Table I). However, lycopene and β-carotene were accumulated at levels 1.2- and 1.7-fold higher in dsm2-1 than in the wild type (Table I). Lutein, a product of α-carotene hydroxylase, accumulated at a level 1.3-fold higher in the mutant than in the wild type. After moderate drought stress, the xanthophyll level was significantly reduced in the mutant, but lycopene and β-carotene contents were slightly increased in the mutant compared with their controls (Table I). Considering that DSM2 was induced dramatically by drought, DSM2 may be a major player in the OsBCH family for producing xanthophyll under drought stress. In the DSM2 overexpression lines, the xanthophyll level was increased by 1.4-fold compared with the control after the stress, but there was only a slight decrease in the amount of lycopene and β-carotene compared with the negative transgenic control, presumably owing to the increased conversion of β-carotene to zeaxanthin rather than to lutein (Table I). These results were consistent with the predicted function of DSM2 as a BCH.
Table I. Carotenoid composition and ABA content in leaves of dsm2-1 and the DSM2 overexpression line (DSM2-OE) quantified by HPLC and HPLC-mass spectrometry.
Carotenoid notes are as follows: a, seedling in normal conditions; ba, before drought stress at the reproductive stage; bb, drought stress for 3 d at the reproductive stage; bc, drought stress for 10 d at the reproductive stage; bd, recovery for 4 d at the reproductive stage. Asterisks indicate significance (t test) at * P < 0.05 and ** P < 0.01. Values are means ± sd (n = 3). The unit is ng g−1 leaf tissue.
| Carotenoid | Wild Type | dsm2-1 | DSM2-OE |
| Lycopenea | 7.87 ± 0.96 | 9.23 ± 0.84** | 7.64 ± 0.76 |
| α-Carotenea | 3.25 ± 0.43 | 4.87 ± 0.51** | 3.21 ± 0.29 |
| Luteina | 132.64 ± 9.7 | 168.3 ± 10.24** | 133.74 ± 8.93 |
| β-Carotenea | 42.98 ± 5.19 | 65.73 ± 7.35** | 41.75 ± 5.85* |
| Xanthophylla | 51.17 ± 4.15 | 23.63 ± 2.73** | 68.83 ± 6.01** |
| ABAba | 10.58 ± 1.92 | 9.81 ± 1.01 | 10.79 ± 1.46 |
| ABAbb | 59.28 ± 4.76 | 43.83 ± 3.06** | 60.68 ± 4.02 |
| ABAbc | 103.73 ± 4.95 | 81.83 ± 5.92** | 107.43 ± 5.79 |
| ABAbd | 22.86 ± 3.98 | 21.75 ± 1.63 | 22.05 ± 2.27 |
We also measured the endogenous ABA level in the dsm2-1 mutant, and the result showed that the ABA content was slightly lower than in the wild type before stress but was significantly (P < 0.01) lower than in the wild type after drought stress (Table I). This result suggested that DSM2 contributes significantly to ABA synthesis, especially under drought stress, at the step of zeaxanthin synthesis. However, the ABA level was not significantly enhanced in the DSM2 overexpression lines.
DSM2 Contributes to Photosynthetic Efficiency and NPQ Capacity
Due to the photoprotective function of xanthophylls, plants deprived of zeaxanthin often suffered from photobleaching damage (Niyogi, 1999; Tian et al., 2003). To determine the effect of reduced xanthophylls in the dsm2 on Fv/Fm and NPQ, chlorophyll fluorescence was measured in rice seedlings of a reference mutant, phs3-1 (impaired in the synthesis of lycopene), the two allelic dsm2 mutants, and the DSM2 overexpression line. The dsm2 and phs3-1 mutants showed significantly slower induction and lower amplitude of NPQ compared with the controls, whereas the overexpression line showed slightly higher NPQ induction than the control (Fig. 5A). The dsm2 and phs3-1 mutants also had significant lower Fv/Fm ratios than their controls, while the overexpression line showed an increased Fv/Fm ratio compared with the negative transgenic control (Fig. 5B), suggesting that the change in the xanthophyll cycle has a significant effect on the transfer efficiency of absorbed light energy to the PSII reaction center. This result is consistent with the predicted outcome of the reduced xanthophyll cycle in the dsm2 mutants and with a previous report on the reduced Fv/Fm and NPQ in phs3-1 (Fang et al., 2008).
Figure 5.
Photochemistry measurement of the mutant and overexpression lines at the four-leaf stage. A, NPQ of chlorophyll fluorescence as a function of actinic light intensity (μmol photons m−2 s−1). B, Maximal efficiency of PSII photochemistry (Fv/Fm). Lines are as follows: 1, XS11; 2, phs3-1; 3, ZH11; 4, dsm2-1; 5, Huayang; 6, dsm2-2; 7, negative transgenic line; 8, DSM2 overexpression. All plants were dark adapted for 30 min prior to light exposure. Values are means ± sd (n = 12). ** P < 0.01 (t test).
Increased Sensitivity of dsm2 to Oxidative Stress
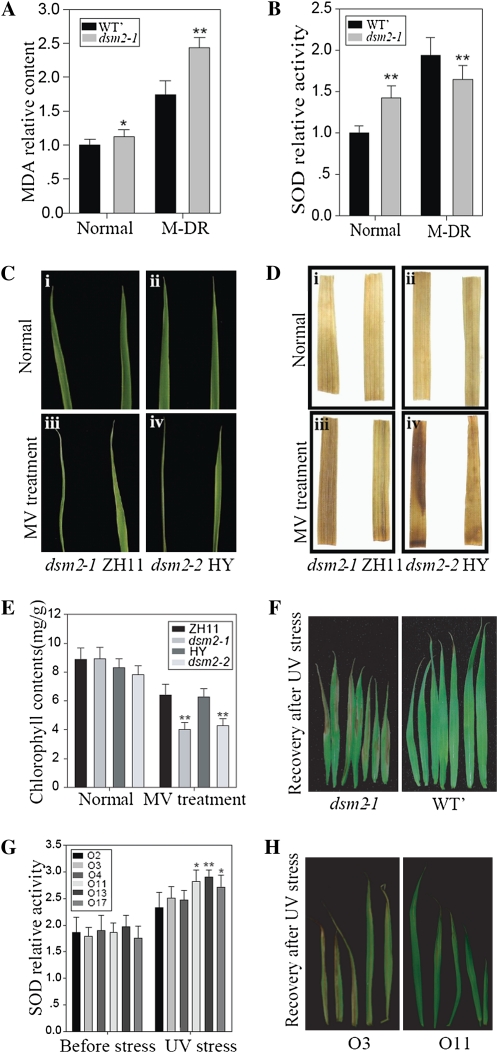
A decrease in the xanthophyll cycle can lead to increased vulnerability of plants to oxidative damages (Davison et al., 2002). We detected a significantly higher level of malondialdehyde, an important intermediate in ROS scavenging that is toxic to plant cells if overaccumulated (Mittler, 2002; Apel and Hirt, 2004), in the dsm2-1 mutant than in the wild type after drought stress (Fig. 6A). We also measured superoxide dismutase (SOD) activities in the soluble proteins extracted from dsm2 and the wild type before and after drought stress. Compared with the wild type, the SOD activity in the mutant was slightly higher before the stress but was significantly reduced after the stress (Fig. 6B).
Figure 6.
The performance of dsm2 mutant and overexpression lines under oxidative stress. A and B, Relative malondialdehyde (MDA) content (A) and relative SOD activity (B) in leaves at the reproductive stage under normal and moderate drought stress (M-DR). C, Leaf phenotype of the mutant at the three-leaf stage in the normal condition and 30 μm MV stress for 2 d. D, Diaminobenzidine staining for H2O2 level in unstressed (i, ii) and MV-stressed (iii, iv) leaves of wild-type and mutant plants. E, Chlorophyll content before and after MV treatment. F, Leaf phenotype of the dsm2-1 mutant after 20 h of UV irradiation and recovery. G, Relative SOD activity in the leaves of three DSM2 overexpression lines (O11, O13, O17) and three negative lines (O2, O3, O4) before and after the UV light stress. H, The leaf appearance of a DSM2-overexpressing line (O13) compared with that of the control (O3) after UV light stress. WT’, Wild type. Values are means ± sd (n = 3). ** P < 0.01.
We tested the response of the dsm2 mutant to oxidative stress by application of methylviologen (MV), which is a well-known oxidative stress inducer that inhibits electron transport during photosynthesis and generates H2O2 in chloroplasts under light (Cheng, 2006). Two days after 30 μm MV treatment, necrosis was observed on the leaves of dsm2-1 and dsm2-2, whereas all the leaves of the wild type remained green. A significant reduction of chlorophyll content was also detected in the mutants (Fig. 6, C and E; Supplemental Fig. S5). The accumulation of H2O2 was further checked by diaminobenzidine staining. Under normal growth conditions, no obvious staining was observed in either dsm2-1 or the control plants. After 30 μm MV stress for 2 d, heavier accumulation of H2O2 was observed in the mutant than in the wild type (Fig. 6D). These results suggested that the enhanced sensitivity of the dsm2 mutant to oxidative stress may result from a reduced ROS-scavenging ability. To confirm this, seedlings of dsm2-1, DSM2 overexpression line, and the wild type at the two-leaf stage were exposed to UV irradiation for 20 h, a condition that can also lead to oxidative stress. The result showed that the mutant was more sensitive to the UV light-stimulated oxidative stress than the wild type, as indicated by more severe growth inhibition and necrosis (Fig. 6F; Supplemental Fig. S5). In contrast, the DSM2 overexpression transgenic plants grew significantly better and had higher SOD activity than the control after UV light stress (Fig. 6, G and H). These results further establish the importance of DSM2 in oxidative stress resistance.
Decreased Stomatal Closure and Increased Water Loss of the dsm2 Mutant under Dehydration Stress
It is well recognized that ABA promotes stomatal closure to avoid water loss under drought stress. Because dsm2 was partially impaired for the biosynthesis of an ABA precursor and ABA content was also reduced in the dsm2 mutant, we measured the water loss rate of detached leaves in wild-type and dsm2 plants. The dsm2 mutant lost water slightly faster than the wild type (Fig. 7A). We also recorded lower relative water content in the dsm2-1 leaves than in the wild type under drought condition (Fig. 7B). However, compared with the control, the DSM2 overexpression line showed no significant difference in water loss rate (Fig. 7A) or relative water content (data not shown). This result prompted us to check the density and aperture of stomatal pores, and we observed that significantly (P < 0.01) more stomatal pores were opened in the dsm2-1 mutant under drought stress conditions (Fig. 7, C and D). In contrast, the DSM2 overexpression line showed no significant difference in the density and aperture of stomatal pores (data not shown).
Figure 7.
Water loss, relative water content, and stomatal conductance assay. A, Water loss assays for the leaves of the dsm2-1, wild-type (WT’), and DSM2-overexpressing lines were performed within 29 h. B, Relative water content (RWC) of rice leaves after 10 d of drought stress in PVC tubes. C, The aperture stomata observed with a scanning electron microscope. D, Statistical analysis of opening stomata. Values are means ± sd (n = 6 for water loss rate, n = 3 for other measurements). ** P < 0.01.
Knockout of DSM2 Influences the Transcript Levels of Some Stress-Related Genes
To gain further insight into the mechanism of the drought sensitivity of dsm2, transcript levels of 16 well-characterized stress-responsive genes were checked in the dsm2-1 mutant and DSM2 overexpression lines under normal and drought stress conditions. Three bZIP (for basic leucine zipper) transcription factor genes that have been proposed to mediate dehydration responses, OsTRAB1, OsbZIP23, and OsbZIP72 (Kobayashi et al., 2005; Xiang et al., 2008; Lu et al., 2009), were slightly down-regulated and up-regulated, respectively, in the dsm2-1 mutant and the DSM2 overexpression line after the drought stress (Fig. 8). Two peroxidase genes, POX22.3 and POX8.1, that are induced by drought stress (Ning et al., 2010) showed significantly stronger induction by drought stress in the overexpression line compared with the wild type and the mutant (Fig. 8). ABA accumulation often leads to feedback regulation of the expression of ABA synthesis genes (Li et al., 2008). Therefore, we checked a few genes involved in ABA biosynthesis, including OsPDS, OsZDS, OsCRTISO, OsLCY, OsTATC/ZEP, and OsNCED4 (for their positions in the pathway, see Supplemental Fig. S1), and found that only the immediate upstream gene OsLCY was significantly affected (down-regulated in the mutant and up-regulated in the overexpression line) under nonstress conditions. The expression levels of the genes positioned far upstream or downstream of the β-carotene hydroxylation step, such as OsPDS, OsZDS, OsCRTISO, and OsNCED4, were essentially unaffected in either the mutant or the overexpression line under normal conditions. After drought stress, only OsLCY and OsTATC showed obvious feedback regulation (stronger induction in the overexpression line but weaker induction in the mutant), whereas the other genes for carotenoid biosynthesis showed no obvious feedback regulation either in the dsm2-1 mutant or in the overexpression line (Fig. 8). We also checked a few well-characterized drought resistance-related genes (e.g. OsSNAC1, OsSKIPa, OsRAB16b, OsPP2C, OsP5CS, OsRD22), but all of them showed no significant difference in transcript levels in either the mutant or the overexpression line compared with the control under both normal and drought stress conditions. These results suggest that disruption of DSM2 only affects the expression of some stress-responsive genes and the genes immediately upstream or downstream of the β-carotene hydroxylation step for ABA synthesis.
Figure 8.
Expression of 16 stress-related genes in dsm2-1, the DSM2 overexpression line, and the control detected by real-time PCR. The y axis shows relative expression level normalized to the wild type (WT’) under normal conditions. The putative stress-related genes encode diverse proteins, including late embryogenesis abundant protein, ABA biosynthesis enzymes, protein phosphatase, zinc finger, and bZIP transcription factors. DR, Drought treatment.
Distinctive Expression Patterns of the BCH Family in Rice
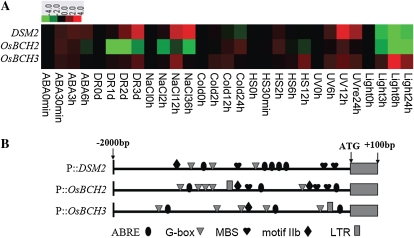
In the rice genome, there are two homologs of DSM2/OsBCH1, designated OsBCH2 (LOC_Os04g48880) and OsBCH3 (LOC_Os10g38940), showing 82% and 75% identity to OsBCH1, respectively. We compared the expression patterns of the three members of the rice BCH family (Fig. 9A). Under drought stress, the DSM2 transcript level was increased, reaching 7- to 8-fold induction at day 3 after treatment, but the expression of OsBCH2 was decreased significantly 2 d after drought stress, and slight induction was observed for OsBCH3. Compared with the strong induction (9- to 10-fold) of DSM2 by UV light, OsBCH2 and OsBCH3 were only slightly induced. However, OsBCH3 was strongly induced by light, whereas DSM2 and OsBCH2 were repressed by light. Cold stress caused slight suppression of DSM2, but OsBCH2 and OsBCH3 were slightly induced; they were also induced by heat stress, which had no effect on DSM2 expression. The distinctive expression pattern of DSM2 compared with those of the other two members under abiotic stress implies that DSM2 may play a distinctive role in stress responses. In addition, DSM2 showed a distinctive expression profile compared with those of OsBCH2 and OsBCH3 during vegetative and reproductive development, whereas OsBCH2 and OsBCH3 showed similar expression patterns in most of the tissues and organs (Supplemental Fig. S6).
Figure 9.
Distinctive expression features within the OsBCH family under stress treatments. A, The expression profiles of the three OsBCH members under abiotic stresses, including cold (4°C), heat (42°C), salt (200 mm NaCl), light after dark, UV light and drought, and ABA (200 μm). B, The cis-acting elements of 2.1-kb genomic regions upstream of the transcriptional start of the corresponding genes predicted with PlantCARE. MBS, MYB binding site; motif IIb, abscisic acid-responsive element; LTR, low-temperature-responsive element. [See online article for color version of this figure.]
The distinctive expression patterns of rice BCH members may imply a divergence in the promoters of these genes. Therefore, we compared proximal promoter regions (–2,000 to +100 bp relative to the initiation of transcription) and noticed distinctive enrichment of a few stress-responsive cis-elements, such as ABRE and MYB binding site MBS in the promoter of DSM2, and enrichment of the light response-related G-box in the promoters of OsBCH2 and OsBCH3 (Fig. 9B). The difference in its promoter further supported the distinctive role of DSM2 of the rice BCH family in stress responses.
DISCUSSION
Sessile plants have evolved sophisticated mechanisms to deal with the ever-changing environment during their growth and development. ABA and its precursor carotenoids play important roles in plant responses to stresses, and carotenoids such as zeaxanthin can directly decrease oxidative damages (Davison et al., 2002; Johnson et al., 2008). In Arabidopsis, overexpression of the BCH gene resulted in the increased conversion of β-carotene to xanthophylls and enhanced the tolerance of high-light stress (Davison et al., 2002). Tobacco plants overexpressing a BCH gene (crtZ) showed less lipid peroxidation than the wild type after UV light treatment (Götz et al., 2002). In contrast to the intensive molecular and genetic studies of zeaxanthin biosynthesis (BCH) genes in Arabidopsis, the BCH gene has not been reported in rice. Here, we report that the rice BCH gene DSM2 plays a critical role in drought resistance, as supported by data from both the mutant and overexpression lines. To the best of our knowledge, this is the first report of a BCH gene conferring drought resistance in plants with direct evidence.
Mechanism of Drought Resistance Conferred by DSM2
BCHs function in the enzymatic reactions to produce zeaxanthin, a main member of the xanthophyll cycle and an upstream substrate for ABA synthesis. Previous analysis predicted that Arabidopsis CYP97A3 and CYP97C1 are chloroplast proteins based on chloroplast-targeting sequences (Tian et al., 2003; Inoue, 2004; Tian and DellaPenna, 2004; Nambara and Marion-Poll, 2005). Proteomics analysis identified three hydroxylases (CYP97A3, CYP97C1, CYP97B3) in the Arabidopsis chloroplast proteome (Zybailov et al., 2008). In this study, DSM2 was confirmed to localize in chloroplast by transient expression in Arabidopsis mesophyll protoplasts and western blot of chloroplast protein. Although we failed to obtain adequate soluble DSM2 protein expressed in E. coli to perform an enzymatic assay in vitro, the results of chloroplast localization of the protein and the measurements of carotenoids and ABA strongly support DSM2 as a functional BCH in rice.
Increasing evidence suggests that carotenoids are required for plant response to dehydration stress (Thompson et al., 2007; Jayaraj and Punja, 2008). The hypersensitivity of the dsm2 mutant to drought stress and the significant changes of carotenoids (especially zeaxanthin) under the stress suggest that DSM2 plays a critical role in dehydration tolerance via carotenoids. In this study, significant reductions of the xanthophyll cycle and endogenous ABA level were detected in the dsm2 mutant. In plants, the xanthophyll cycle plays key roles in photoprotection (Niyogi et al., 1998, 2001) and could enhance the ability of ROS scavenging. In Arabidopsis, the b1b2 double mutant had a reduced xanthophyll cycle and insufficient endogenous ABA production (Tian et al., 2003; Tian and DellaPenna, 2004; Kim et al., 2009). The dehydration and increased ABA trigger the closing of stomata to control the water loss from plants via transpiration to the atmosphere (Schroeder et al., 2001). Most ABA-deficient mutants, such as osaba1 and psh3-1, lose water faster than the control (Agrawal et al., 2001; Fang et al., 2008). Compared with these reported ABA-deficient mutants, the dsm2 mutant showed less reduction of ABA synthesis. However, the dsm2 mutant still showed significantly reduced stomatal closure and lost water faster than the wild type under the drought stress. In addition, the drought-induced expression levels of a few ABA-dependent stress-responsive genes in the mutant are significantly lower than in the wild type. Together, these findings suggest that the drought-hypersensitive phenotype of dsm2 resulted from the combination of reductions of the xanthophyll cycle and ABA level.
Carotenoids perform a variety of critical roles in chloroplast photosystem structure, light harvesting, and photoprotection (Green and Durnford, 1996; Tracewell et al., 2001). Carotenoid-defective mutants, such as psh1, psh2, psh3, and psh4 of rice, b1b2lut1lut5 quadruple mutant of Arabidopsis, and tomato wf, displayed albino phenotypes (Galpaz et al., 2006; Fang et al., 2008; Kim et al., 2009). Under strong light or oxidative stress conditions, photosynthetic electron transport in the thylakoid membrane often generates excessive ROS, which can be subsequently scavenged by local carotenoids. Although ROS can act as signal molecules, for example, mediating intensive carotenoid synthesis during chromoplast differentiation (Bouvier et al., 1998), excessive ROS induced by severe stresses can cause cell damage (Lokstein et al., 2002; Mittler et al., 2004). In this study, we found that the dsm2 mutant was sensitive to oxidative stress. The dsm2 mutant had slightly increased relative SOD activity under normal conditions, which might be explained by a transient feedback stimulation of the ROS-scavenging system caused by the defect in the xanthophyll cycle. After drought stress, however, the relative SOD activity in the mutant was significantly lower than in the wild type, which suggests that ROS scavenging in the mutant cannot be induced as efficiently as in the wild type to cope with the increasing drought-caused oxidative stress, mainly due to the significantly reduced induction of the xanthophyll cycle. The increased sensitivity of dsm2 to oxidative stress was confirmed by applying UV light and MV to the mutant. In addition, the expression changes of two peroxidase genes, POX22.3 and POX8.1, before and during the stress are consistent with the sensitivity phenotype of dsm2 to oxidative stresses. These results together demonstrate the important role of DSM2 in oxidative stress tolerance.
To verify the function of DSM2 in stress tolerance and evaluate its effect on improving drought resistance in rice, we generated DSM2 overexpression transgenic rice. When the overexpression lines were treated with drought, salt, UV light, cold, and heat stresses, significant differences were observed for drought, salt, and UV light stresses. These results suggest that DSM2 functions mainly in dehydration and oxidative stresses. The level of zeaxanthin, the enzymatic product of DSM2, and a few parameters related to the xanthophyll cycle (e.g. NPQ, Fv/Fm, SOD) were significantly higher as expected in the overexpression lines than in the wild type under the stress conditions, further supporting the role of DSM2 in stress resistance through the control of the xanthophyll pool. Unexpectedly, however, the ABA level in the DSM2 overexpression rice showed no significant change under normal conditions, which contrasts to the results of overexpression of other rate-limiting genes for ABA biosynthesis. Nevertheless, the level of ABA in the overexpression rice was slightly higher than in the control after the drought stress, which might be explained by a positive effect of the increased zeaxanthin level on the downstream reactions in the ABA synthesis pathway when the downstream genes are induced under the stress conditions. In addition, we noted that the root growth of the overexpression lines under drought conditions was slightly better than in the wild-type control (data not shown), which may also have contributed to the improved drought resistance.
Functional Redundancy and Specificity of the BCH Family
The BCHs are widely distributed in all photosynthetic plants and some photosynthetic and nonphotosynthetic primitive organisms (Kim et al., 2009). Carotenoids participate not only in ABA biosynthesis but also in chloroplast development (Fang et al., 2008). The dsm2 mutant seems not like the mutants of other genes for carotenoid synthesis in rice, such as psh1, psh2, psh3, and psh4, which are single-copy genes in the genome, and displayed strong phenotypic changes related to ABA deficiency such as preharvest sprouting (Fang et al., 2008). For comparison, we exposed psh3-1 to the stress treatments and found that it was also sensitive to drought stress (Supplemental Fig. S7). In the dsm2 mutant, a significantly reduced ABA level was detected under the drought stress conditions, but no preharvest sprouting phenotype was observed. In addition, the dsm2 mutant showed weaker defects in Fv/Fm and NPQ capacity than the psh3-1 mutant (Fig. 5). These results may suggest that the loss of function of DSM2 can be partially compensated by other members in the BCH family.
In Arabidopsis, two BCH mutants, b1 and b2 for BCH1 and BCH2, respectively, have been reported. Both BCH1 and BCH2 had high activity toward β-rings but weak activity toward ε-rings of β-carotene in the photosynthetic apparatus (Tian et al., 2003). However, unlike dsm2, which was markedly impaired in NPQ, the NPQ capability of the b2 mutant was similar to the wild type, and knockout of the BCH2 gene had no impact on carotenoid composition. In the b1 mutant, only about a 10% reduction in NPQ was detected. The difference of NPQ reduction in b1 and b2 was mainly due to the expression of BCH1 rather than BCH2 (Pogson et al., 1998; Lokstein et al., 2002). Genetic and molecular biology studies suggested that carotene hydroxylases can be partially complemented by each other. However, the tomato mutant wf with a defect in a BCH gene resulted in an 80% reduction of total carotenoid concentration in petals (Galpaz et al., 2006). In rice, the carotene hydroxylase genes have a different dynamic expression pattern (Supplemental Fig. S6). These results suggest that, despite functional redundancy of the BCH family at the protein or enzyme level, different members may function with different amplitudes and thus cause distinctive phenotypes.
In Arabidopsis, the b1b2, lut1b1, and lut1b2 double mutants had lower Fv/Fm and NPQ than b1 and b2 single mutants, and the lut1b1b2 triple mutant had a very rapid initial induction of NPQ, suggesting that photoprotection may be an important function of BCHs (Tian et al., 2003; Fiore et al., 2006). In this study, the single mutant dsm2 (defect in OsBCH1) showed a very significant reduction in zeaxanthin as well as Fv/Fm and NPQ, which was not significantly changed in the Arabidopsis b1 and b2 single mutants. In fact, the reduction of zeaxanthin synthesis in dsm2-1 was even stronger than that in the b1b2 double mutant. This implies that DSM2 may be a major enzyme for zeaxanthin biosynthesis in rice.
The stress-induced expression profile of DSM2 is distinctive from the other two members of the BCH family. DSM2 was strongly induced in vascular tissues by drought stress as well as other dehydration stresses. In addition, OsBCH2 and OsBCH3 showed high expression at the seedling stage, whereas DSM2 had a high expression level in mature tissues, especially at the panicle development stage (Supplemental Fig. S6). The distinctive expression pattern suggests that DSM2 plays an indispensable role in response to stresses, especially dehydration stresses. In fact, the mutant or RNA interference plants for the two DSM2 homologs, OsBCH2 and OsBCH3, showed no obvious change in drought resistance (data not shown). A distinctive phenotype of a mutant in the BCH family resulting from distinctive expression has also been reported in tomato. The tomato wf mutant, impaired in the gene CrtR-B2 specifically expressed in flower and fruit, exhibits a white-flower phenotype that cannot be complemented by the homologous gene CrtR-B1 (Galpaz et al., 2006).
Different expression profiles often result from sequence variation in the promoters. Enrichment of putative ABRE and MYB binding sites was found in the DSM2 promoter. These elements have been well characterized for their roles in response to salt and drought stress (Yamaguchi-Shinozaki and Shinozaki, 2005; Zhang et al., 2005). OsBCH3 contains enriched G-box elements, and this gene was indeed induced by light. These results suggest that the three rice BCH genes have undergone selection in their promoters and DSM2 has evolved more specifically in stress responses than the other two members.
Because the CYP97 and BCH families are closely related for hydroxylation of carotene, we further checked the evolutionary relationship of the two families. Both families have three members in the rice genome. In Arabidopsis, there are three CYP97 and two BCH members (Tian et al., 2004; Kim et al., 2009). According to the unrooted phylogenetic tree constructed with BCH and CYP97 protein sequences from 30 organisms including bacteria, algae, and monocot and dicot plants (Supplemental Fig. S8; Supplemental Tables S1 and S2), the BCHs can be classified into three subgroups: (1) monocots, (2) dicots, and (3) cyanobacteria and algae. There are multiple members in each plant species, but only a single gene in cyanobacteria and algae. Gene duplications of monocot BCH members appear to have occurred after the split of monocot and dicot plants. The CYP97s can be classified into four subgroups: (1) cyanobacteria, (2) monocots, (3) dicots, and (4) algae. Interestingly, the rice CYP97 members are distributed in all the subgroups except the cyanobacterial subgroup, which may imply that amplification of CYP97 ancestral genes occurred before the evolutionary divergence of algae and higher plants. Interestingly, phylogenetic analysis of CYP97 suggested that homologs of the CYP97 ancestor occurred in photosynthetic bacteria before the divergence of green algae and plants (Supplemental Fig. S8). The phylogenetic results suggest that BCH may be an important ancestral gene family exposed to strong selective pressure during evolution.
In conclusion, this study reports characterization of a BCH gene (DSM2) in rice. This gene plays a critical role in stress resistance, especially for drought and oxidative stresses, via control of the xanthophyll cycle and ABA level. Overexpression of DSM2 in rice resulted in significantly increased drought resistance, suggesting that it is a promising candidate gene for drought improvement in crops. The distinctive expression pattern of the DSM2 gene compared with the other members of the family under stress conditions and the dsm2 mutant phenotype suggests an indispensable role of DSM2 in drought resistance. Further research is needed to understand how the BCH members in rice function coordinately to fine-tune the level of xanthophylls and ABA in different stress responses and developmental processes.
MATERIALS AND METHODS
Plant Materials, Stress Treatments, and Physiological Measurements
Mutant dsm2-1 rice (Oryza sativa; ZH11 background) was obtained from the Rice Mutant Database (http://rmd.ncpgr.cn/; Zhang et al., 2006), and its allelic mutant dsm2-2 (Hwayoung background) was ordered from the POSTECH Rice T-DNA Insertion Sequence Database (http://www.postech.ac.kr/life/pfg/risd/; Jung et al., 2003; Jeong et al., 2006). The homozygous mutant and the wild-type genotype segregated from the heterozygous mutant were identified by PCR analysis using a pair of genomic primers flanking the insertion site and a primer on the T-DNA.
To check the transcript levels of genes under stress, ZH11 rice plants were grown in a greenhouse with a 14-h-light/10-h-dark cycle. Seedlings at the four-leaf stage were treated with ABA or abiotic stresses. ABA treatment was conducted by spraying 200 μm ABA on leaves followed by sampling at 0, 0.5, 3, and 6 h after spraying. For UV light stress, seedlings were transferred to a tissue culture room with UV light (emission peak at 254 nm, 1,100 μW cm−2 at plant level [TUV30W; Philips]), sampled at 0, 6, and 12 h, and recovered at 24 h. For cold and heat shock stresses, seedlings were transferred, respectively, to a growth chamber at 4°C and sampled at 0, 2, 12, and 24 h and at 42°C and sampled at 0, 0.5, 2, 6, and 12 h after treatment. For drought stress, the seedlings were grown for 4 d without water and sampled when the leaves showed slight rolling (noted as day 1 of the sampling regime). For salt stress, the seedlings were irrigated with 200 mm NaCl solution and sampled at the designated time. For light inducement, rice was germinated and grown in a dark room to the two-leaf stage and then placed in an incubator for continuous light treatment and sampled at 0, 3, 8, and 24 h.
For drought testing of mutant and overexpression lines, seeds of T1 or T2 overexpression lines and dsm2 mutants were germinated on Murashige and Skoog medium with 50 mg L−1 hygromycin. For drought stress at the seedling stage, overexpression (or mutant) and control plants, each occupying half of each pot, were grown to the four-leaf stage, and water supply was stopped to allow the development of drought stress. After severe drought stress (all leaves wilted) and recovery, the survival of plants was photographed and recorded. Drought stress testing at the reproductive stage was performed under two growth conditions: one in a refined paddy field facilitated with a movable rain-off shelter, and the other in PVC pipes (1 m in height and 0.2 m in diameter). The details of drought treatment and trait measurement were the same as in a previous report (Yue et al., 2006). Water loss rate of detached leaves, relative water content, and photosynthesis rate were measured as in our previous study (Hu et al., 2006). Stomatal closure was monitored by scanning electron microscopy (JSM-6390LV; JEOL). Chlorophyll fluorescence was measured in seedling leaves using a Hansatech FMS-2 chlorophyll fluorometer. Twelve plants were measured for each genotype. For UV light stress, two-leaf stage seedlings were exposed to UV irradiation for 20 h and then recovery in the tissue culture room.
Plasmid Construction and Rice Transformation
To generate the DSM2 overexpression constructs, the full-length cDNA of DSM2 was amplified from ZH11 rice by reverse transcription (RT)-PCR. The PCR product was cloned into TOPO-D entry vector and introduced into destination vector pCB2004H (under the control of the 35S promoter) by LR reaction following the manufacturer’s instructions (Invitrogen). To generate the DSM2-FLAG overexpression constructs, the full open reading frame fragment was inserted into the p1301U-FLAG vector as described previously (Sun and Zhou, 2008). To investigate the expression profile of DSM2, a genomic DNA sequence from DSM2 promoter regions (–2,000 to +100 bp relative to the initiation of transcription) was cloned into vector DX2181, placed in front of the GFP reporter gene. These constructs were introduced into japonica ZH11 rice by Agrobacterium tumefaciens-mediated transformation (Lin and Zhang, 2005).
Subcellular Localization Assays of DSM2
To investigate the subcellular localization of the DSM2 protein, the full open reading frame of DSM2 was cloned into pM999-33 vector, fused with the GFP reporter gene. Plasmids were purified using Qiagen columns according to the manufacturer’s protocol. The plasmids were introduced into Arabidopsis (Arabidopsis thaliana) protoplasts according to a previously reported method (Yoo et al., 2007) with minor modifications by using 5 μg of plasmids. The expression of the fusion construct was monitored after 20 h of incubation in a dark room, and images were captured with a confocal microscope (TCS SP2; Leica).
To confirm the chloroplast localization of DSM2 by western blot, 5 g of four-leaf-stage leaves from the DSM2-FLAG transgenic seedlings was used for chloroplast isolation following the protocol provided in the Chloroplast Isolation Kit (Sigma). The chloroplast proteins were isolated by lysis buffer (10 mm Tricine, 2 mm EDTA, 2 mm dithiothreitol, 10% glycerol, and 0.0025% phenylmethylsulfonyl fluoride), separated on a 12% SDS-PAGE gel, and then transferred to a polyvinylidene difluoride membrane. The membrane was incubated with anti-FLAG antibody, and western blotting was conducted according to a standard protocol.
Quantification of Carotenoids and ABA
The quantification of carotenoids was performed as described previously (Fraser et al., 2000). Briefly, a reverse-phase YMC 5-μm C30 column (250 × 4.6 mm) with a methanol/tert-methyl butyl ether-based mobile phase was used for HPLC with Agilent 1100 series. Using chromatography, the elution was monitored continuously from 200 to 600 nm by the detector. Peaks were normalized relative to the internal standard as described (Schaub et al., 2005). For ABA extraction, 100 mg of four-leaf-stage seedlings before and after drought stress was extracted two times with 2 mL of extracted buffer (80% [v/v] methanol containing 1 mM butylated hydroxytoluene). Quantification of ABA was performed by an Applied Biosystems 4000Q-TRAR liquid chromatography-mass spectrometry system with stable isotope-labeled ABA as a standard from OlChemIm according to previously described methods (Agrawal et al., 2001; Luo et al., 2006).
Quantitative PCR and Northern-Blot Analysis
Total RNA was isolated from rice leaves using Trizol reagent (Invitrogen). The first-strand cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Real-time PCR was performed on an optical 96-well plate in an ABI Prism 7500 real-time PCR system (Applied Biosystems) by using SYBR Premix ExTaq reagent (TaKaRa). The relative expression level of the gene was determined with the rice Actin1 gene as an internal control. The relative expression levels were identified as described previously (Livak and Schmittgen, 2001). For semiquantitative RT-PCR, reactions were performed in 50-μL volumes with the following protocol: one cycle of 94°C for 4 min and 26 cycles of 94°C for 30 s, 50°C for 30 s, and 68°C for 50 s. The gene-specific primers are listed in Supplemental Table S3. For northern-blot analysis, 15 μg of total RNA was transferred to a Hybond nylon membrane (Amersham) and hybridized with a 32P-labeled DSM2-specific probe, and the images were captured by autoradiography on Fujifilm FLA-5100.
Biochemical Assays Related to Oxidative Stresses
SOD activity was measured essentially as described previously (DeLong et al., 2002) with the reaction containing 0.05 m potassium phosphate (pH 7.8), 13 mm Met, 0.01 mm EDTA, 0.002 mm riboflavin, and 0.075 mm nitroblue tetrazolium. The activity of SOD was quantified by recording the inhibition of nitroblue tetrazolium in the reaction with protein extract and the control without protein extract by using a DU640 spectrophotometer (Beckman) at a wavelength of 560 nm. For quantification of malondialdehyde, 0.5 g of leaf tissue was ground in 5% TCA and centrifuged at 3,000 rpm for 10 min, and then 2 mL of the supernatant, mixed with 0.67% thiobarbituric acid, was boiled for 30 min and measured using the DU640 spectrophotometer at wavelengths of 450, 532, and 600 nm. The final quantity of malondialdehyde (μmol L−1) was calculated by the following formula: 6.45 × (A532 – A600) – 0.56 × A450. Chlorophyll content was measured according to the method reported by Ritchie (2006).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AK287823.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ABA biosynthesis pathway in higher plants.
Supplemental Figure S2. Additional data on drought sensitivity of the dsm2 mutant.
Supplemental Figure S3. dsm2-1 is sensitive to salt stress.
Supplemental Figure S4. Additional data on drought resistance performance of the DSM2 overexpression lines.
Supplemental Figure S5. Additional data on the performance of the dsm2 mutant under oxidative stress.
Supplemental Figure S6. Expression profiles of six carotene hydroxylase genes in the tissues and organs covering a whole life cycle of rice.
Supplemental Figure S7. The drought-sensitive phenotype of psh3-1 mutants at the seedling stage.
Supplemental Figure S8. Phylogenetic analysis of BCH and CYP97 subfamilies.
Supplemental Table S1. Accession numbers for the BCH homologs used for the phylogenetic analysis.
Supplemental Table S2. Accession numbers for the CYP97 homologs used phylogenetic analysis.
Supplemental Table S3. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Changyin Wu (Huazhong Agricultural University, Wuhan, China), Gynheung An (Pohang University of Science and Technology, Korea), and Chengcai Chu (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, China) for providing the mutants dsm2-1, dsm2-2, and phs3-1, respectively, and Jan Xu and Rongjian Ye (Huazhong Agricultural University) for providing plasmids pM999-33 and DX2181, respectively. We also thank Kehui Cui (Huazhong Agricultural University) for kind help in using the chlorophyll fluorometer.
References
- Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H. (2001) Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion: tagging of a zeaxanthin epoxidase gene and a novel ostatc gene. Plant Physiol 125: 1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Park S, Jeong DH, Lee DY, Kang HG, Yu JH, Hur J, Kim SR, Kim YH, Lee M, et al. (2003) Generation and analysis of end sequence database for T-DNA tagging lines in rice. Plant Physiol 133: 2040–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Barrero JM, Piqueras P, González-Guzmán M, Serrano R, Rodríguez PL, Ponce MR, Micol JL. (2005) A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J Exp Bot 56: 2071–2083 [DOI] [PubMed] [Google Scholar]
- Bick JA, Lange BM. (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415: 146–154 [DOI] [PubMed] [Google Scholar]
- Bouvier F, Backhaus RA, Camara B. (1998) Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J Biol Chem 273: 30651–30659 [DOI] [PubMed] [Google Scholar]
- Boyer JS. (1982) Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Cheng Q. (2006) Structural diversity and functional novelty of new carotenoid biosynthesis genes. J Ind Microbiol Biotechnol 33: 552–559 [DOI] [PubMed] [Google Scholar]
- Conti A, Pancaldi S, Fambrini M, Michelotti V, Bonora A, Salvini M, Pugliesi C. (2004) A deficiency at the gene coding for zeta-carotene desaturase characterizes the sunflower non dormant-1 mutant. Plant Cell Physiol 45: 445–455 [DOI] [PubMed] [Google Scholar]
- Davison PA, Hunter CN, Horton P. (2002) Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418: 203–206 [DOI] [PubMed] [Google Scholar]
- DeLong JM, Prange RK, Hodges DM, Forney CF, Bishop MC, Quilliam M. (2002) Using a modified ferrous oxidation-xylenol orange (FOX) assay for detection of lipid hydroperoxides in plant tissue. J Agric Food Chem 50: 248–254 [DOI] [PubMed] [Google Scholar]
- Destefano-Beltrán L, Knauber D, Huckle L, Suttle JC. (2006) Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol Biol 61: 687–697 [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A. (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6: 78–84 [DOI] [PubMed] [Google Scholar]
- Fang J, Chai C, Qian Q, Li C, Tang J, Sun L, Huang Z, Guo X, Sun C, Liu M, et al. (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J 54: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. (2006) Seed dormancy and the control of germination. New Phytol 171: 501–523 [DOI] [PubMed] [Google Scholar]
- Fiore A, Dall’osto L, Fraser PD, Bassi R, Giuliano G. (2006) Elucidation of the beta-carotene hydroxylation pathway in Arabidopsis thaliana. FEBS Lett 580: 4718–4722 [DOI] [PubMed] [Google Scholar]
- Fraser PD, Pinto ME, Holloway DE, Bramley PM. (2000) Technical advance: application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24: 551–558 [DOI] [PubMed] [Google Scholar]
- Galpaz N, Ronen G, Khalfa Z, Zamir D, Hirschberg J. (2006) A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell 18: 1947–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Mohanty N, Yamamoto HY. (1994) Epoxidation of zeaxanthin and antheraxanthin reverses non-photochemical quenching of photosystem II chlorophyll a fluorescence in the presence of trans-thylakoid delta pH. FEBS Lett 350: 271–274 [DOI] [PubMed] [Google Scholar]
- Götz T, Sandmann G, Römer S. (2002) Expression of a bacterial carotene hydroxylase gene (crtZ) enhances UV tolerance in tobacco. Plant Mol Biol 50: 129–142 [DOI] [PubMed] [Google Scholar]
- Green BR, Durnford DG. (1996) The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 47: 685–714 [DOI] [PubMed] [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV. (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8: 183–187 [DOI] [PubMed] [Google Scholar]
- Hable WE, Oishi KK, Schumaker KS. (1998) Viviparous-5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol Gen Genet 257: 167–176 [DOI] [PubMed] [Google Scholar]
- Havaux M, Bonfils JP, Lütz C, Niyogi KK. (2000) Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol 124: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103: 12987–12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WN. (2007) The non-mevalonate pathway of isoprenoid precursor biosynthesis. J Biol Chem 282: 21573–21577 [DOI] [PubMed] [Google Scholar]
- Inoue K. (2004) Carotenoid hydroxylation: P450 finally! Trends Plant Sci 9: 515–517 [DOI] [PubMed] [Google Scholar]
- Isaacson T, Ronen G, Zamir D, Hirschberg J. (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj J, Punja ZK. (2008) Transgenic carrot plants accumulating ketocarotenoids show tolerance to UV and oxidative stresses. Plant Physiol Biochem 46: 875–883 [DOI] [PubMed] [Google Scholar]
- Jeong DH, An S, Park S, Kang HG, Park GG, Kim SR, Sim J, Kim YO, Kim MK, Kim SR, et al. (2006) Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. Plant J 45: 123–132 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Davison PA, Ruban AV, Horton P. (2008) The xanthophyll cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana. FEBS Lett 582: 262–266 [DOI] [PubMed] [Google Scholar]
- Jung KH, Hur J, Ryu CH, Choi Y, Chung YY, Miyao A, Hirochika H, An G. (2003) Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol 44: 463–472 [DOI] [PubMed] [Google Scholar]
- Kim J, DellaPenna D. (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc Natl Acad Sci USA 103: 3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith JJ, Tian L, Dellapenna D. (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50: 463–479 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T. (2005) Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J 44: 939–949 [DOI] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I. (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Li F, Vallabhaneni R, Wurtzel ET. (2008) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol 146: 1333–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Matthews PD, Burr B, Wurtzel ET. (1996) Cloning and characterization of a maize cDNA encoding phytoene desaturase, an enzyme of the carotenoid biosynthetic pathway. Plant Mol Biol 30: 269–279 [DOI] [PubMed] [Google Scholar]
- Lin YJ, Zhang Q. (2005) Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep 23: 540–547 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lokstein H, Tian L, Polle JE, DellaPenna D. (2002) Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta 1553: 309–319 [DOI] [PubMed] [Google Scholar]
- Lu G, Gao C, Zheng X, Han B. (2009) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229: 605–615 [DOI] [PubMed] [Google Scholar]
- Luo A, Qian Q, Yin H, Liu X, Yin C, Lan Y, Tang J, Tang Z, Cao S, Wang X, et al. (2006) EUI1, encoding a putative cytochrome P450 monooxygenase, regulates internode elongation by modulating gibberellin responses in rice. Plant Cell Physiol 47: 181–191 [DOI] [PubMed] [Google Scholar]
- Miki D, Shimamoto K. (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45: 490–495 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Ning J, Li X, Hicks LM, Xiong L. (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152: 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK. (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Shih C, Soon Chow W, Pogson BJ, Dellapenna D, Björkman O. (2001) Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res 67: 139–145 [DOI] [PubMed] [Google Scholar]
- North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A. (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50: 810–824 [DOI] [PubMed] [Google Scholar]
- Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ. (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Niyogi KK, Björkman O, DellaPenna D. (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA 95: 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RJ. (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89: 27–41 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Gacio MdC, Matilla-Vázquez MA, Matilla AJ. (2009) Seed dormancy and ABA signaling: the breakthrough goes on. Plant Signal Behav 4: 1035–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub P, Al-Babili S, Drake R, Beyer P. (2005) Why is golden rice golden (yellow) instead of red? Plant Physiol 138: 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ. (2001) Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JA. (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131: 1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T. (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7: 41–48 [DOI] [PubMed] [Google Scholar]
- Singh M, Lewis PE, Hardeman K, Bai L, Rose JK, Mazourek M, Chomet P, Brutnell TP. (2003) Activator mutagenesis of the pink scutellum1/viviparous7 locus of maize. Plant Cell 15: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zhou DX. (2008) Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc Natl Acad Sci USA 105: 13679–13684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Mulholland BJ, Jackson AC, McKee JM, Hilton HW, Symonds RC, Sonneveld T, Burbidge A, Stevenson P, Taylor IB. (2007) Regulation and manipulation of ABA biosynthesis in roots. Plant Cell Environ 30: 67–78 [DOI] [PubMed] [Google Scholar]
- Tian L, DellaPenna D. (2004) Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch Biochem Biophys 430: 22–29 [DOI] [PubMed] [Google Scholar]
- Tian L, Magallanes-Lundback M, Musetti V, DellaPenna D. (2003) Functional analysis of beta- and epsilon-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 15: 1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D. (2004) The Arabidopsis LUT1 locus encodes a member of the cytochrome p450 family that is required for carotenoid epsilon-ring hydroxylation activity. Proc Natl Acad Sci USA 101: 402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracewell CA, Vrettos JS, Bautista JA, Frank HA, Brudvig GW. (2001) Carotenoid photooxidation in photosystem II. Arch Biochem Biophys 385: 61–69 [DOI] [PubMed] [Google Scholar]
- Vallabhaneni R, Gallagher CE, Licciardello N, Cuttriss AJ, Quinlan RF, Wurtzel ET. (2009) Metabolite sorting of a germplasm collection reveals the Hydroxylase3 locus as a new target for maize provitamin A biofortification. Plant Physiol 151: 1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45: 523–539 [DOI] [PubMed] [Google Scholar]
- Wu C, Li X, Yuan W, Chen G, Kilian A, Li J, Xu C, Li X, Zhou DX, Wang S, et al. (2003) Development of enhancer trap lines for functional analysis of the rice genome. Plant J 35: 418–427 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Tang N, Du H, Ye H, Xiong L. (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148: 1938–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK. (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13: 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Zhu JK. (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10: 88–94 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yue B, Xue W, Xiong L, Yu X, Luo L, Cui K, Jin D, Xing Y, Zhang Q. (2006) Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172: 1213–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, Wang S. (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res 34: D745–D748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ruan J, Ho TH, You Y, Yu T, Quatrano RS. (2005) Cis-regulatory element based targeted gene finding: genome-wide identification of abscisic acid- and abiotic stress-responsive genes in Arabidopsis thaliana. Bioinformatics 21: 3074–3081 [DOI] [PubMed] [Google Scholar]
- Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ. (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.