Abstract
Nitric oxide (NO) has been identified as a signal molecule that interplays with reactive oxygen species in response to heavy metal stresses. Roles of NO in regulating cadmium toxicity and iron deficiency have been proposed; however, the function of NO in zinc (Zn) tolerance in plants remains unclear. Here, we investigated NO accumulation and its role in plant Zn tolerance. Zn-induced NO production promoted an increase in reactive oxygen species accumulation in Solanum nigrum roots by modulating the expression and activity of antioxidative enzymes. Subsequently, programmed cell death (PCD) was observed in primary root tips. Inhibiting NO accumulation by 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (a specific NO scavenger) or NG-nitro-l-arginine-methyl ester (a NO synthase inhibitor) prevented the increase of superoxide radical and hydrogen peroxide as well as the subsequent cell death in the root tips, supporting the role of NO in Zn-induced PCD in the root tips. Zn-induced NO production affected the length of primary roots, the number of lateral roots, and root hair growth and thereby modulated root system architecture and activity. Investigation of metal contents in Zn-treated roots suggests that NO is required for metal (especially iron) uptake and homeostasis in plants exposed to excess Zn. Taken together, our results indicate that NO production and the subsequent PCD in root tips exposed to excess Zn are favorable for the S. nigrum seedling response to long-term Zn toxicity by modulating root system architecture and subsequent adaptation to Zn stress.
Heavy metal contamination is a serious problem for the environment. Some metallic elements, such as zinc (Zn), are essential micronutrients and play a role as enzyme cofactors in many metabolic reactions. However, uptake of high concentrations of Zn is found to be toxic to plant growth and development. High concentrations of Zn (260–16,000 mg kg−1) have been found in the soil near smelting sites (Bi et al., 2006), and Zn contamination has been of increasing concern in these regions due to its threat to agriculture and human health (Bi et al., 2006).
Zn homeostasis is a tightly regulated process because Zn can be both essential and deleterious to plants depending on its concentration. The effects of Zn on plants have been widely reported (Broadley et al., 2007; Wang et al., 2009), including tolerance to Zn accumulation and Zn deficiency as well as the protective effects of Zn in plants. Zn is closely involved in protein synthesis and nitrogen metabolism; the growth of Zn-deficient plants is markedly inhibited. Zn is also a constituent of copper/zinc superoxide dismutase (Cu/Zn SOD). Zn deficiency reduces antioxidative enzyme activity and thereby results in reactive oxygen species (ROS) accumulation and oxidative damage (Sharma et al., 2004). Tolerance to Zn accumulation in plants is a complex phenomenon. Aside from Zn deficiency, excess Zn can also inhibit plant growth and development by disequilibrating the uptake and redistribution of mineral nutrition and by disturbing the antioxidant defense system and metabolic processes such as photosynthesis, transpiration, and antioxidative enzyme activity. Recent studies have shown that Zn toxicity affects the activity of antioxidative enzymes, such as SOD, catalase (CAT), and ascorbate peroxidase (APX), in plants (Wójcik et al., 2006; Tewari et al., 2008). The mechanisms of Zn toxicity are not fully understood; however, they may involve competition for catalytic sites or for transporter proteins (González-Guerrero et al., 2005). Zn toxicity also inhibits the uptake of other nutrient elements, such as iron (Fe). Deficiency of these elements can lead to ROS accumulation and oxidative stress (Bonnet et al., 2000). Excess Zn may bind to proteins and lead to the displacement of other ions, such as Fe2+, from protein-binding sites. Plants exposed to excess Zn become Fe deficient (Wintz et al., 2003). However, the effects of Zn stress on root system development have not been elucidated.
Nitric oxide (NO) is a free radical gas that has emerged as an important signaling molecule in plants (Neill et al., 2002). NO accumulation in roots mediates auxin-induced lateral root formation (Correa-Aragunde et al., 2004), adventitious root growth (Tewari et al., 2008), and root hair development (Lombardo et al., 2006). Graziano and Lamattina (2007) have reported that root hair proliferation induced by Fe deficiency is involved in NO accumulation in tobacco (Nicotiana tabacum). It has also been indicated that NO protects plant cells against oxidative stress by reducing ROS accumulation (Wink and Mitchell, 1998; Xu et al., 2010). NO enhances the tolerance of plants to heavy metal stresses (Yang et al., 2006; Sun et al., 2007; Zhang et al., 2008; Xu et al., 2009). However, very little is known about the level of Zn-mediated NO accumulation in plants and the physiological and molecular mechanisms of the effects of NO on tolerance to Zn toxicity.
Programmed cell death (PCD) is an active process of cellular suicide that is essential for development and stress responses in plants. Diverse abiotic stresses, such as salt, drought, nutrient deficiency, and cadmium (Cd) toxicity, can induce PCD in plants. Salt stress has been reported to induce PCD in root apical meristem cells (Huh et al., 2002). Subbaiah and Sachs (2003) have demonstrated that flooding stress induces PCD-like root tip death in maize (Zea mays) and pea (Pisum sativum) plants. Duan et al. (2010) have shown that drought induces PCD in Arabidopsis (Arabidopsis thaliana) root tips. De Michele et al. (2009) have reported that Arabidopsis cell suspension cultures undergo PCD when exposed to Cd stress and that NO is involved in this process. Many studies have indicated that PCD plays a role in the developmental plasticity of plant architecture (Duan et al., 2010). Excess Zn-induced growth inhibition and root death have been found in various plant species (Lingua et al., 2008; Ozdener and Aydin, 2010). However, whether Zn-induced root death occurs through PCD and its underlying mechanisms are poorly understood.
In this study, we used a Zn-hyperaccumulator, Solanum nigrum, to study the effects of excess Zn on root system architecture and the roles of NO and ROS in these effects. S. nigrum is known to hyperaccumulate Zn and Cd in natural soil or in soils contaminated with Zn or Cd. In recent years, physiological characteristics of S. nigrum under Zn or Cd stress have been reported (Wei et al., 2004; Sun et al., 2007; Marques et al., 2008; Xu et al., 2009). The aim of this work was to study plant tolerance to excess Zn and the function of NO produced in Zn-treated plants. Our findings support the model in which NO contributes to rapid ROS accumulation and subsequent PCD in root tips in response to heavy metal stresses; our results also indicate that NO is an important regulator of Zn-modulated root system architecture. Potential mechanisms involved in this process are discussed.
RESULTS
Excess Zn Induces NO Accumulation in S. nigrum Roots
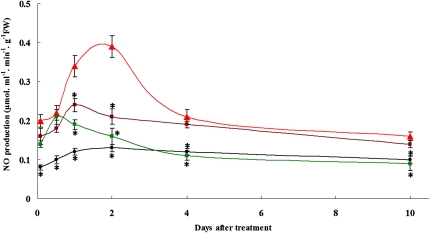
To elucidate the correlation between NO accumulation and Zn tolerance, we measured NO production under Zn stress (Fig. 1). For visualization of NO localization in roots, we also used a specific fluorescent probe, 4,5-diaminofluorescein diacetate (DAF-2DA; Supplemental Figs. S1 and S2; Supplemental Materials and Methods S1). DAF-2DA has been successfully used to detect NO production in plants (Gould et al., 2003; Correa-Aragunde et al., 2004; Corpas et al., 2006; Besson-Bard et al., 2009). A basal NO level was detected along the roots of plants grown in Hoagland solution (Fig. 1; Supplemental Fig. S1). Excess Zn increased NO levels in roots (Fig. 1) and induced a marked increase of NO fluorescence intensity from the meristem zone to the elongation zone of the roots (Supplemental Fig. S1A). Supplementation with a NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), or a NO synthase (NOS) inhibitor, NG-nitro-l-arginine-methyl ester (l-NAME), almost completely inhibited the fluorescence (Supplemental Fig. S2), indicating that the increase of fluorescence intensity was specifically induced by NO. The Zn-induced NO production in the roots was further verified by monitoring the dose response and time course of NO accumulation in Zn-treated roots. No significant differences in NO levels were detected in control roots. Treatment with 200 or 400 μm Zn2+ triggered an increase of NO production within 2 h; the increase was sustained for 2 d, after which the production gradually declined. After 10 d of treatment with 200 or 400 μm Zn2+, NO levels were similar to levels in the control roots. Compared with the 200 μm Zn2+ treatment, the 400 μm Zn2+ treatment induced a slightly higher increase of NO production within 1 d of treatment but a significantly stronger increase at 2 d of treatment (Fig. 1; Supplemental Fig. S1B).
Figure 1.
NO production in S. nigrum roots exposed to Zn2+ or to Zn2+ and Fe2+. Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with 200 or 400 μm ZnCl2 in the presence or absence of 200 μm Fe-EDTA. NO content was determined by the methemoglobin method as described in “Materials and Methods.” Symbols are as follows: black diamonds, control; purple squares, 200 μm ZnCl2; red triangles, 400 μm ZnCl2; green squares, 400 μm ZnCl2 plus 200 μm Fe-EDTA. Asterisks indicate values significantly different from those of the roots treated with 400 μm ZnCl2 alone: * Student’s t test with P < 0.05. FW, Fresh weight.
Besson-Bard et al. (2009) have reported that NO production in Cd-treated roots is related to Cd-induced Fe deficiency. To examine whether Fe deficiency is also involved in Zn-induced NO accumulation, we analyzed the impact of Fe2+ supply on NO production in Zn-treated roots. Treatment with 200 μm Fe-EDTA induced a slight increase of NO production in plants grown in Hoagland solution (data not shown). Compared with the 400 μm Zn2+ treatment alone, supplementation with Fe2+ did not affect the NO level in roots within 1 d of treatment; however, a significant reduction in NO production was observed after 1 d of treatment (Fig. 1). This result is discussed below.
Zn-Induced NO Affects the Expression and Activity of Antioxidative Enzymes
The effects of Zn toxicity and NO accumulation on the antioxidant activity of certain key enzymes have been broadly reported. Several studies have demonstrated the effects of Zn stress on the activity of antioxidative enzymes (SOD, APX, and CAT) in plants (Wójcik et al., 2006; Tewari et al., 2008; Wang et al., 2009). Vital et al. (2008) have found that NO inhibits SOD activity in cotton (Gossypium hirsutum) callus. De Michele et al. (2009) have reported that Cd-induced NO production negatively affects the activity of CAT and APX in Cd-treated Arabidopsis suspension cells. To test if Zn-induced NO accumulation in S. nigrum roots affects the expression and activity of antioxidative enzymes, we measured the transcript levels and activity of SOD, CAT, and APX as well as their changes with PTIO or l-NAME treatment. We used 400 μm Zn2+ in the study because this concentration induced a stronger response in NO production compared with 200 μm Zn2+.
Semiquantitative reverse transcription (RT)-PCR analysis of Zn-treated S. nigrum roots showed differential effects of Zn on the expression of these antioxidative enzymes (Table I; Supplemental Fig. S3). Excess Zn down-regulated the expression of manganese SOD1 (MnSOD1) and MnSOD2 at 2, 4, and 10 d of treatment; supplementation with PTIO or l-NAME further down-regulated their expression. In contrast, excess Zn up-regulated the expression of Cu/ZnSOD2 and FeSOD2 at 2 and 4 d of treatment. After 10 d of treatment, Cu/ZnSOD2 expression was markedly down-regulated, whereas FeSOD2 expression was up-regulated; supplementation with PTIO or l-NAME down-regulated the expression of these genes in Zn-treated roots at all time points. Zn treatment up-regulated the expression of CAT1, CAT2, cAPX, and pAPX; supplementation with PTIO or l-NAME further up-regulated their expression in Zn-treated roots at 2 and 4 d of treatment but markedly down-regulated the expression at 10 d of treatment.
Table I. Expression and enzyme activity of SOD, CAT, and APX in S. nigrum roots exposed to 400 μm ZnCl2 with or without PTIO or l-NAME.
+ and – represent with or without 0.2 mm PTIO or 0.5 mm l-NAME supplementation, respectively. ↑ and ↓ represent up-regulation/elevation or down-regulation/decrease of the expression and activity of antioxidative enzymes, respectively. △ represents no significant difference observed.
| Enzyme | 2 d | 4 d | 10 d | |||||||||
| Gene Expression | Enzyme Activity | Gene Expression | Enzyme Activity | Gene Expression | Enzyme Activity | |||||||
| PTIO | − | + | − | + | − | + | − | + | − | + | − | + |
| Total SOD | ↑ | ↓ | ↑ | ↓ | ↓ | ↓ | ||||||
| MnSOD | ↓ | ↓ | △ | ↓ | ↓ | ↓ | △ | ↓ | ↓ | ↓ | △ | ↓ |
| FeSOD | ↑ | ↓ | △ | ↓ | ↑ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ |
| Cu/ZnSOD | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ |
| CAT | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ |
| APX | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ |
We next examined the activity of these antioxidative enzymes. As shown in Table I and Supplemental Figure S4, total SOD activity increased in roots when seedlings were treated with 400 μm Zn2+ for 2 and 4 d but decreased at 10 d of treatment. Supplementation with PTIO or l-NAME significantly decreased SOD activity. Cu/ZnSOD is the major component (53%) of total SOD, and its activity behaves in a similar pattern to total SOD activity in roots. Excess Zn did not affect FeSOD activity in the first 2 d but markedly reduced its activity at 4 and 10 d of treatment; supplementation with PTIO or l-NAME further reduced its activity in roots. The slight decrease in MnSOD activity in Zn-stressed roots was not statistically significant, but inhibition of NO production by PTIO or l-NAME markedly decreased its activity. Zn stress also increased the activity of CAT and APX at every analyzed time point. Supplementation with PTIO or l-NAME further augmented their activity in roots at 2 and 4 d of treatment but decreased their activity at 10 d of treatment. These data indicate that Zn stress increased the activity of most of the antioxidants at 2 and 4 d, which was reversed in the presence of PTIO or l-NAME for SOD activity, suggesting that NO increases SOD activity. However, the increased CAT and APX activity in the presence of the NO scavenger or NOS inhibitor suggests that NO inhibits the activity of CAT and APX.
Enhanced SOD activity, however, should also decrease superoxide radical (O2−) levels, but our data showed that although NO increased SOD activity, it enhanced O2− production in roots of S. nigrum seedlings. Tewari et al. (2008) reported that NO activates NADPH oxidase (NOX) activity, resulting in increased O2− production. To further confirm if NOX is also involved in Zn-induced O2− production and the roles of NO in the process, we analyzed NOX activity in S. nigrum roots under Zn stress as well as their changes with PTIO or l-NAME treatment (Supplemental Fig. S5; Supplemental Materials and Methods S1). In support of our data, Zn treatment improved NOX activity, whereas supplementation with PTIO or l-NAME markedly inhibited NOX activity, suggesting that Zn-induced NO production enhanced O2− production by mediating NOX activity.
NO Plays a Role in Modulating the Levels of Hydrogen Peroxide and O2−
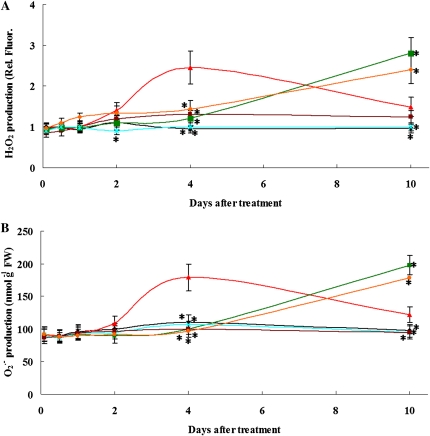
Because Zn toxicity affects antioxidative enzyme activity and NO is involved in this effect, we further investigated the production of hydrogen peroxide (H2O2) and O2− in roots exposed to excess Zn and the role of NO in modulating the levels of H2O2 and O2−. Two-week-old seedlings grown in Hoagland solution were treated with 400 μm Zn2+ or 400 μm Zn2+ plus 0.2 mm PTIO or 400 μm ZnCl2 plus 0.5 mm l-NAME from 2 to 10 d. After the treatment, the roots were specifically stained for H2O2 and O2−. The 2,7-dichlorofluorescin diacetate (DCFH-DA) fluorescent probe and nitroblue tetrazolium (NBT) staining have been successfully used for H2O2 and O2− detection in plants (Ramel et al., 2009; Xu et al., 2010). As shown in Figure 2, 400 μm Zn2+-induced H2O2 and O2− accumulation (157.8% and 62.7% higher than those in untreated control plants, respectively) was detected in the roots after 4 d of treatment (for representative images, see Supplemental Figs. S6 and S7). Supplementation with PTIO or l-NAME markedly inhibited the production of H2O2 and O2− compared with the 400 μm Zn2+ treatment alone in the roots at 4 d of treatment. These data indicate that Zn-induced NO production increases H2O2 and O2− levels, which is consistent with our previous data demonstrating that NO increases SOD activity and decreases CAT and APX activity. However, a marked elevation of H2O2 and O2– production was observed in PTIO- or l-NAME-supplemented roots at 10 d of Zn treatment. In contrast, the levels of H2O2 and O2– in Zn-treated roots significantly decreased at 10 d. Identical results were obtained from H2O2 level detection by 3-diaminobenzidine staining (Supplemental Fig. S8; Supplemental Materials and Methods S1).
Figure 2.
ROS production in roots of 2-week-old S. nigrum seedlings grown in Hoagland solution exposed to Zn2+ or to Zn2+ plus PTIO or Zn2+ plus l-NAME. A, H2O2 production was measured by the DCFH-DA method. Values are normalized against the levels in untreated controls (2 h) that are given a value of 1. B, O2− production was quantified as described in “Materials and Methods.” Symbols are as follows: black diamonds, control; blue crosses, 0.2 mm PTIO; purple squares, 0.5 mm l-NAME; red triangles, 400 μm ZnCl2; green squares, 400 μm ZnCl2 plus 0.2 mm PTIO; orange diamonds, 400 μm ZnCl2 plus 0.5 mm l-NAME. Asterisks indicate values significantly different from those of the roots treated with 400 μm ZnCl2 alone: * Student’s t test with P < 0.05. Images of H2O2 and O2− detection are presented in Supplemental Figures S6 and S7, respectively. FW, Fresh weight; Rel. Fluor., relative fluorescence units.
NO Plays a Role in Zn-Induced PCD in S. nigrum Roots
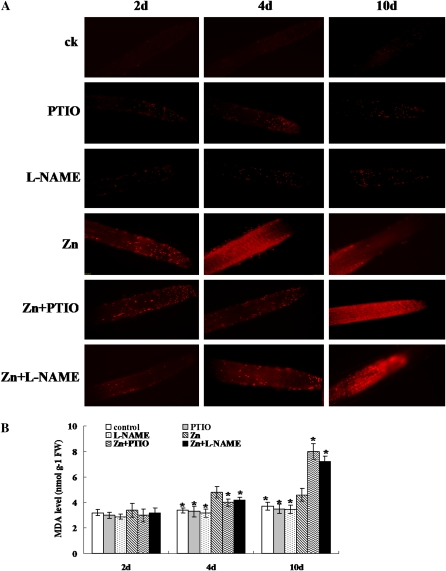
The results above indicate that Zn stress can induce ROS accumulation in roots. To test if the ROS accumulation leads to oxidative damage and cell death in roots, we examined the plasma membrane (PM) integrity in root tips using propidium iodide (PI) staining. PI is a membrane-impermeable dye that binds to nucleotides; it is generally excluded from living cells. A PI-positive nucleus is a strong indication of the loss of membrane integrity (De Cnodder et al., 2005). Consistent with the phenomenon of ROS accumulation in the roots, the results of PI staining showed that exposure to excess Zn led to significant cell death in the primary root tips at 4 d of treatment, especially from the back end of the meristem zone to the elongation zone (Fig. 3A). Inhibiting NO production by PTIO or l-NAME not only inhibited ROS production (Fig. 2; Supplemental Figs. S6–S8) but also reduced cell death in the root tips at 4 d of treatment (Fig. 3A). However, at 10 d of treatment, compared with the Zn treatment alone, supplementation with PTIO or l-NAME markedly increased ROS accumulation (Fig. 2; Supplemental Figs. S6–S8) and increased cell death in the root tips from the meristem zone to the forepart of the root hair zone. One of the possible causes of membrane injury was speculated to be the decrease in membrane fluidity and the loss of function produced by lipid peroxidation, which could be estimated from the malondialdehyde (MDA) content (Barclay and McKersie, 1994). To further test if the cell death in the root tips was due to oxidative damage, we measured MDA levels in the roots. As shown in Figure 3B, Zn treatment markedly increased the MDA level by 41.2% and 24.3% at 4 and 10 d of treatment, respectively, compared with the controls. Supplementation with PTIO or l-NAME markedly reduced the MDA level in the roots at 4 d of the Zn treatment but markedly increased the MDA level at 10 d of the treatment.
Figure 3.
Detection of cell death and oxidative damage in the roots of 2-week-old S. nigrum seedlings grown in Hoagland solution treated with 400 μm ZnCl2 (Zn) or 400 μm ZnCl2 plus 0.2 mm PTIO (Zn+PTIO) or 400 μm ZnCl2 plus 0.5 mm l-NAME (Zn+l-NAME). A, Effect of PTIO or l-NAME on PM integrity. Roots were excised and then stained with 3 μg mL−1 PI for 1 min as described in “Materials and Methods.” B, MDA content in roots of S. nigrum seedlings. ck, Untreated control; PTIO, 0.2 mm PTIO; l-NAME, 0.5 mm l-NAME; FW, fresh weight.
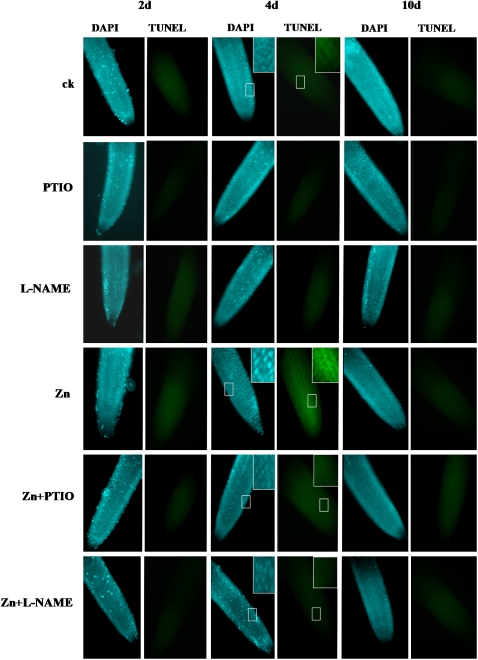
Zn-induced cell death in root tips can occur through either necrosis or PCD. To examine whether PCD is involved in the cell death in root tips, we investigated the chromatin condensation and the internucleosomal fragmentation of DNA by 4,6-diamidino-2-phenylindole (DAPI) staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, respectively. Only weak DAPI (weak fluorescence and round, homogenously stained nuclei) and TUNEL signals were detected in the roots of seedlings grown in Hoagland solution; nearly no DAPI- or TUNEL-positive nuclei were detected (Fig. 4). However, a marked increase of DAPI fluorescence and condensed and granular nuclei staining and TUNEL-positive signals were detected in the root tips from the meristem zone to the elongation zone at 4 d of the Zn treatment, whereas supplementation with PTIO or l-NAME markedly inhibited the DAPI- and TUNEL-positive signals in these regions, indicating that NO induces PCD in root tips. Noticeably, supplementation with PTIO or l-NAME significantly increased oxidative damage and cell death in the root tips, indicated by the MDA level and PI staining at 10 d of the Zn treatment, although it did not induce PCD in the root tips (Figs. 3 and 4).
Figure 4.
Characterization of cell death in primary roots of Zn-treated S. nigrum seedlings. DAPI and TUNEL staining was done in primary root cells of 2-week-old S. nigrum seedlings grown in Hoagland solution treated with 400 μm ZnCl2 (Zn) or 400 μm ZnCl2 plus 0.2 mm PTIO (Zn+PTIO) or 400 μm ZnCl2 plus 0.5 mm l-NAME (Zn+l-NAME). Insets show closeup observations of chromatin condensation. ck, Untreated control; PTIO, 0.2 mm PTIO; l-NAME, 0.5 mm l-NAME.
NO Accumulation Is Required to Modulate Root System Development under Zn Toxicity and Zn Tolerance Conditions
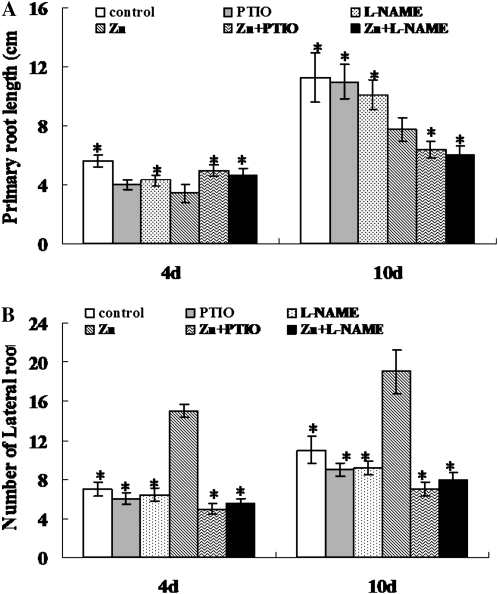
To investigate the physiological mechanisms underlying the roles of NO in Zn tolerance, 2-week-old S. nigrum seedlings were treated with 400 μm ZnCl2 with or without PTIO or l-NAME. As shown in Figure 5 and Supplemental Figure S9, excess Zn inhibited seedling growth. In the presence of PTIO or l-NAME, the primary roots were longer than those of seedlings grown with Zn treatment alone at 4 d of treatment. However, supplementation with PTIO or l-NAME markedly reduced the primary root growth when seedlings were treated for 10 d (Fig. 5A), and the inhibitory effect of PTIO or l-NAME was more significant thereafter (data not shown).
Figure 5.
Effect of PTIO or l-NAME on primary root growth (A) and number of lateral roots (B) of the S. nigrum roots treated with 400 μm ZnCl2. Asterisks indicate values significantly different from those of the roots treated with Zn alone: * Student’s t test with P < 0.05. PTIO, 0.2 mm PTIO; l-NAME, 0.5 mm l-NAME; Zn, 400 μm ZnCl2; Zn+PTIO, 400 μm ZnCl2 plus 0.2 mm PTIO; Zn+l-NAME, 400 μm ZnCl2 plus 0.5 mm l-NAME.
Zn stress also induced lateral root formation. The number of lateral roots in ZnCl2-treated plants increased by 114.2% and 72.7% after 4 and 10 d of treatment, respectively, compared with the number in control plants. Scavenging NO by PTIO completely counteracted the effect of Zn on lateral root formation at both time points. Using the NOS inhibitor l-NAME, we observed a similar effect (Fig. 5B). These results indicate that NO is involved in Zn-modulated root system development.
The results above show that although supplementation with PTIO or l-NAME promoted primary root elongation within 4 d of treatment, it markedly inhibited primary root growth and lateral root development at 10 d of treatment, suggesting that early-stage NO production is favorable for plant response to long-term Zn toxicity. Therefore, we wonder if NO affects root system activity under long-term Zn stress. For this purpose, we used bromocresol purple as a pH indicator to examine the effect of NO on rhizosphere acidification under long-term Zn stress (Supplemental Fig. S10; Supplemental Materials and Methods S1). Rhizosphere acidification is an indicator of root system activity. Supplementation with PTIO markedly inhibited rhizosphere acidification in Zn-treated S. nigrum roots, indicating that Zn-induced NO accumulation improves root system activity and therefore is favorable for plant response to Zn toxicity. Inhibition of NO production by a NOS inhibitor, l-NAME (0.5 mm), exerted similar effects as PTIO on the growth of seedlings exposed to excess Zn, supporting the role of NO in modulating root system development and activity.
Inhibiting NO Production Leads to the Reduction of Zn, Fe, Cu, and Mn Accumulation in Roots Exposed to Excess Zn
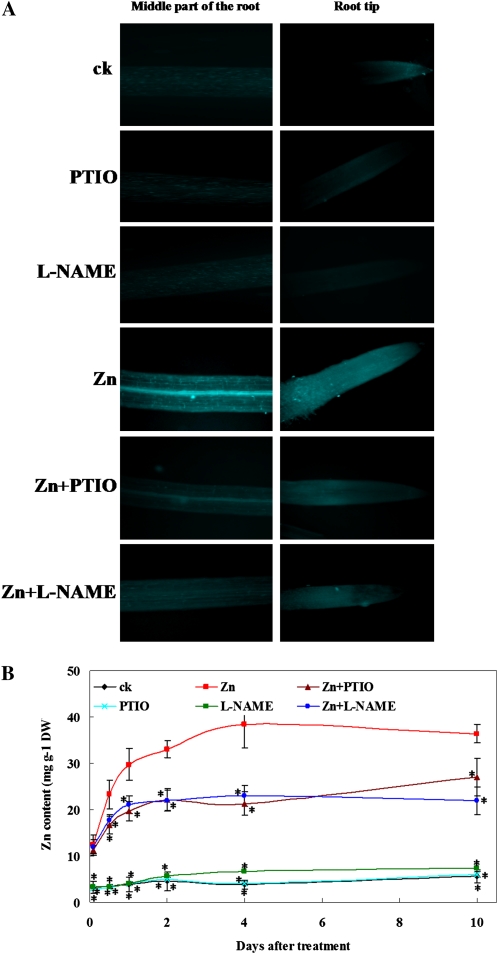
NO-mediated metal ion uptake in plants has been reported (Besson-Bard et al., 2009). To investigate whether NO production in Zn-treated plants is related to Zn accumulation and modulates the uptake of other metal ions in roots exposed to excess Zn, we investigated the distribution of intracellular free Zn in roots by labeling the intracellular Zn with a membrane-permeant Zn-specific fluorophore, Zinquin-ethyl-ester (Zinquin), and we performed the analysis by fluorescence microscopy. We also measured metal contents in the seedling roots exposed to 400 μm ZnCl2 in the presence or absence of PTIO or l-NAME for 2 h, 12 h, 1 d, 2 d, 4 d, and 10 d. After the treatment, the metal contents in roots were measured by inductively coupled plasma mass spectroscopy (ICP-MS).
As shown in Figure 6A, a low fluorescence intensity was observed in the roots grown in Hoagland solution, whereas a strong increase of Zn fluorescence intensity was observed in the roots of Zn-treated S. nigrum, especially in the vascular tissues of the roots. Supplementation with PTIO or l-NAME markedly reduced the blue fluorescence, indicating that inhibiting NO production reduced Zn accumulation in the roots. ICP-MS analysis yielded the identical result of an abrupt decrease of Zn content in the Zn-treated roots supplemented with PTIO or l-NAME. Quantification of Zn content revealed that in the presence of PTIO or l-NAME, the Zn content in the roots was markedly reduced after 12 h of treatment (Fig. 6B).
Figure 6.
Effect of PTIO or l-NAME on Zn accumulation in S. nigrum roots. A, Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with Zn2+ or Zn2+ plus PTIO or Zn2+ plus l-NAME for 4 d. Visualization of Zn accumulation in S. nigrum roots was detected with the Zinquin fluorescent probe. The treated roots were stained with 25 μm Zinquin (Sigma) for 1 h as described in “Materials and Methods.” Images were obtained by confocal microscopy. B, Zn content in the roots was measured by ICP-MS right after treatment. Asterisks indicate values significantly different from those of the roots treated with Zn alone: * Student’s t test with P < 0.05. ck, Untreated control; PTIO, 0.2 mm PTIO; l-NAME, 0.5 mm l-NAME; Zn, 400 μm ZnCl2; Zn+PTIO, 400 μm ZnCl2 plus 0.2 mm PTIO; Zn+l-NAME, 400 μm ZnCl2 plus 0.5 mm l-NAME; DW, dry weight.
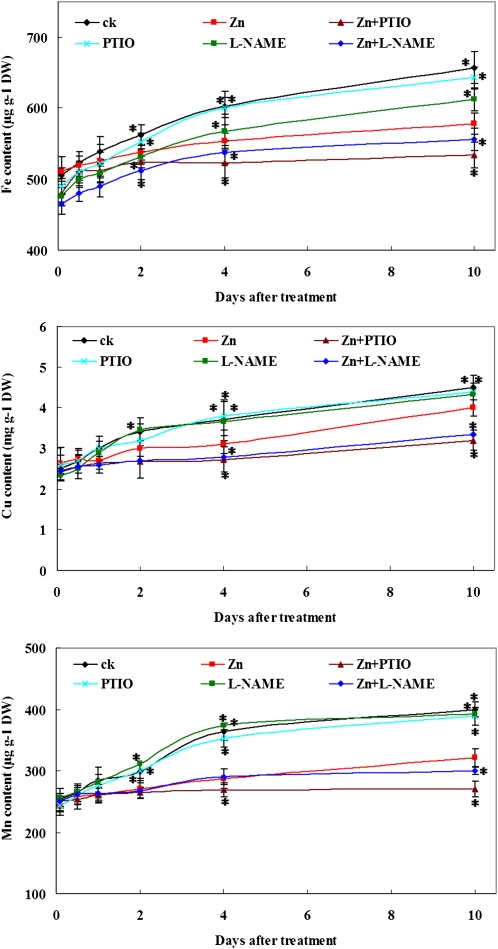
ICP-MS analysis indicated that the contents of Fe, Mn, and Cu in Zn-treated roots did not significantly decrease within 1 d of treatment. However, compared with that in plants grown in Hoagland solution, a significant reduction in the content of these metals was observed after 4 d of treatment: Fe (22%), Mn (26%), and Cu (19%). Inhibiting NO production by PTIO or l-NAME further decreased the contents of these metals in roots (Fig. 7).
Figure 7.
Effect of PTIO or l-NAME on metal contents of Zn-treated S. nigrum roots. Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with Zn2+ or Zn2+ plus PTIO or Zn2+ plus l-NAME. Metal contents in the roots were measured by ICP-MS right after treatment. Asterisks indicate values significantly different from those of the roots treated with Zn alone: * Student’s t test with P < 0.05. ck, Untreated control; PTIO, 0.2 mm PTIO; l-NAME, 0.5 mm l-NAME; Zn, 400 μm ZnCl2; Zn+PTIO, 400 μm ZnCl2 plus 0.2 mm PTIO; Zn+l-NAME, 400 μm ZnCl2 plus 0.5 mm l-NAME; DW, dry weight.
Scavenging NO Reduces the Ferric-Chelate Reductase Activity in Zn-Treated Roots
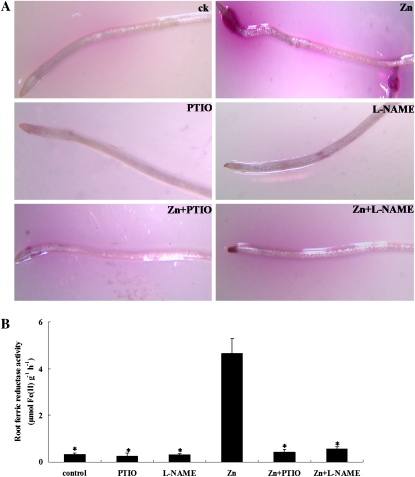
Excess Zn leads to Fe deficiency in roots. To detect the association between Zn-induced NO production and Fe assimilation in Zn-treated plants, we examined ferric-chelate reductase activity in roots. As shown in Figure 8, ferric-chelate reductase activity was greatly increased by the Zn treatment (14-fold). Localization of Fe-reduction activity in the roots analyzed by an agar-coated assay showed that ferric-chelate reductase activity was detected more extensively in the Zn-treated roots, whereas the plants grown in Hoagland solution displayed very low ferric-chelate reductase activity (Fig. 8A). However, supplementation with PTIO or l-NAME markedly reduced ferric-chelate reductase activity in the Zn-treated plants (31.2- and 32.1-fold, respectively) compared with the Zn treatment alone (Fig. 8B).
Figure 8.
Ferric-chelate reductase activity regulated by NO during Zn toxicity. Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with 400 μm ZnCl2 in the presence or absence of 0.2 mm PTIO or 0.5 mm l-NAME for 4 d. A, Visualization of ferric-chelate reductase activity. B, Root ferric-chelate reductase activity in response to PTIO or l-NAME. Asterisks indicate values significantly different from those of the roots treated with Zn alone: * Student’s t test with P < 0.05. ck, Untreated control; PTIO, 0.2 mm PTIO; l-NAME, 0.5 mm l-NAME; Zn, 400 μm ZnCl2; Zn+PTIO, 400 μm ZnCl2 plus 0.2 mm PTIO; Zn+l-NAME, 400 μm ZnCl2 plus 0.5 mm l-NAME.
DISCUSSION
Various biotic and abiotic stresses, such as pathogen attack, salt, low temperature, and Cd and Fe deficiency, induce the up-regulation of NO production. It is now becoming clear that the NO-regulated stress response is essential for stress tolerance and survival of plants. Exposure to excess Zn leads to various alterations in plant homeostasis and even results in plant death; however, the molecular mechanisms of Zn tolerance and the role of NO in the root sensing and the subsequent signal transduction pathways in response to Zn stress have not been elucidated. Several past studies on NO function in the plant response to heavy metals were based on the use of an exogenous NO donor. However, stress-induced NO might be endogenously produced and, therefore, play specific roles in response to heavy metals (Besson-Bard et al., 2009). In this study, we examined excess Zn-induced NO production in roots. Using both physiological and chemical approaches, we demonstrate that NO affects antioxidative enzyme activity and thereby modulates ROS accumulation in roots when plants are subjected to Zn toxicity. Our data revealed that root hair elongation, PCD in primary root tips, and lateral root formation in response to Zn toxicity involve NO-mediated pathways. Finally, we demonstrate that NO is important in plant tolerance to Zn accumulation in the hyperaccumulator S. nigrum.
NO Production in Zn-Treated S. nigrum Roots Is Partially Related to Zn-Induced Fe Deficiency
To assess the NO production in Zn-treated roots, the dynamic change in the endogenous NO level was measured. We showed that Zn treatment in roots triggered an increase of NO production within several hours; the increased NO level was sustained for 2 d and then gradually declined until 10 d.
The fact that excess Zn decreases Fe uptake (Wintz et al., 2003; Wang et al., 2009) and up-regulates IRT1 expression (van de Mortel et al., 2006), together with the findings that Fe deficiency promotes NO production (Graziano and Lamattina, 2007) and is also involved in Cd-induced NO accumulation in Arabidopsis roots (Besson-Bard et al., 2009), suggest that Zn-induced NO production may also be related to Fe deficiency. To test this hypothesis, we measured Fe content and NO production in Zn-treated roots supplemented with 200 μm Fe2+. Excess Zn did not affect Fe content in the roots within 1 d of treatment (Fig. 7); however, excess Zn markedly induced NO production in the roots within several hours, and the elevation was sustained for 2 d; supplementation with Fe2+ did not affect NO level in the roots in this period (Fig. 1; Supplemental Fig. S1). However, a significant decrease in Fe content was observed after 2 d of treatment (Fig. 7), and supplementation with Fe2+ markedly repressed NO production in the roots (Fig. 1; Supplemental Fig. S1). Notably, although the Fe deficiency was more severe thereafter, it did not induce a constant elevation of NO production in the Zn-treated roots. In contrast, the NO production level returned to normal after 10 d compared with that in the untreated plants (Fig. 1; Supplemental Fig. S1). Although the detailed molecular mechanisms of NO synthesis in Zn-treated plants remains unclear, our results here indicate that the early-stage NO accumulation (within 1 d) in Zn-treated roots is not due to Fe deficiency; however, the Fe deficiency observed at and after 2 d of treatment may act as a signal to stimulate or maintain the NO level for a certain time period (within 10 d) in roots of S. nigrum exposed to excess Zn.
There are different NO sources in plants: the nitrate reductase enzyme (Desikan et al., 2002), the NOS-like enzyme (Guo et al., 2003) and nonenzymatic pathways (Stöhr and Ullrich, 2002). The role of the NOS-like enzyme pathway in NO biosynthesis in vivo has been well studied. Previous studies have shown that l-NAME, which is an l-Arg analog, can inhibit NOS activity in plants (Neill et al., 2002). In this study, we found that supplementation with the NOS inhibitor l-NAME markedly repressed the production of NO in Zn-treated S. nigrum roots (Supplemental Fig. S2). Similar to treatment with the NO scavenger PTIO, l-NAME treatment also altered ROS levels, PCD-mediated processes, and overall seedling growth. These results suggested that NO production in Zn-treated S. nigrum seedlings might be catalyzed through an l-Arg-dependent route.
Excess Zn Differentially Affects the Expression and Activity of SOD, CAT, and APX
Zn-dependent modulation of antioxidative enzyme activity has been reported in Cacanus cajan (Madhava Rao and Sresty, 2000), Mentha pulegium (Candan and Tarhan, 2003), Hydrilla verticillata (Panda and Khan, 2004), Thlaspi caerulescens (Wójcik et al., 2006), Brassica napus (Wang et al., 2009), and Eruca sativa (Ozdener and Aydin, 2010), although there are conflicting results in these studies. The discrepancies may be attributed to differences in plant species and Zn concentrations used and in the time points detected in these studies. To better understand the effects of Zn stress on antioxidative enzyme activity, we tested the expression and activity of SOD, CAT, and APX at different time points in the roots of S. nigrum seedlings. SOD, as a key enzyme in protecting cells against oxidative stress, catalyzes the dismutation of O2− to H2O2 and oxygen. There are three types of SOD in plants according to cofactors: MnSOD, FeSOD, and Cu/ZnSOD. Excess Zn up-regulated the expression and activity of Cu/ZnSOD within 4 d of treatment. Cu/ZnSOD is a major part of total SOD. Therefore, it is not surprising that total SOD activity behaves in a similar pattern to Cu/ZnSOD. Interestingly, excess Zn up-regulated FeSOD expression within 4 d of treatment but down-regulated FeSOD activity. A similar phenomenon has also been observed in Cd-treated pea plants: Cd treatment up-regulated the FeSOD transcript but reduced FeSOD activity (Sandalio et al., 2001; Rodríguez-Serrano et al., 2009). These results suggest a possible post-translational regulation of FeSOD. Considering that FeSOD is sensitive to H2O2, Rodríguez-Serrano et al. (2009) proposed that the posttranslational regulation of FeSOD might be due to oxidation or Fe availability. Our results showed a significant increase of ROS production (Fig. 2; Supplemental Figs. S6–S8) and a decrease of Fe content (Fig. 7) in the roots of S. nigrum seedlings exposed to excess Zn after 2 d of treatment, supporting the hypothesis of Rodríguez-Serrano et al. (2009). CAT catalyzes the dismutation of H2O2 to water and oxygen. APX catalyzes ascorbic acid to form monodehydroascorbate by consuming H2O2. Both the expression and enzyme activity of CAT and APX were elevated in Zn-treated S. nigrum roots, indicating a high H2O2-scavenging capacity in S. nigrum roots. These findings may partially explain the fact that S. nigrum, as a hyperaccumulator, has a strong heavy metal stress tolerance.
Zn-Induced NO Production Modulates ROS Levels by Affecting the Expression and Activity of Antioxidative Enzymes in Roots
Several studies have addressed the importance of NO-modulated antioxidative enzyme activity (Vital et al., 2008; De Michele et al., 2009). However, these studies did not focus on Zn toxicity or its signaling events. In this study, we have shown that NO increases SOD activity but inhibits the activity of CAT and APX. These findings support a role for NO in elevating H2O2 levels. Enhanced SOD activity increases H2O2 production, whereas reduced CAT and APX activity promotes H2O2 accumulation.
Antioxidative enzyme activity is tightly modulated by several factors and signals in plants subjected to excess Zn. Although we have not been able to clearly discern whether the time-course changes in the expression and activity of these antioxidative enzymes are modulated by NO, ROS, or metal ions, and whether these factors interact in these processes, this study showed that Zn-induced NO production is an early-stage response of plants to excess Zn that affects antioxidative enzyme activity and thus modulates ROS accumulation. ROS accumulation in plants, in turn, modulates antioxidative enzyme activity (Vital et al., 2008). Excess Zn disturbs the uptake of mineral nutrition and thereby inhibits antioxidative enzyme activity (Sharma et al., 2004). Taken together, we propose that NO primarily regulates early-stage changes in antioxidative enzymes, whereas, after 10 d of treatment, NO production levels return to normal (compared with untreated plants) and ROS levels and metal ion content may regulate the expression and activity of antioxidative enzyme.
Zn-induced NO production occurred within several hours, and it gradually increased in roots for 2 d. However, ROS (including H2O2 and O2−) accumulation occurred after 4 d of treatment, and supplementation with PTIO or l-NAME markedly inhibited ROS accumulation. This time course supports the hypothesis that NO acts upstream of ROS in the plant response to Zn stress and is in agreement with previous reports showing that NO stimulates ROS production in Arabidopsis suspension cells exposed to Cd toxicity (De Michele et al., 2009).
NO Contributes to the Accumulation of Zn and Other Metals in Roots
Zn is necessary for plants, but excess Zn disturbs both the uptake and distribution of metallic elements in plants (Wang et al., 2009). In this study, long-term growth with 400 μm ZnCl2 led to a decrease in the contents of Fe, Cu, and Mn in S. nigrum roots. Similar results have also been observed in rapeseed (Brassica napus) seedlings (Wang et al., 2009). Several studies have demonstrated that Zn can enter cells by the same transporters used by Fe, Mn, and Cu (Wintz et al., 2003). Excess Zn may compete with these elements for the transporters and cause a reduction in both the uptake and the accumulation of these metallic elements. For example, the Fe transporters IRT1 and IRT2 are also responsible for transporting Zn in plants (Wintz et al., 2003). Several studies have shown that NO can modulate the expression of metal transporters, such as IRT1, and therefore affect the accumulation of these metals in plants (Parani et al., 2004; Besson-Bard et al., 2009). In this study, scavenging or inhibiting NO further reduced the Zn, Fe, Cu, and Mn contents in the roots of S. nigrum seedlings at 10 d of treatment, supporting a positive role of NO in mineral nutrition absorption. Ferric-chelate reductase activity is an indicator of Fe uptake capability of plants subjected to Fe deficiency. Graziano and Lamattina (2007) have observed that NO enhances FRO expression and ferric-chelate reductase activity in tomato (Solanum lycopersicum) roots. Robinson et al. (1999) have found that rhizosphere acidification enhances ferric-chelate reductase activity. Our results are in agreement with these findings. Excess Zn induced rhizosphere acidification in S. nigrum seedlings and enhanced ferric-chelate reductase activity in roots, whereas inhibiting NO production by PTIO or l-NAME almost completely inhibited ferric-chelate reductase activity, supporting the role of NO in promoting Fe assimilation under Zn stress.
Zn-Induced NO Production and Subsequent PCD in Root Tips Modify Root System Architecture and Improve Zn Tolerance
Results from our data indicate that Zn toxicity inhibits primary root growth, whereas it promotes lateral root formation. Similar morphological changes in the root system have also been noted in Cd- and NO-treated plants. Correa-Aragunde et al. (2004) found that NO modulates the expression of cell cycle regulatory genes during lateral root development. The application of exogenous NO increases the number of tomato lateral roots, whereas depletion of intracellular NO arrests lateral root initiation. Besson-Bard et al. (2009) reported that NO is responsible for the Cd-induced growth inhibition of primary roots in Arabidopsis. However, the underlying physiological and molecular mechanisms have not been completely elucidated. In this study, inhibiting NO production by PTIO or l-NAME alleviated Zn-induced inhibition of primary root growth at 4 d of treatment and reduced lateral root formation, indicating a role of NO in Zn-induced inhibition of primary root growth and the promotion of lateral root formation.
Inhibition of primary root growth can occur through either necrosis or PCD in root tips. Several studies have proposed that heavy metal stress disrupts cell membrane integrity, resulting in cell death in roots. Pan et al. (2001) have reported that aluminum stress induces PCD in barley (Hordeum vulgare) root tip cells. A moderate concentration of Cd was shown to induce apoptosis in onion (Allium cepa) root apical cells (Behboodi and Samadi, 2004), whereas high concentrations of Cd induce necrosis (Behboodi and Samadi, 2004; Garnier et al., 2006). These studies suggest that heavy metal-induced PCD is beneficial for plants to survive under these adverse conditions. Inhibitory effects of excess Zn on root growth have been reported (Rout and Das, 2003; Broadley et al., 2007; Lingua et al., 2008; Wang et al., 2009), but the underlying mechanisms of root growth inhibition under Zn stress and its biological role remain largely unknown. Wiseman et al. (2010) have reported that Zn accumulation induces endothelial cell apoptosis by disrupting cellular antioxidant capacity. In this study, we found that Zn toxicity led to oxidative damage and cell death in roots of S. nigrum seedlings and that supplementation with PTIO or l-NAME reduced cell death within 4 d of treatment, suggesting that NO is involved in Zn-induced cell death in roots. We next examined whether Zn toxicity-induced cell death occurs through PCD and whether NO is involved in this process. As expected, Zn-induced cell death in S. nigrum roots indeed occurred through PCD, as evidenced by DAPI and TUNEL staining. The majority of the cell death occurred in the elongation zone and the meristematic cells of S. nigrum root tips (Fig. 3A), indicating that Zn-induced cell death largely occurs in undifferentiated cells and thereby inhibits primary root elongation. Supplementation with PTIO or l-NAME inhibited PCD, indicating that NO is required for Zn-induced PCD in primary root tips. These findings explain the phenomenon that Zn-induced NO production induces growth arrest in primary roots of Zn-treated plants. De Michele et al. (2009) have reported that NO stimulates ROS production in Arabidopsis suspension cells and thus leads to PCD. Duan et al. (2010) have found that drought treatment induces high ROS production in root meristematic cells and thereby results in PCD in Arabidopsis root tips. Our results are in agreement with these findings, which demonstrated that NO-induced ROS production likely precedes PCD. Considering the spatial and temporal correlation between ROS accumulation and PCD occurrence in the root tips, we propose that NO-induced ROS (including O2− and H2O2) accumulation plays a key role in PCD induction. Taken together, these findings suggest that Zn-mediated morphological changes to the root system might be caused by NO-mediated lateral root development and PCD in primary root tips. Interestingly, although PTIO or l-NAME alleviated Zn-induced inhibition of primary root growth at 4 d of treatment, it further inhibited primary root growth at 10 d of treatment, and the inhibitory effect of PTIO was more significant thereafter. These results suggest that NO is beneficial for plant adaptation to Zn stress. NO promoted the formation of root hairs (Supplemental Fig. S9A) and lateral roots (Fig. 5B) and induced PCD in primary root tips of Zn-treated plants. The occurrence of PCD in primary root tips changed the growth pattern of primary roots and further promoted lateral root development.
Several studies indicated that the morphological alterations that result in an increased surface area, such as the formation of root hairs and lateral roots, provide the basis for physiological reactions to abiotic stresses (Schmidt et al., 2000). To better understand the physiological effect of NO-modulated changes in root system architecture on plant growth, we tested root system activity under Zn stress by examining rhizosphere acidification. Rhizosphere acidification is closely related to the activity of PM H+-ATPase and is also an indicator of root system activity. NO functions as a second messenger in enhancing PM H+-ATPase activity and has been observed in salt-induced reed (Phragmites communis) calluses (Zhao et al., 2004) and maize seedlings (Zhang et al., 2006). Several studies indicate that ATPase-mediated rhizosphere acidification enhances the activity of the PM-bound reductase FRO2, increases the expression of the Fe transporter IRT1, and therefore improves the capacity of mineral nutrition absorption (Toulon et al., 1992; Eide et al., 1996; Fox et al., 1996; Robinson et al., 1999; Schmidt et al., 2000). Our study showed that supplementation with PTIO or l-NAME prevented the rhizosphere acidification in response to excess Zn, suggesting that Zn-induced NO production improves H+-ATPase activity and root system activity and therefore is favorable for the plant response to long-term Zn toxicity.
Root traits are closely related to stress tolerance of plants. Duan et al. (2010) have reported that drought-induced PCD in primary root tips provides a protective mechanism by modifying root system architecture in response to severe water deficiency. Our results support the hypothesis that the inhibition of primary root growth by NO-induced PCD at the early stage of Zn stress (within 4 d) is an adaptive response of plants subjected to Zn toxicity. After 10 d of treatment, we found that inhibiting NO production by PTIO or l-NAME led to severe oxidative damage and cell death (but not through PCD; Figs. 3 and 4) in roots and markedly inhibited seedling growth (Supplemental Fig. S9B). These findings are in agreement with previous reports showing that NO enhances the tolerance to salt (Zhang et al., 2006), Cd (Laspina et al., 2005), and Fe (Martin et al., 2009) deficiency in plants. However, they are contradictory to the result of Besson-Bard et al. (2009) showing that NO contributes to the Cd sensitivity of root growth in all stages of treatment (until 6 weeks). This discrepancy might be attributed to differences in plant species and heavy metals used in these studies. S. nigrum, as a heavy-metal hyperaccumulator, exhibits a higher Zn/Cd tolerance than Arabidopsis; therefore, it may respond to heavy metal stress more rapidly and, subsequently, change its root system architecture. After adaptation to adverse conditions by modulating root system architecture, plant growth was gradually recovered. In contrast, scavenging or inhibiting NO production disturbed the modulation of root system architecture under Zn toxicity and therefore was disadvantageous for the plant’s long-term adaptation to excess Zn (Supplemental Fig. S11). The major questions to be further addressed concern what factors act upstream of NO in this signal transduction pathway and the origin of NO in roots exposed to excess Zn.
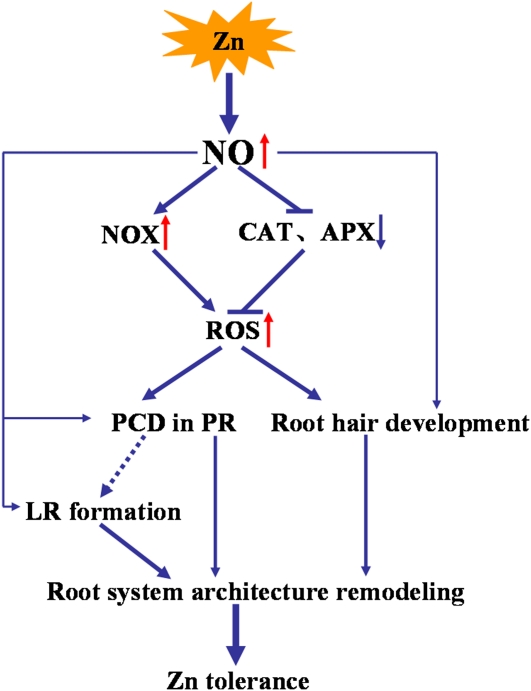
In conclusion, our data indicate that NO, as a key player under conditions of Zn stress, plays an important role in Zn tolerance in the hyperaccumulator S. nigrum (Fig. 9). Zn-induced NO production promoted an increase in ROS accumulation in roots by modulating the activity of NOX and antioxidative enzymes. Subsequently, PCD was observed in primary root tips. Zn-induced NO production also affected the number of lateral roots and root hair growth and thereby modulated root system architecture and activity. These findings show that NO-mediated plant responses increased Zn tolerance through morphological and physiological changes in the roots. Such an understanding is helpful for the breeding and cultivation of a Zn hyperaccumulator and provides insights into novel strategies for phytoremediation.
Figure 9.
NO plays an important role in Zn tolerance in the hyperaccumulator S. nigrum. LR, Lateral root; PR, primary root.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Solanum nigrum seeds were kindly provided by the National Southwest Germplasm Resources Laboratory of China (IP: SCSB-C-003073). To obtain seedlings, the seeds were sown under sterile conditions in petri dishes containing Murashige and Skoog medium (Murashige and Skoog, 1962) and solidified with 0.8% (w/v) agar (Sigma). Cultures were maintained at 22°C to 25°C under a 16-h photoperiod. Seven-day-old seedlings were transferred into Hoagland solution (Hoagland and Arnon, 1950) for plant growth under the same culture condition. Treatment with Fe-EDTA, ZnCl2, PTIO (Sigma), and l-NAME (Sigma) was applied to 2-week-old seedlings grown in Hoagland solution. The culture solution was replaced every 2 d.
Measurement of NO Production
Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with 200 or 400 μm ZnCl2 in the presence or absence of 200 μm Fe-EDTA for 2 h, 12 h, 24 h, 2 d, 4 d, or 10 d. NO content was determined using the methemoglobin method as described by Zhao et al. (2004). Treated roots (5 g) were incubated with 100 units of CAT and 100 units of SOD for 5 min to remove endogenous ROS. Oxyhemoglobin was subsequently added to a final concentration of 5 mm. After a 2-min incubation, the NO concentration was measured spectrophotometrically by measuring the conversion of oxyhemoglobin to methemoglobin. Oxyhemoglobin has an absorbance peak at 415 nm, whereas methemoglobin has an absorbance peak at 406 nm. The amount of methemoglobin that was produced was calculated with an extinction coefficient of 39 mm−1 cm−1. The NO concentration is expressed as μmol mL−1 min−1 g−1 fresh weight.
Measurement of ROS Production
Excess Zn-induced H2O2 accumulation in root tips was monitored using the fluorescent probe DCFH-DA. Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with 400 μm ZnCl2, 400 μm ZnCl2 plus 0.2 mm PTIO, or 400 μm ZnCl2 plus 0.5 mm l-NAME for 2 h, 12 h, 1 d, 2 d, 4 d, or 10 d. The treated roots were incubated with 10 μm DCFH-DA (Beyotime) in phosphate-buffered saline (PBS) buffer for 30 min at 37°C, washed three times in PBS buffer, and then viewed with a Leica laser scanning confocal microscope (excitation, 488 nm; emission, 530 nm). H2O2 production was measured using a Luminescence Spectrometer with 490-nm excitation and 530-nm emission filters. Data were expressed as relative fluorescence units.
O2− levels were monitored by staining for 20 min in a solution of 2 mm NBT in 20 mm phosphate buffer (pH 6.1). The reaction was stopped by transferring the seedlings into distilled water. O2− content was quantified using the method of Ramel et al. (2009). The NBT-stained plantlets were ground in liquid nitrogen; the obtained powder was solubilized in 2 m KOH-dimethyl sulfoxide (1:1.16, v/v) and then centrifuged for 10 min at 12,000g. The A630 was immediately measured and compared with a standard curve obtained from known amounts of NBT in the KOH-dimethyl sulfoxide mixture.
Antioxidative Enzyme Activity
Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with 400 μm ZnCl2, 400 μm ZnCl2 plus 0.2 mm PTIO, or 400 μm ZnCl2 plus 0.5 mm l-NAME for 2 h, 12 h, 1 d, 2 d, 4 d, or 10 d. The treated roots (0.5 g) were ground in liquid nitrogen to extract total protein. The obtained powder was suspended in 3 mL of extraction buffer containing 50 mm sodium phosphate buffer (pH 7.5), 1% (w/v) polyvinylpyrrolidone, and 0.1 mm EDTA. To determine APX activity, the root powder was suspended in 50 mm HEPES buffer (pH 7.5) containing 1% (w/v) polyvinylpyrrolidone and 0.5 mm ascorbate. After centrifugation (30 min, 12,000g), the supernatant was used to measure enzyme activity.
SOD activity was determined by recording the inhibition of the formazan formation rate by the enzyme (Dhindsa et al., 1981). The reaction mixture consisted of 50 mm phosphate buffer (pH 7.8), 10 mm Met, 25 mm NBT, 0.1 mm EDTA, 50 mm sodium carbonate, 2 μm riboflavin, and 100 μL enzyme in a volume of 3 mL. Reactions were conducted at 25°C under a light intensity of 120 μmol m−2 s−1 for 15 min. To distinguish the SOD isoforms Cu/ZnSOD, FeSOD, and MnSOD, the sensitivity of Cu/ZnSOD to cyanide (3 mm) as well as the sensitivity of Cu/ZnSOD and FeSOD to H2O2 (5 mm) were measured. The absorbance was recorded at 560 nm. One unit of SOD activity was defined as the amount of enzyme required to inhibit the reduction rate of NBT by 50%.
CAT activity was determined by measuring the decrease in H2O2 A240. The reaction mixture contained 50 mm phosphate buffer (pH 7.0), 12.5 mm H2O2, and 50 μL enzyme in a volume of 3 mL. The activity was estimated with an extinction coefficient of 39.4 mm−1 cm−1 and expressed as μmol H2O2 min−1 mg−1 protein.
APX activity was estimated following the H2O2-dependent oxidation of ascorbate as a decrease of A290. The reaction mixture contained 50 mm phosphate buffer (pH 7.0), 0.5 mm ascorbic acid, 0.1 mm EDTA, 150 μm H2O2, 100 μL of enzyme extract, and water in a volume to 3 mL. The activity was estimated with an extinction coefficient of 2.8 mm−1 cm−1 and expressed as nmol ascorbate oxidized min−1 mg−1 protein.
Detection of Cell Death by PI Staining
To assess the cell death level in root tips under Zn stress, the roots were immersed in 3 μg mL−1 PI dissolved in distilled water for 1 min. After washing, the samples were examined with a fluorescence microscope using an excitation wavelength of 546 nm. For each treatment, at least 10 roots were analyzed using a compound microscope (Zeiss Axioskop).
Oxidative Damage (Membrane Liquid Peroxidation)
Excess Zn-induced oxidative damage (membrane liquid peroxidation) was estimated by measuring MDA levels in roots. Fresh roots were homogenized in 0.1% (w/v) TCA solution. After centrifugation (15 min, 12,000g), an aliquot of the supernatant was added to 0.5% thiobarbituric acid in 20% TCA and heated at 90°C for 30 min. After cooling on ice, the mixture was centrifuged at 8,000g for 5 min. The absorbance was recorded at 532 and 600 nm. The MDA concentration was calculated from the difference between absorbance values at 532 and 600 nm (Ben Amor et al., 2005).
DAPI Staining
For DAPI staining, we incubated the roots with 1 μg mL−1 DAPI (Beyotime) in PBS buffer with 1% (v/v) Triton X-100 for 25 min, followed by three washes in PBS buffer. The image was viewed microscopically with a UV light filter.
TUNEL Assay
Roots undergoing PCD were detected using an in situ apoptosis detection kit (Takara) according to the manufacturer’s instructions with a few modifications. Briefly, the roots were fixed in 4% formaldehyde-PBS (pH 7.4) at 4°C for 12 h. After washing three times in PBS buffer for 10 min, the roots were immersed in 70% ethanol at −20°C for 48 h and then washed three times in PBS buffer for 30 min on ice. After washing, the roots were incubated at 37°C for 90 min with terminal deoxynucleotidyl transferase and fluorescein-conjugated nucleotides. The roots were examined with a Leica laser scanning confocal microscope (excitation, 488 nm; emission, 530 nm).
RT-PCR Analysis of Gene Expression
Total RNA was isolated from S. nigrum roots using TRIZOL (GIBCO/BRL Life Technologies). For the semiquantitative RT-PCR, we accurately quantified the RNA concentration using spectrophotometry. The cDNA was synthesized from DNase-treated total RNA using the Reverse Transcription System Kit (Promega) and oligo(dT) primers. We performed control reactions using the 18S RNA primers to ensure that an equal amount of RNA was used in each set of reactions. We optimized the cycle numbers to ensure that the amplification reaction was performed in the exponential phase. Gene expression was quantified using Photoshop CS2 version 9.0. Specific primers for each gene are listed in Supplemental Table S1.
Phenotypic Analysis of Zn2+ and NO Effects
Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with ZnCl2 (400 μm) in the presence or absence of PTIO (0.2 mm) or l-NAME (0.5 mm) for 4 or 10 d. Images of the seedlings were digitized using a scanner (Epson Perfection 1670) after treatment. Primary root length and the number of lateral roots were measured at the indicated times using ImageJ software, version 1.38.
In Situ Zn Localization
For visualization of Zn distribution in intact roots of S. nigrum, seedlings were treated with ZnCl2 (400 μm) with or without PTIO (0.2 mm) or l-NAME (0.5 mm) for 4 d. The treated roots were stained with 25 μm Zinquin (Sigma) for 1 h in the dark. Zinquin fluorescence was visualized with a Leica laser scanning confocal microscope (excitation, 368 nm; emission, 490 nm).
ICP-MS Analysis
Two-week-old S. nigrum seedlings grown in Hoagland solution were treated with 400 μm ZnCl2, 400 μm ZnCl2 plus 0.2 mm PTIO, or 400 μm ZnCl2 plus 0.5 mm l-NAME for 2 h, 12 h, 1 d, 2 d, 4 d, or 10 d. The treated roots were immersed in a solution containing 1 mm EDTA for 2 h and thoroughly rinsed with distilled water. The samples were oven dried at 75°C for 48 h. The dried plant tissues were ground and digested in concentrated nitric acid for 2 to 3 d at room temperature. The samples were then boiled for 1 to 2 h until completely digested. After adding 4 mL of Millipore-filtered deionized water and a brief centrifugation, the contents of Zn, Fe, Mn, and Cu were determined using ICP-MS. Each experiment was repeated at least three times.
Ferric-Chelate Reductase Activity Analysis
The ferric-chelate reductase activity in roots was quantified using the ferrozine assay (Schmidt et al., 2000). The assay solution contained 0.5 mm FeNaEDTA, 0.5 mm CaSO4, and 0.25 mm ferrozine. Localization of ferric-chelate reductase activity was determined by embedding the roots in an agar (0.7%, w/v) medium containing the assay solution (Graziano and Lamattina, 2007). Photographs were obtained after 30 min. Reduction activity was measured by following the changes at 562 nm. Reduction rate was calculated using an extinction coefficient of 27.9 mm−1 cm−1.
Statistical Analysis
For each treatment, 15 roots were analyzed; all the experiments were repeated at least three times. The results are presented as means ± sd. For statistical analysis, we used Student’s t test (P < 0.05).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers X67238, FJ402833, FJ402834, FJ402835, FJ402836, FJ402837, FJ402838, FJ979918, and FJ979919.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Detection of NO production in S. nigrum roots by the DAF-2DA fluorescent probe.
Supplemental Figure S2. Effect of PTIO or l-NAME on NO production in Zn-treated S. nigrum roots.
Supplemental Figure S3. Effect of PTIO or l-NAME on the expression of MnSOD1, MnSOD2, Cu/ZnSOD2, FeSOD, CAT1, CAT2, cAPX, and pAPX.
Supplemental Figure S4. Effect of PTIO or l-NAME on the activity of total SOD, Cu/ZnSOD, FeSOD, MnSOD, CAT, and APX in Zn-treated S. nigrum roots.
Supplemental Figure S5. Effect of PTIO or l-NAME on NADPH oxidase activity in Zn-treated S. nigrum roots.
Supplemental Figure S6. Visualization of H2O2 detected with the DCFH-DA fluorescent probe.
Supplemental Figure S7. Visualization of O2− by NBT staining.
Supplemental Figure S8. Visualization and quantification of H2O2 by the 3-diaminobenzidine method.
Supplemental Figure S9. Effects of PTIO or l-NAME on root hair elongation and plantlet height of S. nigrum seedlings under Zn toxicity.
Supplemental Figure S10. Inhibitory effect of PTIO or l-NAME on rhizosphere acidification in Zn-treated S. nigrum roots.
Supplemental Figure S11. Effect of PTIO or l-NAME on the seedling growth of S. nigrum under Zn toxicity.
Supplemental Table S1. List of the primers for RT-PCR analysis of the genes.
Supplementary Material
Acknowledgments
We thank Prof. Dr. Dezhu Li for providing the S. nigrum seeds. We truly appreciate the time that Prof. Dr. Karl-Josef Dietz and the anonymous reviewers spent on helping to clarify confusions and modify the paper. We thank Prof. Junming Li and Dr. Wensheng Zhang for helping with confocal microscopy and Prof. Dr. Xia Li for her helpful discussion and guidance.
References
- Barclay KD, McKersie BD. (1994) Peroxidation reactions in plant membranes: effects of free fatty acids. Lipids 29: 877–883 [DOI] [PubMed] [Google Scholar]
- Behboodi BS, Samadi L. (2004) Detection of apoptotic bodies and oligonucleosomal DNA fragments in cadmium-treated root apical cells of Allium cepa Linnaeus. Plant Sci 167: 411–416 [Google Scholar]
- Ben Amor N, Ben Hamed K, Debez A, Grignonb C, Abdelly C. (2005) Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci 168: 889–899 [Google Scholar]
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D. (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Feng X, Yang Y, Qiu G, Li G, Li F, Liu T, Fu Z, Jin Z. (2006) Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environ Int 32: 883–890 [DOI] [PubMed] [Google Scholar]
- Bonnet M, Camares O, Veisseire P. (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv. Apollo). J Exp Bot 346: 945–953 [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. (2007) Zinc in plants. New Phytol 173: 677–702 [DOI] [PubMed] [Google Scholar]
- Candan N, Tarhan L. (2003) Changes in chlorophyll-carotenoid contents, antioxidative enzyme activities and lipid peroxidation levels in Zn-stressed Mentha pulegium. Turk J Chem 27: 21–30 [Google Scholar]
- Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA. (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224: 246–254 [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde NM, Graziano ML, Lamattina L. (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218: 900–905 [DOI] [PubMed] [Google Scholar]
- De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP. (2005) Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: a matter of apoplastic reactions. New Phytol 168: 541–550 [DOI] [PubMed] [Google Scholar]
- De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, Sanità di Toppi L, Lo Schiavo F. (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150: 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S. (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99: 16314–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Throne TA. (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32: 93–101 [Google Scholar]
- Duan YF, Zhang WS, Li B, Wang YN, Li KX, Sodmergen, Han C, Zhang Y, Li X. (2010) An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol 186: 681–695 [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93: 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TC, Shaff JE, Grusak MA, Norvell WA, Chen Y, Chaney RL, Kochian LV. (1996) Direct measurement of 59Fe-labeled Fe2+ influx in roots of Pisum sativum using a chelator buffer system to control free Fe2+ in solution. Plant Physiol 111: 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier L, Simon-Plas F, Thuleau P, Agnel JP, Blein JP, Ranjeva R, Montillet JL. (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29: 1956–1969 [DOI] [PubMed] [Google Scholar]
- González-Guerrero M, Azcón-Aguilar C, Mooney M, Valderas A, MacDiarmid CW, Eide DJ, Ferrol N. (2005) Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet Biol 42: 130–140 [DOI] [PubMed] [Google Scholar]
- Gould K, Lamotte O, Klinguer A, Pugin A, Wendehenne D. (2003) Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ 26: 1851–1862 [Google Scholar]
- Graziano M, Lamattina L. (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52: 949–960 [DOI] [PubMed] [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM. (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302: 100–103 [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347, Berkeley [Google Scholar]
- Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM. (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J 29: 649–659 [DOI] [PubMed] [Google Scholar]
- Laspina NV, Groppa MD, Tomaro ML, Benavides MP. (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169: 323–330 [Google Scholar]
- Lingua G, Franchin C, Todeschini V, Castiglione S, Biondi S, Burlando B, Parravicini V, Torrigiani P, Berta G. (2008) Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ Pollut 153: 137–147 [DOI] [PubMed] [Google Scholar]
- Lombardo MC, Graziano M, Polacco JC, Lamattina L. (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AP, Oliveira RS, Rangel AO, Castro PM. (2008) Application of manure and compost to contaminated soils and its effect on zinc accumulation by Solanum nigrum inoculated with arbuscular mycorrhizal fungi. Environ Pollut 151: 608–620 [DOI] [PubMed] [Google Scholar]
- Martin M, Colman MJR, Gómez-Casati DF, Lamattina L, Zabaleta EJ. (2009) Nitric oxide accumulation is required to protect against iron-mediated oxidative stress in frataxin-deficient Arabidopsis plants. FEBS Lett 583: 542–548 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT. (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53: 1237–1247 [PubMed] [Google Scholar]
- Ozdener Y, Aydin BK. (2010) The effect of zinc on the growth and physiological and biochemical parameters in seedlings of Eruca sativa (L.) (rocket). Acta Physiol Plant 32: 469–476 [Google Scholar]
- Pan JW, Zhu MY, Chen H. (2001) Aluminum-induced cell death in root-tip cells of barley. Environ Exp Bot 46: 71–79 [DOI] [PubMed] [Google Scholar]
- Panda SK, Khan MK. (2004) Changes in growth and superoxide dismutase activity in Hydrilla verticillata L. under abiotic stress. Braz J Plant Physiol 16: 115–118 [Google Scholar]
- Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman DW, Goldman SL. (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J 2: 359–366 [DOI] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G. (2009) Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol 9: 28–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, Del Río LA, Sandalio LM. (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150: 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout GR, Das P. (2003) Effect of metal toxicity on plant growth and metabolism. I. Zinc. Agronomie 23: 3–11 [Google Scholar]
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA. (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52: 2115–2126 [DOI] [PubMed] [Google Scholar]
- Schmidt W, Tittel J, Schikora A. (2000) Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol 122: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PN, Kumar P, Tewari RK. (2004) Early signs of oxidative stress in wheat plants subjected to zinc deficiency. J Plant Nutr 27: 449–461 [Google Scholar]
- Madhava Rao KV, Sresty TV. (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157: 113–128 [DOI] [PubMed] [Google Scholar]
- Stöhr C, Ullrich WR. (2002) Generation and possible roles of NO in plant roots and their apoplastic space. J Exp Bot 53: 2293–2303 [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Sachs MM. (2003) Molecular and cellular adaptations of maize to flooding stress. Ann Bot (Lond) 91: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BT, Jing Y, Chen KM, Song LL, Chen FJ, Zhang LX. (2007) Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J Plant Physiol 164: 536–543 [DOI] [PubMed] [Google Scholar]
- Tewari RK, Kim SY, Hahn EJ, Paek KY. (2008) Involvement of nitric oxide-induced NADPH oxidase in adventitious root growth and antioxidant defense in Panax ginseng. Plant Biotechnol Rep 2: 113–122 [Google Scholar]
- Toulon V, Sentenac H, Thibaud JB, Davidian JC, Moulineaz C, Grignon C. (1992) Role of apoplast acidification by the H+ pump: effect on the sensitivity to pH and CO2 of iron reduction by roots of Brassica napus L. Planta 186: 212–218 [DOI] [PubMed] [Google Scholar]
- van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Ver Loren van Themaat E, Koornneef M, Aarts MGM. (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142: 1127–114716998091 [Google Scholar]
- Vital SA, Fowler RW, Virgen A, Gossett DR, Banks SW, Rodriguez J. (2008) Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environ Exp Bot 62: 60–68 [Google Scholar]
- Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP. (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75: 1468–1476 [DOI] [PubMed] [Google Scholar]
- Wei SH, Zhou QX, Wang X, Cao W, Ren LP, Song YF. (2004) Potential of weed species applied to remediation of soils contaminated with heavy metals. J Environ Sci (China) 16: 868–873 [PubMed] [Google Scholar]
- Wink DA, Mitchell JB. (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25: 434–456 [DOI] [PubMed] [Google Scholar]
- Wintz H, Fox T, Wu YY, Feng V, Chen WQ, Chang HS, Zhu T, Vulpe C. (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278: 47644–47653 [DOI] [PubMed] [Google Scholar]
- Wiseman DA, Sharma S, Black SM. (2010) Elevated zinc induces endothelial apoptosis via disruption of glutathione metabolism: role of the ADP translocator. Biometals 23: 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik M, Skórzynska-Poli E, Tukiendorf A. (2006) Organic acids accumulation and antioxidant enzyme activities in Thlaspi caerulescens under Zn and Cd stress. Plant Growth Regul 48: 145–155 [Google Scholar]
- Xu J, Yin HX, Li X. (2009) Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep 28: 325–333 [DOI] [PubMed] [Google Scholar]
- Xu J, Yin HX, Liu XJ, Li X. (2010) Salt affects plant Cd-stress responses by modulating growth and Cd accumulation. Planta 231: 449–459 [DOI] [PubMed] [Google Scholar]
- Yang JD, Yun JY, Zhang TH, Zhao HL. (2006) Presoaking with nitric oxide donor SNP alleviates heat shock damages in mung bean leaf discs. Bot Stud 47: 129–136 [Google Scholar]
- Zhang LP, Mehta SK, Liu ZP, Yang ZM. (2008) Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol 49: 411–419 [DOI] [PubMed] [Google Scholar]
- Zhang YY, Wang LL, Liu YL, Zhang Q, Wei QP, Zhang WH. (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224: 545–555 [DOI] [PubMed] [Google Scholar]
- Zhao LQ, Zhang F, Guo JK, Yang YL, Li BB, Zhang LX. (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134: 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.