Abstract
The symbiotic associations between rhizospheric fungi and plants have enormous environmental impact. Fungi are crucial to plant health as antagonists of pathogens and herbivores and facilitate the uptake of soil nutrients. However, little is known about the plant products obtained by fungi in exchange or how they are transported through the symbiotic interface. Here, we demonstrate that sucrose and raffinose family oligosaccharides in root exudates are important for rhizosphere competence in the insect pathogen Metarhizium robertsii (formerly known as Metarhizium anisopliae). We identified mutants in the Metarhizium raffinose transporter (Mrt) gene of M. robertsii that grew poorly in root exudate and were greatly reduced in rhizosphere competence on grass roots. Studies on sugar uptake, including competition assays, revealed that MRT was a sucrose and galactoside transporter. Disrupting MRT resulted in greatly reduced or no growth on sucrose and galactosides but did not affect growth on monosaccharides or oligosaccharides composed entirely of glucose subunits. Consistent with this, expression of Mrt is exclusively up-regulated by galactosides and sucrose. Expressing a green fluorescent protein gene under the control of the Mrt promoter confirmed that MRT was expressed by germlings in the vicinity of grass roots but not in surrounding bulk soil. Disrupting Mrt did not reduce virulence to insects, demonstrating that Mrt is exclusively involved in M. robertsii’s interactions with plants. To our knowledge, MRT is the first oligosaccharide transporter identified and characterized in a fungus and is unique to filamentous fungi, but homologous genes in Magnaporthe, Ustilago, Aspergillus, Fusarium, Epichloe, and Penicillium species indicate that oligosaccharide transport is of widespread significance.
The rhizosphere is the narrow zone of soil directly influenced by root secretions. It is the site of complex interactions between plants, bacteria, fungi, protists, nematodes, and insects (Bais et al., 2006) that are important for nutrient cycling, ecosystem functioning, and carbon sequestration (Singh et al., 2004). Fungi in particular are crucial to plant growth and health as nutrient solubilizers, phytase producers, and antagonists of plant pathogens and insects (Bridge and Spooner, 2001; Hu and St. Leger, 2002; Marx, 2004; Harman and Shoresh, 2007).
It is generally accepted that the large microbial population in the rhizosphere is supported by a very complex mixture of relatively labile organic compounds (amino acids, organic acids, sugars, phenolics, and various secondary metabolites) in the root exudate (Walker et al., 2003). These compounds can have negative as well as positive interactions with the microbial community and influence the relationship between microbes and insects (Li and Holdom, 1995; Ganade and Brown, 1997). However, due to the complexity of root exudates, identifying the roles of different components in these rhizospheric processes has been highly problematic (Walker et al., 2003).
The ascomycete Metarhizium robertsii ARSEF2575 (formerly known as Metarhizium anisopliae var anisopliae; Bischoff et al., 2009) is ubiquitous in the soil community, where it establishes mutualistic interactions with plants as a rhizospheric fungus (Hu and St. Leger, 2002) and is a potent insect pathogen (Prior, 1992; Roberts and St. Leger, 2004). The distribution of genetic groups of M. robertsii depends on their adaptations to specific soils and plant types rather than their pathogenicity to insects (Bidochka et al., 1998), but applying Metarhizium to seed increases the yield of field corn (Zea mays), possibly in part by killing soil insects (Kabaluk and Ericsson, 2007). Metarhizium also increases plant growth in insect-free microcosms in a multifactorial manner that involves mobilizing nutrients (O’Brien, 2009) and inhibition of plant pathogens (Kang et al., 1996; Ownley et al., 2010). M. robertsii, therefore, provides an unusually versatile model system for studying complicated root-insect-fungus interactions.
The most informative approach for evaluating the importance of root exudate production in establishing rhizosphere competence will involve comparisons with mutant fungi that cannot colonize the rhizosphere. The inability of M. robertsii ΔMad2 (disrupted in an adhesin gene) to adhere to plant roots provided a clear test of the importance of root interactions (Wang and St. Leger, 2007). In this study, we screened over 20,000 transformants in a M. robertsii genome-wide random DNA insertion library. A mutant with poor growth in root exudate was disrupted in a novel sugar transporter gene (Metarhizium raffinose transporter [Mrt]). Disrupting the gene had no effect on virulence to insects, but rhizosphere competency was greatly reduced. Further characterization showed that Mrt encodes a transporter, unique to ascomycete and basidiomycete filamentous fungi, that is essential for growth on heterologous oligosaccharides, thus providing an explanation for the role of Mrt in establishing rhizosphere competence. The distribution of genes that are highly conserved with Mrt in diverse soil-living and plant pathogenic fungi indicates that the occurrence of oligosaccharide transport is widespread.
RESULTS
Characterization of the Sugar Transporter MRT in M. robertsii
Using Agrobacterium tumefaciens-mediated transformation as described previously (Fang et al., 2004) with pFBARGFP (Fang et al., 2009b), we generated a set of 20,328 T-DNA-tagged strain ARSEF2575 mutants. In order to identify mutants putatively compromised in rhizosphere competence, transformants were individually inoculated on agar plates containing soybean (Glycine max) root exudate at 0.03 mg mL−1, to match the concentration of dissolved organic matter within millimeters from an individual root surface (Wenzel et al., 2001). One transformant (M545) showed no apparent growth on root exudate (Supplemental Fig. S1). Both the left and right flanking sequences of the T-DNA insert in M545 displayed significant similarity (<2e−19) to a sugar transporter gene (XP_002484658) from Talaromyces stipitatus, and we designated the disrupted gene as Mrt (GenBank accession no. GQ167043).
The open reading frame of Mrt is 1,671 bp long, not interrupted by introns, and encodes a predicted plasma membrane protein containing 556 amino acid residues. The deduced MRT has a region located between amino acid residues Trp-56 and Phe-513 that shows significant similarity (1e−75) to a sugar porter domain (Pfam00083). As deduced by TMHMM version 2.0 (Krogh et al., 2001), MRT is a 12-transmembrane domain (TMD) topology transporter of the major facilitator superfamily with intracellular termini, a large extracellular loop (between TMD1 and TMD2), and a relatively long central loop that is shared by many sugar transporters (Supplemental Fig. S2). Most sugar transporters have a five-element fingerprint signature (Attwood et al., 2003). However, MRT contains only three elements (elements 2, 4, and 5; Supplemental Fig. S2). Close homologs of MRT (≤2e−156) were identified in diverse ascomycete and basidiomycete fungi but not in zygomycetes and chytridiomycetes, suggesting that they arose in a common ancestor of ascomycetes and basidiomycetes after they diverged from zygomycetes. The homologs were nearly the same length and almost identical in predicted structure (Supplemental Table S1). A phylogenetic tree (Supplemental Fig. S3) confirmed that MRT and these homologs formed a separate clad distinct from a large group that includes many well-characterized hexose transporters and a group that includes Srt1, a highly specific Suc transporter from Ustilago maydis (Wahl et al., 2010). MRT shows low similarity (up to 28%) with animal, plant, and bacterial sugar transporters. Therefore, MRT-like transporters are members of a novel family of transporters that are exclusive to fungi.
MRT Is Essential for Growth on Raffinose Family Oligosaccharides
For functional analysis, we disrupted Mrt to get M. robertsii ΔMrt. Complementing ΔMrt and M545 with a genomic clone of Mrt produced strains indistinguishable from the wild-type strain as determined by all the phenotypic assays conducted in this study; unless otherwise indicated, data for the complemented transformants are not presented.
To measure the ability of MRT to transport carbohydrates, we compared germination rate of the wild type with that of ΔMrt in basal salt medium (BS; 0.1% KH2PO4, 0.025% Na2SO4, 0.05% KCl, 0.0125% MgSO4·7H2O, 0.00625% CaCl2, and 0.3% NaNO3; Fang et al., 2006) supplemented with each of the carbohydrates listed in Table I. The ΔMrt mutant germinated and grew at similar rates as the wild type on monosaccharides and oligomers composed entirely of repeated Glc subunits (glucooligosaccharides). In contrast, melezitose (a trisaccharide composed of Glc attached to an isomer of Suc) and raffinose family oligosaccharides (RFOs [raffinose, stachyose, and verbascose]) did not increase germination and growth of ΔMrt above the very low levels seen in medium containing no carbon sources (e.g. water or BS; Table I; Supplemental Fig. S4). Germination of ΔMrt on disaccharides such as Suc and lactose that contain two different monosaccharides was significantly (P < 0.001) reduced compared with the wild type (Table I).
Table I. Carbohydrate consumption and in vitro growth on root exudate.
| Carbohydrate | Germination Rate in BS Plus Carbohydrate (1%) | ||
| Wild Type | ΔMrt | ΔMrt Complemented with Mrt | |
| % | |||
| BS only | 8.0 ± 0.2 | 7.8 ± 0.5 | 8.0 ± 0.6 |
| Monosaccharides | |||
| Fru | 27.6 ± 1.2 | 26.2 ± 1.8 | 27.3 ± 1.7 |
| Man | 58.2 ± 3.2 | 56.2 ± 2.7 | 58.1 ± 2.9 |
| Oxylose | 24.8 ± 2.2 | 22.4 ± 1.9 | 25.1 ± 2.5 |
| Sorbose | 24.8 ± 1.3 | 25±1.4 | 24.7 ± 1.9 |
| Glc | 36.4 ± 2.3 | 34.8 ± 2.9 | 36.5 ± 3.1 |
| Gal | 42.4 ± 2.5 | 41.4 ± 2.8 | 42.3 ± 2.6 |
| Homologous disaccharides or oligosaccharides composed of Glc | |||
| Maltose | 35.6 ± 1.9 | 34.4 ± 2.3 | 35.0 ± 2.1 |
| Trehalose | 35.2 ± 2.1 | 33±3.3 | 34.5 ± 2.7 |
| Cellobiose | 29.8 ± 2.2 | 28.9 ± 3.4 | 29.3 ± 2.9 |
| Maltotriose | 25.5 ± 2.1 | 24.9 ± 2.4 | 25.3 ± 2.2 |
| Maltotetraose | 26.3 ± 2.5 | 25.8 ± 1.9 | 25.9 ± 2.8 |
| Maltopentaose | 26.9 ± 2.2 | 26.2 ± 3.3 | 27.1 ± 3.2 |
| Maltohexaose | 25.8 ± 2.9 | 26.1 ± 1.7 | 25.9 ± 1.8 |
| Maltoheptaose | 25.1 ± 0.9 | 25.5 ± 1.3 | 24.9 ± 2.1 |
| Heterologous dioligosaccharides composed of a mixture of monosaccharides | |||
| Suc | 35.2 ± 2.3 | 12.2 ± 2.3 | 35.3 ± 2.4 |
| Lactose | 34.8 ± 2.1 | 21.6 ± 2.1 | 34.9 ± 2.2 |
| Raffinose | 31.2 ± 3.1 | 7.4 ± 0.7 | 30.9 ± 2.9 |
| Melezitose | 30.2 ± 2.8 | 8.2 ± 0.2 | 31.1 ± 3.1 |
| Stachyose | 31.9 ± 2.7 | 7.6 ± 0.3 | 31.5 ± 2.5 |
| Verbascose | 29.9 ± 2.3 | 7.8 ± 0.6 | 29.8 ± 2.1 |
| Root exudate alone (dissolved in sterile distilled water) | |||
| 1 mg mL−1 | 100 | 100 | 100 |
| 0.1 mg mL−1 | 85 ± 1.2 | 53.6 ± 1.8 | 84.5 ± 1.5 |
| 0.01 mg mL−1 | 28 ± 2.1 | 8.1 ± 1.5 | 28.1 ± 1.9 |
| 0 mg mL−1 | 7.8 ± 1.9 | 7.9 ± 1.2 | 8.0 ± 1.5 |
These results suggest that MRT is M. robertsii’s sole transporter for heterologous oligosaccharides and a major transporter for heterologous disaccharides. We confirmed its transporter activity by assaying the uptake of labeled oligosaccharides and disaccharides (Fig. 1). Unlike ΔMrt, wild-type hyphae took up fluorescently labeled raffinose, melezitose, stachyose, and verbascose, consistent with MRT being the only transporter for heterologous oligosaccharides. Neither wild-type nor ΔMrt hyphae took up labeled maltotriose, maltotetraose, maltopentaose, maltohexaose, or maltoheptaose. Both ΔMrt and the wild type grew with these homologous oligosaccharides as sole carbon sources, suggesting that they are hydrolyzed extracellularly.
Figure 1.
Uptake assays of fluorescently labeled disaccharides, heterologous oligosaccharides, and homologous oligosaccharides by the wild type (WT) and the Mrt disruption mutant (ΔMrt). Neither ΔMrt nor the wild type can transport any homologous oligosaccharides, and the image of maltotriose was used as a representative of maltotetraose, maltopentaose, maltohexaose, and maltoheptaose. [See online article for color version of this figure.]
To further assess MRT specificity, labeled raffinose uptake was assayed in the presence of a 10-fold excess of unlabeled sugars used as competitors. Verbascose, stachyose, raffinose, melezitose, Suc, and lactose greatly inhibited the transportation of labeled raffinose by MRT, but monosaccharides (Fru, Glc, and Gal) and glucooligosaccharides (trehalose, cellobiose, maltose, maltotriose, and maltotetraose) had no effect (Fig. 2). This inhibition pattern confirms that MRT has most affinity for heterologous dioligosaccharides.
Figure 2.
Competition assay. Hyphae expressing MRT were incubated with a 10-fold excess of each unlabeled competitor 5 min prior to adding fluorescently labeled raffinose. Inhibitions were indicated by greatly reduced fluorescence as compared with the control incubated with labeled raffinose alone. [See online article for color version of this figure.]
The addition of nigericin (Na+, K+ ionophore) to BS + raffinose greatly reduced the germination rate of the wild-type strain, and carbonyl cyanide 3-chlorophenylhydrazone (H+ ionophore) blocked germination completely (Supplemental Fig. S5). The wild-type strain produced an asporogenic fluffy colony with raffinose on a Na+-free medium (pH = 7.0) in which choline chloride replaced NaCl (Supplemental Fig. S5). This indicates that MRT activity is dependent on the electrochemical membrane potential (i.e. MRT is a secondary active transporter).
Expression of Mrt by M. robertsii Is Induced by Heterologous Disaccharides and Oligosaccharides
Regulation of Mrt expression was studied by quantitative reverse transcription PCR (qPCR) using total RNA isolated from mycelia grown on different carbohydrates (Table I). Mrt was constitutively expressed at low levels in nutrient-rich medium (Sabouraud dextrose broth [SDB]). Expression of Mrt did not change when mycelia were transferred from SDB into BS supplemented with a monosaccharide or a polymer of Glc (Table I). However, the expression of Mrt was increased approximately 10-fold by heterologous disaccharides or oligosaccharides (Suc, lactose, raffinose, stachyose, melezitose, and verbarose). There were no significant differences between RFOs and other heterologous oligosaccharides in their ability to induce the expression of Mrt (P > 0.38).
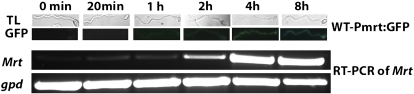
The time course for Mrt expression was also examined by following GFP fluorescence driven by the Mrt promoter in WT-Pmrt:GFP. Spores of WT-Pmrt:GFP that had been germinated in Glc fluoresced within 2 h of transfer to BS + raffinose. GFP fluorescence peaked at 4 h and then remained stable. The GFP fluorescent signal was consistent with the amount of qPCR product, as this showed a 10-fold increase between 0 and 4 h (Fig. 3).
Figure 3.
Top panel, time course of induction of the Mrt-gfp reporter in BS supplemented with raffinose. TL, Transmitted light. Bottom panel, expression assayed with qPCR after mycelium was transferred from SDB into BS supplemented with raffinose. [See online article for color version of this figure.]
MRT and Rhizosphere Competency of M. robertsii
The involvement of Mrt in the rhizosphere competency of M. robertsii was initially investigated by measuring germination rates of the wild type and ΔMrt in root exudate as sole carbon and nitrogen sources. At a high concentration of root exudate (1 mg mL−1), ΔMrt and the wild type germinated at similar rates, and both achieved 100% germination after 20 h of incubation. With 0.1 mg mL−1 root exudate, 85% ± 1.2% of wild-type spores and 53.6% ± 1.8% of ΔMrt spores had germinated by 20 h. At 0.01 mg mL−1, 28% ± 2.1% of the wild-type spores had germinated, but the germination rate of ΔMrt (8.1% ± 1.5%) was the same as that on water alone (7.9% ± 1.2%). The lower concentrations of root exudate (0.01–0.1 mg mL−1) probably approximate most closely to conditions in nature (Wenzel et al., 2001).
To investigate rhizospheric interactions, the roots of grass seedlings were embedded in 1% low-melting-point agarose supplemented with 107 mL−1 of the parental strains (wild-type) spores, ΔMrt spores, or WT-Pmrt:GFP spores, and the germination behavior around the root (less than 3 mm) or far from the root (more than 10 mm) was observed over 24 h. Less than 8% of wild-type spores germinated when they were more than 10 mm from a root, whereas 60% ± 2.1% of spores that were less than 3 mm from a root germinated. Of the germinated wild-type spores, 96% ± 3.5% of the germlings grew toward the root. In contrast, only 29% ± 2.7% of ΔMrt spores less than 3 mm from a root germinated, and 93% ± 2.3% of these germlings grew toward the root. The rate of germ tube growth by the wild type was approximately 6-fold greater than that of ΔMrt. All wild-type germ tubes produced at least one lateral branch, consistent with efficient colonization and utilization of resources, but ΔMrt germlings never exhibited branching. Boosted hyphal growth with branching is a common response of rhizosphere fungi to the chemical signals they use to locate plant roots (Harrison, 2005).
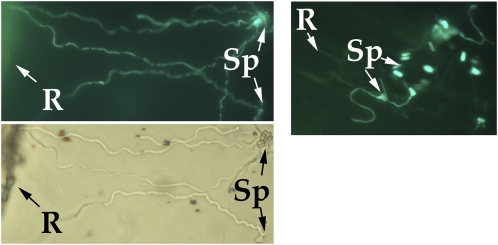
Transformants harboring the promoter probe construct (WT-Pmrt:GFP) had the same germination behavior as the wild type. Strong GFP fluorescence was only detected in spores germinating in close vicinity to roots (Fig. 4), demonstrating that heterologous dioligosaccharides were being released from roots into the agarose.
Figure 4.
Left panel, induction of the Mrt-gfp reporter by germlings growing toward grass roots embedded in 1% agarose containing 107 WT-Pmrt:GFP spores mL−1 (top, fluorescence; bottom, transmitted light). Right panel, induction of the Mrt-gfp reporter by spores and germlings in the vicinity of a grass root growing in soil. Penetrant hyphae are growing in the intercellular spaces of the root epidermal cells. Photographs were taken following incubation for 24 h at 27°C in agarose or 1 month in the soil. R, Root; Sp, spores. [See online article for color version of this figure.]
We then determined if heterologous dioligosaccharides play an important part in supporting growth and rhizosphere competency of M. robertsii in the soil root interphase. Spores of the wild type, ΔMrt, or WT-Pmrt:GFP were inoculated into soil microcosms containing grasses. Spores and germlings of WT-Pmrt:GFP fluoresced in the rhizospheric soil (Fig. 4) but not in spores from the bulk soil, confirming the localized distribution of the heterologous dioligosaccharides in soil as well as M. robertsii’s ability to react to them. Although M. robertsii produces appressoria on insect cuticle, we did not observe WT-Pmrt:GFP producing appressoria against plant roots, although fluorescent hyphae had penetrated the superficial cell layers of the roots and were growing around the cells (Fig. 4), suggesting that they were restricted to the intercellular spaces. The rhizosphere competency of the wild type and ΔMrt were compared by counting colony-forming units (CFUs). In the first month, levels of the wild type and ΔMrt in the rhizospheric and bulk soil samples stayed constant. However, counts of the wild type had increased 8-fold at 2 months after inoculation and 45-fold at 3 months after inoculation. In contrast, numbers of ΔMrt CFUs did not change significantly for the first 2 months but had increased 4-fold by 3 months (Fig. 5). At 3 months, counts of the wild-type strain in rhizospheric soil were 11-fold higher than those of ΔMrt, but counts of the wild type and ΔMrt in bulk soil were not significantly different (P = 0.33).
Figure 5.
Rhizosphere competency of the Mrt disruption mutant (white bars) and wild-type M. robertsii (black bars). Rhizosphere competency was measured by counting the number of CFUs in rhizospheric soils from grass roots. In 3 months, the number of CFUs of the wild type or the Mrt disruption mutant in the bulk soil had not significantly changed (P > 0.1).
Insect Bioassay
The virulence of ΔMrt against larval Manduca sexta was the same as that of the wild type, with LT50 values (time taken to kill 50% of the insects) around 4.5 d following topical application of 1 × 107 spores mL−1.
DISCUSSION
The symbiotic relationships between rhizospheric fungi and plants have an enormous impact on terrestrial ecosystems (Smith and Read, 1997). Fungi facilitate the uptake of soil nutrients by plants and, in exchange, obtain various products of photosynthesis, thus representing a large sink for atmospheric plant-fixed CO2 (seedlings typically exude about 30%–40% of their fixed carbon as root exudates; Whipps, 1990; Schüssler et al., 2006). However, it is not known which of the compounds in plentiful supply in root exudate, such as common sugars, organic acids, and amino acids, play the most important roles in supporting fungal growth in the rhizosphere, and the means by which they are transported through the symbiotic interface is also poorly understood (Schüssler et al., 2006). To date, only the monosaccharide transporter GfMST1 from the glomeromycotan fungus Geosiphon pyriformis has been implicated in symbiotic processes (Schüssler et al., 2006), although proliferation of the basidiomycete pathogen U. maydis in maize requires the specific Suc transporter Srt1 (Wahl et al., 2010). Monosaccharide (hexose) and disaccharide transporters are the only sugar transporters characterized in filamentous fungi (Nehls et al., 1998; Voegele et al., 2001; Delgado-Jarana et al., 2003; Vankuyk et al., 2004; Wei et al., 2004; Forment et al., 2006; Schüssler et al., 2006; Jackson-Hayes et al., 2008; Wahl et al., 2010). The molecular genetics of sugar sensing and transport in filamentous fungi, therefore, is a significantly understudied area, as sugar-related aspects contribute to the central role of fungi in every terrestrial ecosystem and in their crucial roles in industry, medicine, agriculture, and basic science.
This work presents consistent evidence that a novel type of oligosaccharide transporter (MRT) is important to the rhizosphere competency of the filamentous ascomycete M. robertsii. In the absence of isotope-labeled sugars, we used 2-anthranilic acid (2-AA) as a label, and it is possible that the addition of 2-AA could alter the properties of the sugar and therefore uptake by MRT. This does not appear to have happened, presumably because 2-AA only labels the reducing end of an oligosaccharide and does not alter the structural integrity or conformation of the sugars, nor does it increase steric hindrance (Bigge et al., 1995). In addition, the Mr of 2-AA (137) is smaller than that of a hexose (approximately 180), which would not be beyond the transportation limit of MRT, because MRT can transport raffinose (Suc + Gal), stachyose (raffinose + Gal), and verbascose (stachyose + Gal). The results of the uptake experiments with 2-AA-labeled sugars were entirely consistent with uptake competition assays and with growth experiments, which showed that ΔMrt is unable to grow on the heterologous oligosaccharides that successfully competed with the labeled sugars in uptake assays. Furthermore, the transporter is specifically induced by heterologous oligosaccharides, consistent with the transporter functioning in their transport.
M. robertsii is known to be able to grow on a wide variety of carbon sources, including many sugars, amino acids, and organic acids that are present in root exudate (Li and Holdom, 1995). The big impact on rhizospheric growth of knocking out a single transporter, therefore, is quite surprising, but sugars are the most abundant component of root exudates (Jaeger et al., 1999) and MRT is the major and perhaps only route for the uptake of multiple heterologous β-galactosides (e.g. lactose), α-galactosides (e.g. raffinose), and the α-glucoside Suc. M. robertsii germinates best in complex media, since raffinose (1%) as sole carbon source produces only 31% germination after 20 h, as compared with 100% germination on 1 mg mL−1 root exudate. However, unlike the wild type, ΔMrt does not germinate in an ecologically relevant concentration of root exudate (0.01 mg mL−1). Oligosaccharide transporters often have broad specificity with some selectivity toward particular types of sugars (Saint-Pol et al., 1999). Bacterial transporters able to take up both α- and β-galactosides are common, but bacterial galactoside transporters are reported not to take up Suc (Okazaki et al., 1997). Interestingly, from the point of view of convergent evolution of plants and fungi, some sugar transporters from plant roots can transport Suc as well as galactosides (Jaeger et al., 1999). The broad specificity of MRT seems well attuned to M. robertsii’s rhizospheric competence, as root exudates usually contain high levels of Suc and RFOs, particularly raffinose, the tetrasaccharide stachyose, and the pentasaccharide verbascose (composed of Gal, Fru, and Glc), all of which are transported by MRT. However, while Suc is particularly abundant at the growing root tip, galactosides such as raffinoses are absent from root tips and instead are distributed along the root (Jaeger et al., 1999; Bringhurst et al., 2001). The ability to take up these diverse nutrients suggests that M. robertsii will not be limited by nutrient dispersal to spatially and temporally distinct areas. This may be key for a fungus when initiating or maintaining rhizospheric competence.
Utilizing an Mrt promoter-reporter construct to precisely reveal the spatial and temporal patterns of MRT activity enabled us to use M. robertsii as a biosensor for sugar levels. Sugars are present at high enough concentrations within 3 mm of the root to induce MRT expression, and MRT is able to catalyze the uptake of sufficient root-derived sugars to greatly enhance growth, as shown by the comparatively poor performance of ΔMrt. The failure of surrounding bulk soil to trigger the reporter and induce germination is consistent with a steep concentration gradient in inducing sugars exiting from the root surfaces into the rhizospheric soils. Disruption of Mrt did not alter the virulence of M. robertsii against its insect host M. sexta. Hence, MRT appears to be exclusively involved in M. robertsii’s existence at the root interphase.
The best studied plant symbionts are the obligate biotrophic mycorrhizial fungi (Glomales, Zygomycetes). Functional analysis of their genes is not a trivial task, and very few have been deleted, the hexose transporter GfMST1 from G. pyriformis being an exception (Schüssler et al., 2006). We did not find MRT genes in any zygomycete (by homology search algorithms), but glomeromycotan fungi rely on plant enzymes to hydrolyze Suc and then take up hexoses directly from the inner cortex (Franken and Requena, 2001). While plants infected with rhizosphere-competent ascomycetes usually do not have the complex structures associated with most mycorrhizal infection, they also occupy a nutritional niche in or on the plant and develop an active cross talk with their plant hosts (Vinale et al., 2008). Suc and RFOs are abundant in root exudates. Low-level constitutive expression of the transporter will allow filamentous hyphal growth in a chemotaxic manner, “testing” the compounds released, and will also induce the production of the transporter. The transporter, therefore, could function as a root-induced receptor enabling the fungus to recognize a root and grow toward the chemical it emits, as determined by an increased concentration gradient of oligosaccharides. The majority of the ΔMrt that succeeded in germinating also orientated toward the root, indicating that M. robertsii is receptive to multiple root-related nutrients, but the number of wild-type germlings that established a nutritional relationship with the plant was much higher. To date, very few other fungal genes have been implicated in rhizosphere competence and plant colonization, even in the best studied rhizosphere-competent ascomycete, Trichoderma harzianum (Harman and Shoresh, 2007). It is currently known that attachment of Trichoderma species to the root by appressoria-like structures is mediated by hydrophobins (Viterbo and Chet, 2006). In M. robertsii, adhesion to roots is achieved by a specialized adhesin (Wang and St. Leger, 2007), and we did not find homologs of MRT or the U. maydis Suc transporter Srt1 in Trichoderma species, which may recognize plant structures by secreting enzymes that cause the release of plant cell wall constituents, including presumably monosaccharides (Woo and Lorito, 2007). Trichoderma species mostly colonize the intercellular spaces of roots, where they are restricted to epidermal cell layers (Harman and Shoresh, 2007). Metarhizium shows a similar pattern of colonization, at least of grass roots, but the differences in protein composition are consistent with rhizosphere competency having evolved independently in the two hypocrealean pyrenomycete genera Metarhizium and Trichoderma. They raise the interesting experimental possibility of switching genes between rhizosphere-competent strains of Metarhizium and Trichoderma to determine if that increases their ability to colonize roots.
The absence of a homolog of Srt1 in Trichoderma is not unexpected, as Magnaporthe, Fusarium, and Epichloe also lack homologs of Srt1. The absence of MRT homologs in Trichoderma is exceptional, as they are highly conserved in most ascomycete and basidiomycete fungi, including species of Magnaporthe, Fusarium, Aspergillus, Ustilago, Epichloe, and Penicillium that are plant pathogens, opportunistic plant symbionts, or soil-dwelling saprophytes (Supplemental Table S1). This suggests that MRT homologs may be generally important for fungal survivorship in association with plants, because Suc and raffinose are the most abundant soluble sugars within plants as well as being important components of their root exudates (Trugo et al., 1995).
MATERIALS AND METHODS
Gene Cloning and Disruption
The flanking sequences of mutants generated by the insertion of T-DNA into the genome of Metarhizium robertsii were cloned by Y-shaped adaptor-dependent extension as described (Fang et al., 2005). The primers employed in this study and their usages are given in Supplemental Table S2. PCR products were cloned into pGEM-T Easy (Promega) for sequence confirmation.
To construct the Mrt disruption plasmid, the 5′-end and 3′-end of Mrt were cloned by PCR and inserted into the XbaI and SpeI sites, respectively, of the plasmid pFBARGFP (Fang et al., 2009b). The disruption mutant (ΔMrt) was obtained utilizing Agrobacterium tumefaciens. To complement M545 and ΔMrt, the genomic sequence of Mrt was cloned, inserted into the XbaI site of pFBENGFP, and transformed into M545 and ΔMrt as described (Supplemental Fig. S6; Fang et al., 2006).
Sugar Uptake Competition Assay
Since isotope-labeled heterologous oligosaccharides are not commercially available, we labeled sugars with 2-AA essentially as described in the 2-AA Labeling Kit (Sigma; PP0530). As MRT is the only transporter for raffinose in the wild type, we tested MRT specificity using a sugar uptake competition assay as described (Lam et al., 1994). Spores of the wild type were germinated in a petri dish with BS + raffinose to induce MRT. The growth medium was discarded and the germlings washed with sterile water before adding a 1% solution of each unlabeled competitor in BS. After 5 min, BS containing 0.6% fluorescently labeled raffinose was added to give a final concentration of 0.1% labeled raffinose, and the intensity of fluorescence in each treatment after 15 min was compared with controls incubated with labeled raffinose without competitors.
Sugar Consumption Assay
The ability of ΔMrt and the wild type to consume different sugars was analyzed by incubating 1.5 × 106 spores in water, BS, or BS plus a carbohydrate (1%; Table I). Germination rates at 27°C were checked every 3 h. Growth rates of ΔMrt and the wild type were compared on BS agar plates supplemented with different carbohydrates.
Expression Pattern of Mrt
Mycelial inoculum from SDB cultures (Fang et al., 2009b) was incubated for 10 h in BS supplemented with different carbohydrates. RNA was isolated using the Plant RNeasy Kit (Qiagen), and qPCR analysis was conducted as described previously (Fang et al., 2009b).
To study how roots effect the expression of Mrt, we constructed transformants expressing GFP under the control of the promoter region (2,000 bp) of Mrt. The promoter was obtained by PCR and cloned into the EcoRI and EcoRV sites of Bargpe1-GFP (Fang and St. Leger, 2010). The egfp cassette was then released by EcoRI and SpeI, blunted with T4 DNA polymerase, and cloned into the EcoRV site of the Ti plasmid Ppk2-bar (Fang et al., 2009b), which was then transformed into the wild-type strain of M. robertsii, producing WT-Pmrt:GFP.
To study the time course of Mrt expression, spores of WT-Pmrt:GFP were incubated for 24 h in BS supplemented with Glc and then in BS supplemented with raffinose. GFP fluorescence was observed at 30-min intervals for 8 h. To obtain roots, grass seeds were grown in autoclaved commercially available grass soil for 3 weeks. Whole plants with 5- to 8-mm-long roots were carefully removed from soil, washed with sterile water, laid on a slide, and covered in 1% low-melting-point agarose (Fisher Scientific) containing 107 mL−1 spores of WT-Pmrt:GFP. The slides were incubated at 27°C, and GFP fluorescence was monitored for up to 24 h.
Rhizosphere Competence Assay
Soybean (Glycine max) root exudate was prepared as described (Barbour et al., 1991). Fungal spores were inoculated into a petri dish containing root exudate (0.01–1 mg mL−1) or sterile water (control). Percentage germination was recorded at 3-h intervals.
Rhizospheric populations of ΔMrt and the wild-type strain were monitored as described by McLean et al. (2004) with modifications. Cube-shaped containers (5.7 L) were filled with grass soil (Scotts Turf Builder Seeding Soil; Scotts Company). Twenty grass seedlings were planted at approximately 5-cm intervals in each container, and the containers were placed in a growth chamber. When the plants were approximately 6 cm high, a spore suspension was evenly spread in the container at the rate of 107 spores m−2. To study population dynamics in bulk soil, spore suspensions were also inoculated into unplanted soil containers. At intervals after inoculation, individual plants and adherent rhizosphere soil were carefully removed and put into a 50-mL tube containing 10 mL of 0.05% Tween 80. The rhizospheric soil was washed off the roots by vigorous vortexing, and the weight of soil in 5 mL was determined as dry weight. Aliquots (100 μL) of the remaining soil solution were then spread on Metarhizium selection plates (Fang et al., 2009a), and CFUs were counted after 10 d of incubation (Hu and St. Leger, 2002). Five plants were individually sampled in each treatment. Rhizosphere competence was recorded as CFUs g−1 soil. This experiment was repeated three times.
Insect Bioassay
M. robertsii was bioassayed against fifth instar Manduca sexta caterpillars (Carolina Biological Supply Company) as described (Wang and St. Leger, 2007), and LT50 values (time taken to kill 50% of the insects) were calculated using the SPSS program.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number GQ167043.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Growth of wild-type M. robertsii and the mutant M545 on an agar plate supplemented with root exudate.
Supplemental Figure S2. Schematic representation of the topology predicted for the primary structure of MRT.
Supplemental Figure S3. Phylogenetic analysis of MRT and its close homologs.
Supplemental Figure S4. Growth of an Mrt disruption mutant and the wild type on BS agar plates supplemented with Glc, Suc, or raffinose.
Supplemental Figure S5. MRT is a secondary active transporter.
Supplemental Figure S6. Confirmation of the disruption of Mrt in M. robertsii by Southern-blot analysis.
Supplemental Table S1. Sequence and structural information about MRT and some of its fungal homologs.
Supplemental Table S2. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Andrew P. MacCabe for help with drawing Supplemental Figure S2.
References
- Attwood TK, Bradley P, Flower DR, Gaulton A, Maudling N, Mitchell AL, Moulton G, Nordle A, Paine K, Taylor P, et al. (2003) PRINTS and its automatic supplement, prePRINTS. Nucleic Acids Res 31: 400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57: 233–266 [DOI] [PubMed] [Google Scholar]
- Barbour WM, Hattermann DR, Stacey G. (1991) Chemotaxis of Bradyrhizobium japonicum to soybean exudates. Appl Environ Microbiol 57: 2635–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidochka MJ, Kasperski JE, Wild GAM. (1998) Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Can J Bot 76: 1198–1204 [Google Scholar]
- Bigge JC, Patel TP, Bruce JA, Goulding PN, Charles SM, Parekh RB. (1995) Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal Biochem 230: 229–238 [DOI] [PubMed] [Google Scholar]
- Bischoff JF, Rehner SA, Humber RA. (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101: 512–530 [DOI] [PubMed] [Google Scholar]
- Bridge P, Spooner B. (2001) Soil fungi: diversity and detection. Plant Soil 232: 147–154 [Google Scholar]
- Bringhurst RM, Cardon ZG, Gage DJ. (2001) Galactosides in the rhizosphere: utilization by Sinorhizobium meliloti and development of a biosensor. Proc Natl Acad Sci USA 98: 4540–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Jarana J, Moreno-Mateos MA, Benítez T. (2003) Glucose uptake in Trichoderma harzianum: role of gtt1. Eukaryot Cell 2: 708–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Feng J, Fan Y, Zhang Y, Bidochka MJ, Leger RJ, Pei Y. (2009a) Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J Invertebr Pathol 102: 155–159 [DOI] [PubMed] [Google Scholar]
- Fang W, Leng B, Xiao Y, Jin K, Ma J, Fan Y, Feng J, Yang X, Zhang Y, Pei Y. (2005) Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol 71: 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Pava-ripoll M, Wang S, St Leger RJ. (2009b) Protein kinase A regulates production of virulence determinants by the entomopathogenic fungus, Metarhizium anisopliae. Fungal Genet Biol 46: 277–285 [DOI] [PubMed] [Google Scholar]
- Fang W, Pei Y, Bidochka MJ. (2006) Transformation of Metarhizium anisopliae mediated by Agrobacterium tumefaciens. Can J Microbiol 52: 623–626 [DOI] [PubMed] [Google Scholar]
- Fang W, St Leger RJ. (2010) RNA binding proteins mediate the ability of a fungus to adapt to the cold. Environ Microbiol 12: 810–820 [DOI] [PubMed] [Google Scholar]
- Fang W, Zhang Y, Yang X, Zheng X, Duan H, Li Y, Pei Y. (2004) Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J Invertebr Pathol 85: 18–24 [DOI] [PubMed] [Google Scholar]
- Forment JV, Flipphi M, Ramón D, Ventura L, MacCabe AP. (2006) Identification of the mstE gene encoding a glucose-inducible, low affinity glucose transporter in Aspergillus nidulans. J Biol Chem 281: 8339–8346 [DOI] [PubMed] [Google Scholar]
- Franken P, Requena N. (2001) Analysis of gene expression in arbuscular mycorrhiza: new approaches and challenges. New Phytol 150: 431–439 [Google Scholar]
- Ganade G, Brown VK. (1997) Effects of below-ground insects, mycorrhizal fungi and soil fertility on the establishment of Vicia in grassland communities. Oecologia 109: 374–381 [DOI] [PubMed] [Google Scholar]
- Harman GE, Shoresh M. (2007) The mechanisms and applications of opportunistic plant symbionts. Vurro M, Gressel J, , Novel Biotechnologies for Biocontrol Agent Enhancement and Management. Springer, Amsterdam, pp 131–157 [Google Scholar]
- Harrison MJ. (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59: 19–42 [DOI] [PubMed] [Google Scholar]
- Hu G, St Leger RJ. (2002) Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl Environ Microbiol 68: 6383–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Hayes L, Hill TW, Loprete DM, Fay LM, Gordon BS, Nkashama SA, Patel RK, Sartain CV. (2008) Two GDP-mannose transporters contribute to hyphal form and cell wall integrity in Aspergillus nidulans. Microbiology 154: 2037–2047 [DOI] [PubMed] [Google Scholar]
- Jaeger CH, III, Lindow SE, Miller W, Clark E, Firestone MK. (1999) Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl Environ Microbiol 65: 2685–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabaluk JT, Ericsson JD. (2007) Metarhizium anisopliae seed treatment increases yield of field corn when applied for wireworm control. Agron J 99: 1377–1381 [Google Scholar]
- Kang SC, Bark YG, Lee DG, Kim H. (1996) Antifungal activities of Metarhizium anisopliae against Fusarium oxysporum, Botrytis cinerea, and Alternaria solani. The Korean Journal of Mycology 24: 49–55 [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305: 567–580 [DOI] [PubMed] [Google Scholar]
- Lam CK, Belanger FC, White JF, Jr, Daie J. (1994) Mechanism and rate of sugar uptake by Acremonium typhinum, an endophytic fungus infecting Festuca rubra: evidence for presence of a cell wall invertase in endophytic fungi. Mycologia 86: 408–415 [Google Scholar]
- Li DP, Holdom DG. (1995) Effects of nutrients on colony formation, growth, and sporulation of Metarhizium anisopliaerobertsii (Deuteromycotina: Hyphomycetes). J Invertebr Pathol 65: 253–260 [Google Scholar]
- Marx J. (2004) The roots of plant-microbe collaborations. Science 304: 234–236 [DOI] [PubMed] [Google Scholar]
- McLean KL, Dodd SL, Sleight BE, Hill RA, Stewart A. (2004) Comparison of the behaviour of a transformed hygromycin resistant strain of Trichoderma atroviride (M1057-hygr) with the wild-type strain (M1057). N Z Plant Prot 57: 72–76 [Google Scholar]
- Nehls U, Wiese J, Guttenberger M, Hampp R. (1998) Carbon allocation in ectomycorrhizas: identification and expression analysis of an Amanita muscaria monosaccharide transporter. Mol Plant Microbe Interact 11: 167–176 [DOI] [PubMed] [Google Scholar]
- O’Brien T. (2009) The saprophytic life of Metarhizium anisopliae. PhD thesis University of Maryland, College Park [Google Scholar]
- Okazaki N, Jue XX, Miyake H, Kuroda M, Shimamoto T, Tsuchiya T. (1997) A melibiose transporter and an operon containing its gene in Enterobacter cloacae. J Bacteriol 179: 4443–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownley BH, Gwinn KD, Vega FE. (2010) Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl 55: 113–128 [Google Scholar]
- Prior C. (1992) Discovery and characterization of fungal pathogens for locust and grasshopper control. Lomer CJ, Prior C, , Biological Control of Locusts and Grasshoppers. CABI International, Wallingford, UK, pp 159–180 [Google Scholar]
- Roberts DW, St Leger RJ. (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54: 1–70 [DOI] [PubMed] [Google Scholar]
- Saint-Pol A, Codogno P, Moore SE. (1999) Cytosol-to-lysosome transport of free polymannose-type oligosaccharides: kinetic and specificity studies using rat liver lysosomes. J Biol Chem 274: 13547–13555 [DOI] [PubMed] [Google Scholar]
- Schüssler A, Martin H, Cohen D, Fitz M, Wipf D. (2006) Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi. Nature 444: 933–936 [DOI] [PubMed] [Google Scholar]
- Singh BK, Millard P, Whiteley AS, Murrell JC. (2004) Unravelling rhizosphere-microbial interactions: opportunities and limitations. Trends Microbiol 12: 386–393 [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. (1997) Mycorrhizal Symbiosis. Academic Press, London [Google Scholar]
- Trugo LC, Farah A, Cabral L. (1995) Oligosaccharide distribution in Brazilian soya bean cultivars. Food Chem 52: 385–387 [Google Scholar]
- Vankuyk PA, Diderich JA, MacCabe AP, Hererro O, Ruijter GJ, Visser J. (2004) Aspergillus niger mstA encodes a high-affinity sugar/H+ symporter which is regulated in response to extracellular pH. Biochem J 379: 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. (2008) Trichoderma-plant-pathogen interactions. Soil Biol Biochem 40: 1–10 [Google Scholar]
- Viterbo A, Chet I. (2006) TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol Plant Pathol 7: 249–258 [DOI] [PubMed] [Google Scholar]
- Voegele RT, Struck C, Hahn M, Mendgen K. (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci USA 98: 8133–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl R, Wippel K, Goos S, Kämper J, Sauer N. (2010) A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol 8: e1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TS, Bais HP, Grotewold E, Vivanco JM. (2003) Root exudation and rhizosphere biology. Plant Physiol 132: 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, St Leger RJ. (2007) The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot Cell 6: 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Vienken K, Weber R, Bunting S, Requena N, Fischer R. (2004) A putative high affinity hexose transporter, hxtA, of Aspergillus nidulans is induced in vegetative hyphae upon starvation and in ascogenous hyphae during cleistothecium formation. Fungal Genet Biol 41: 148–156 [DOI] [PubMed] [Google Scholar]
- Wenzel WW, Wieshammer G, Fitz WJ, Puschenreiter M. (2001) Novel rhizobox design to assess rhizosphere characteristics at high spatial resolution. Plant Soil 237: 37–45 [Google Scholar]
- Whipps JM. (1990) Carbon economy. Lynch JM, , The Rhizosphere. John Wiley & Sons, Essex, UK, pp 59–97 [Google Scholar]
- Woo SL, Lorito M. (2007) Exploring the interaction between fungal antagonists, pathogens and the plant for biocontrol. Vurro M, Gressel J, , Novel Biotechnologies for Biocontrol Agent Enhancement and Management. Springer, Amsterdam, pp 107–130 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.