Abstract
Individuals with C5/C6 spinal cord injury (SCI) have a number of paralyzed muscles in their upper extremities that can be electrically activated in a coordinated manner to restore function. The selection of a practical subset of paralyzed muscles for stimulation depends on the specific condition of the individual, the functions targeted for restoration, and surgical considerations. This paper presents a musculoskeletal model-based approach for optimizing the muscle set used for functional electrical stimulation (FES) of the shoulder and elbow in this population. Experimentally recorded kinematics from able-bodied subjects served as inputs to a musculoskeletal model of the shoulder and elbow, which was modified to reflect the reduced muscle force capacities of an individual with C5 SCI but also the potential of using FES to activate paralyzed muscles. A large number of inverse dynamic simulations mimicking typical activities of daily living were performed that included 1) muscles with retained voluntary control and 2) many different combinations of stimulated paralyzed muscles. These results indicate that a muscle set consisting of the serratus anterior, infraspinatus and triceps would enable the greatest range of relevant movements. This set will become the initial target in a C5 SCI neuroprosthesis to restore shoulder and elbow function.
Index Terms: Functional electrical stimulation (FES), musculoskeletal modeling, neural prostheses, spinal cord injury (SCI)
I. Introduction
Approximately 11 000 new cases of spinal cord injury (SCI) occur each year in the U.S. and a total of 250 000 individuals live today with SCI. Cervical SCI comprises 51% of the total number of SCI cases, with 26% of all SCI cases occurring at the C5/C6 level [1]. Individuals with complete injuries at the cervical level lose control over a number of muscles in their upper extremity and their lower extremities are totally paralyzed. A complete C5/C6 SCI results in hand muscle paralysis and loss of wrist and elbow extension. Paralysis at the more proximal joints is partial. Typically, muscles providing forearm pronation, elbow extension, and shoulder adduction and horizontal flexion are paralyzed, while their antagonists (performing forearm supination, elbow flexion, and shoulder abduction and horizontal extension) retain at least partial voluntary control. Glenohumeral and scapular stability can be severely impaired by paralysis of rotator cuff and serratus anterior muscles, respectively [2]. This paralysis pattern greatly limits the ability to reach specific areas of the arm’s workspace and also prevents mechanical coordination between different motions needed for other functions.
Neuroprostheses are systems that use functional electrical stimulation (FES) to activate paralyzed muscles in a coordinated way so that useful function can be provided. A neuroprosthesis activates the peripheral nervous system by delivering electrical impulses to the motor neurons innervating the paralyzed muscles, thus replacing the electrical signals coming from the brain through the injured spinal cord. Individuals with a C5/C6 SCI have been provided with functional hand grasp through the use of an upper extremity neuroprosthesis, improving their ability to handle objects [3], [4]. However, paralysis and weakness of shoulder and elbow muscles results in a considerable reduction in the person’s ability to perform basic daily activities that require positioning of the arm in space. FES to restore proximal arm function has been explored experimentally but has not been widely deployed into clinical settings. Percutaneous and surface FES systems developed for C4 SCI have used preprogrammed patterns of stimulation of arm muscles to restore simple functions like feeding [5]–[7]. In the C5-C6 SCI population, the conditions are very different than for C4 because many proximal muscles retain voluntary control and there is less proximal denervation [8]. Crago et al. [9] stimulated the triceps muscles of two C6 SCI individuals to restore elbow extension sufficient to allow the users to efficiently move objects within an expanded workspace. Although they demonstrated the basic feasibility of stimulating proximal muscles to restore function, none of these earlier studies systematically investigated the importance of stimulating specific muscles for increasing function while simultaneously maintaining shoulder (glenohumeral and scapular) stability.
The complexity of the shoulder has been a particular challenge for movement restoration. The shoulder mechanism consists of several joints (sternoclavicular, acromioclavicular, and glenohumeral) and an articulation between the medial border of the scapula and the thorax. The coordinated actions of several muscles acting across these joints results in a very large range of shoulder motion and the ability to reach, lift, and move objects by precise placement of the hand [10]. This ability is severely hampered by cervical SCI. It is not practical with existing FES technology to stimulate all the paralyzed muscles of the shoulder and elbow, so some rational technique for selecting a subset of these muscles to maximize restored function while maintaining shoulder stability (both scapular and glenohumeral) must be developed.
In this study, we have modified a musculoskeletal model of the human elbow and shoulder to reflect the effects of paralysis due to C5 SCI and then performed a large number of inverse dynamic simulations to investigate the ability of the remaining “voluntary” muscles, combined with limited subsets of “stimulated” muscles, to provide important functional motions (reaching above shoulder level and across the midline of the body) while maintaining shoulder stability. Such simulations provide a systematic method for evaluating different muscle sets without tedious trial-and-error experiments with human subjects, and provide the capability of customizing the model for specific subjects.
II. Methods
Experiments were conducted during which the 3-D shoulder and elbow kinematics of four able-bodied subjects were recorded during a series of arm movements. These kinematics became the input to an inverse dynamic musculoskeletal model of the shoulder and elbow that was modified to reflect a C5 SCI individual by reducing the maximal forces that each muscle could generate, including setting muscles that are typically paralyzed to have zero maximum force. Furthermore, the potential of FES to generate force in some of the paralyzed muscles was simulated by setting their maximum forces to 50% of able-bodied maximum. The patterns needed from the voluntary and stimulated muscles for each of these movements were obtained by running the model in an inverse dynamic fashion. The effectiveness of a given set of stimulated muscles was assessed by the number of motions that could be successfully simulated by the model for that set (plus the voluntary muscles). The experimental protocol was approved by the Institutional Review Board of MetroHealth Medical Center and all the subjects gave informed consent.
A. Experimental Recording of Shoulder and Elbow Kinematics
Arm movements from four able-bodied subjects were recorded using an Optotrak system (Northern Digital Inc., Waterloo, ON, Canada), as shown in Fig. 1, with the goal of tracking the bony landmarks necessary to generate local coordinate systems and reconstruct the orientation of the shoulder and elbow bone segments (i.e., clavicle, scapula, humerus, and radius-ulna) [11]. Infrared emitting diode (IRED) marker clusters were fixed over the thorax, humerus and forearm of the subject to track the locations of the thorax, humerus, and radius-ulna dynamically. The locations of the scapula and clavicle are difficult to track dynamically, so we used a palpation technique [12] to measure scapular kinematics statically at 15 locations across the range-of-motion of the arm and then estimated the orientations of the scapula and clavicle during the movements based on glenohumeral motions using a regression approach that assumes that the static scapulohumeral rhythm is preserved during normal movements [13]. Subjects performed several movements while the kinematics were measured, including: 1) planar movements such as shoulder abduction/adduction, flexion/extension, horizontal flexion/extension, internal/external rotation and elbow flexion/extension and pronation/supination, 2) reaching movements at the knee level, midlevel, shoulder level, and above-shoulder level, and 3) a set of activities of daily living (ADL) comprised of simulated feeding, drinking, hair brushing, and lifting and relocating objects. All kinematics were recorded at 30 Hz.
Fig. 1.
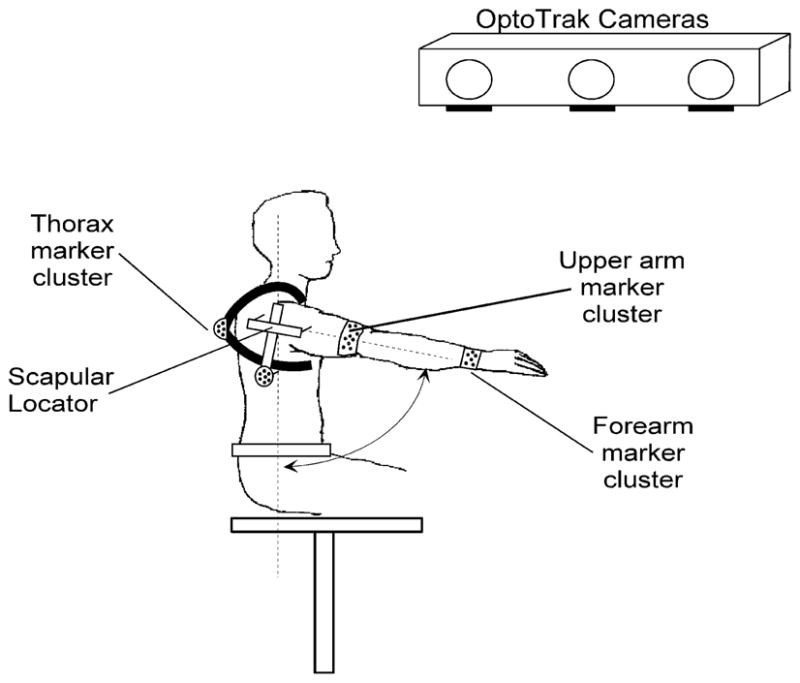
Experimental setup. Marker clusters of eight IREDs track the position and orientation of each segment in the shoulder and elbow. Optotrak infrared camera system registers 3-D location of each marker cluster to reconstruct the orientation of the bone segments in the upper extremity. Scapular locator is used to register the position of the scapula during static trials and regression based on scapulohumeral rhythm is used to reconstruct its orientation during the dynamic trials.
B. Data Processing
The locations of the various IREDs were processed to obtain the orientations of the thorax, clavicle, scapula, humerus, and forearm and then the various elbow and shoulder joint angles. First, an estimate for the location of the glenohumeral center of rotation (GH) was obtained using a regression procedure based on the positions of bony landmarks from the scapula [14] obtained during the static palpation trials. Second, the static trials were used to calculate two regression matrices. One matrix relates humeral orientation to scapular orientation and the other one relates humeral orientation to clavicular orientation. These matrices were used subsequently to estimate the orientation of the scapula and the clavicle from the humerus orientation during each dynamic trial. Third, the locations of specific bony landmarks on the forearm, humerus, and thorax were computed relative to the marker clusters during the movements, and then used to generate local coordinate systems attached to each bone segment. The relative orientations of these local bone coordinate systems were used to compute the various joint angles (clavicular protraction/retraction, elevation/depression, and axial rotation; scapular protraction/retraction, lateral/medial rotation, and forward/backward rotation; glenohumeral plane of elevation, elevation angle, and axial rotation; and elbow flexion/extension, and pronation/supination). Definitions of the bony landmarks, coordinate systems, and Euler Angle order of rotation were done following the International Shoulder Group recommendations for shoulder and elbow recordings [15]. Previous studies as well as our own experience have validated the accuracy and repeatability of the kinematic collection system [16]–[18].
C. Musculoskeletal Model
We used a musculoskeletal model of the shoulder and elbow developed at the Delft University of Technology that consists of the five bones defining these articulations (clavicle, scapula, humerus, radius, and ulna), 29 muscles, and 17 degrees-of-freedom (DOF) distributed over five joints [19]. In many cases, a single muscle is divided into several elements to represent the different mechanical lines of action and their wide anatomical origins and insertions. The specific morphological and muscle contraction parameters for the model and the anatomical location of the muscles, ligaments, tendons, and bones are as previously published [20]. This model has been extensively used for understanding the mechanics of the neuromuscular system [2] and to diagnose and understand pathologies of interest for clinical research [21]–[23].
1) Inverse Dynamic Simulations
In an inverse simulation, the inputs to the model are the kinematics of the recorded movements (i.e., the Euler angles with orientations of each bone segment). Additionally, an external endpoint force vector can be applied to simulate loads at the hand. Kinematic data were digitally resampled at 12 Hz, low-pass filtered at 3 Hz and then scaled appropriately to match the model’s geometry [20] using an optimization approach. Then the inverse dynamic simulations were performed. Simulations using this model solve the equations of motion of the musculoskeletal system to obtain moments at each of the joints, and the joint moments are then distributed as corresponding forces across the muscles spanning each joint using an optimization cost function related to the muscle energy consumption [24]. The outputs of the simulation are the muscle forces required to achieve the input kinematics. Relative force, muscle force divided by the maximal force of each muscle at its corresponding muscle length, was used in this study as an estimate of muscle activation since muscle dynamics were not included in the model. A “voluntary” muscle is partially controlled by an SCI individual and therefore it is described in the model with a nonzero maximum force. A “stimulated paralyzed” muscle is paralyzed in the SCI individual but in the model it will be provided with a nonzero maximum force to simulate the effect of electrical stimulation. When the model is incapable of reproducing a particular movement, the simulation fails and indicates the source of the error. Typically, the maximum available muscle forces is insufficient to balance the moments at each of the joints or the stability of the glenohumeral joint cannot be maintained [21]. In the model, glenohumeral stability is maintained if the joint force vector is directed inside the glenoid fosa. All movements were simulated with and without a 3 kg simulated weight at the endpoint to account for situations where the individual needs to interact with objects in their workspace.
2) Model Adjustment
To reflect the conditions of a C5 SCI subject, the model was modified by decreasing the maximum relative force that could be generated by each muscle. Estimates of the maximum relative muscle forces in a generic C5 SCI individual were obtained by considering the anatomical innervation levels of each muscle, as well as the results of manual muscle tests (MMT) performed previously on 22 individuals with SCI. The maximum relative forces for muscles for which clear and isolated contractions can be elicited were based primarily on the MMT results. The maximum relative forces for less accessible muscles were based on muscles with similar spinal innervation levels that had clear MMT results. Table I shows the values used. Muscles with some voluntary control have nonzero maximum relative forces, while paralyzed muscles have close to zero maximum relative forces. Maximum relative force, even for voluntary muscles, is never greater than 0.5 because a C5 SCI affects almost all the muscles of the upper arm at least partially. Several paralyzed muscles that could be stimulated to produce muscle contractions were included in selected simulations with a maximum relative force of 0.5. If a given “stimulated paralyzed” muscle was represented in the model using multiple muscle elements, these elements were constrained by the inverse simulation optimization procedure to have the same relative muscle force to simulate the effect of electrical stimulation. In SCI, 50% of able-bodied force generating capability is an approximation and best-case scenario [25] since partial denervation and muscle disuse atrophy are present over most of the paralyzed muscles that could be considered for FES.
TABLE I.
Assumed Maximum Available Relative Muscles Forces for a Typical Individual With C5 Spinal Cord Injury
| Partial Voluntary Control | ||
|---|---|---|
| Trapezius Scapular | TS | 0.33 |
| Trapezius Clavicular | TC | 0.5 |
| Deltoid Scapular | DS | 0.33 |
| Deltoid Clavicular | DC | 0.2 |
| Teres Minor | TMi | 0.25 |
| Supraspinatus | SSp | 0.44 |
| Infraspinatus | IS | 0.27 |
| Subscapularis | SSc | 0.24 |
| Levator Scapulae | LS | 0.5 |
| Rhomboids | Rh | 0.5 |
| Biceps Long Head | BL | 0.38 |
| Biceps Short Head | BS | 0.38 |
| Brachialis | BR | 0.38 |
| Brachioradialis | BRd | 0.38 |
| Supinator Hum-Rad | Sup | 0.26 |
| Usually Paralyzed | ||
|---|---|---|
| Serratus Anterior | SA | 0.11 |
| Latissimus Dorsi | LD | 0.01 |
| Coracobrachialis | CB | 0 |
| Pectoralis Major Thoracic | PT | 0 |
| Pectoralis Major Clavicular | PC | 0.08 |
| Pectoralis Minor | PMi | 0 |
| Teres Major | TMj | 0.02 |
| Triceps Long Head | TLo | 0.01 |
| Triceps Medial Head | TMe | 0.01 |
| Triceps Lateral Head | TLa | 0.01 |
| Pronator Teres Hum-Rad | PTh | 0.01 |
| Pronator Teres Uln-Rad | Ptu | 0.01 |
| Pronator Quadratus | PQ | 0.01 |
| Anconeus | An | 0 |
D. Selection of Paralyzed Muscles for Stimulation
Extensive simulations were done with the “voluntary” muscles augmented with different combinations of “stimulated paralyzed” muscles, with and without a 3 kg load at the endpoint. To be included in the simulations, a particular muscle satisfied the following conditions: 1) paralyzed but not denervated in a typical C5 SCI individual, 2) surgically accessible for activation by existing FES technology, and 3) demonstrated to be important contributors to the functional movements examined. This final requirement was quantified by performing simulations with the complete (i.e., able-bodied) model, determining the maximum relative forces required from each muscle during these movements, and then computing (for each muscle) a ratio between this maximum able-bodied force and the maximum relative force available from the C5-adjusted model. If this ratio was greater than 1.0 (i.e., muscle strength was insufficient), this muscle was considered a candidate for “stimulation.” As will be described in Section III, a total of six muscles were ultimately considered for stimulation.
Inverse simulations of the given set of movements were performed for a total of nine conditions: “able-bodied” (i.e., full muscle strength), “C5 SCI” (i.e., reduced forces to reflect paralysis but not FES), “C5 SCI+6FES” (i.e., reduced forces to reflect paralysis but also including simulated FES of all six candidate muscles), and then six separate simulation sets designated as “C5 SCI+6FES-XXX,” where “XXX” represents one of the six muscles in the candidate set. Removing one muscle at a time from the candidate “paralyzed stimulated” set was done to determine if a given muscle was absolutely essential to perform the examined movements. Because the goal of the study was to maximize the functionality provided by an FES system, the number of failed simulations for each muscle set was used as an outcome measure to compare the various sets of “paralyzed stimulated” muscles. Failure rate was defined as the number of failed timesteps in each movement divided by the total number of timesteps in that simulated trial. When averaged across all movements, the failure rate for a particular muscle thus summarizes the relative consequences of excluding a muscle from the FES set.
III. Results
A. Musculoskeletal Model: Kinematic Inputs and Muscle Activation Outputs
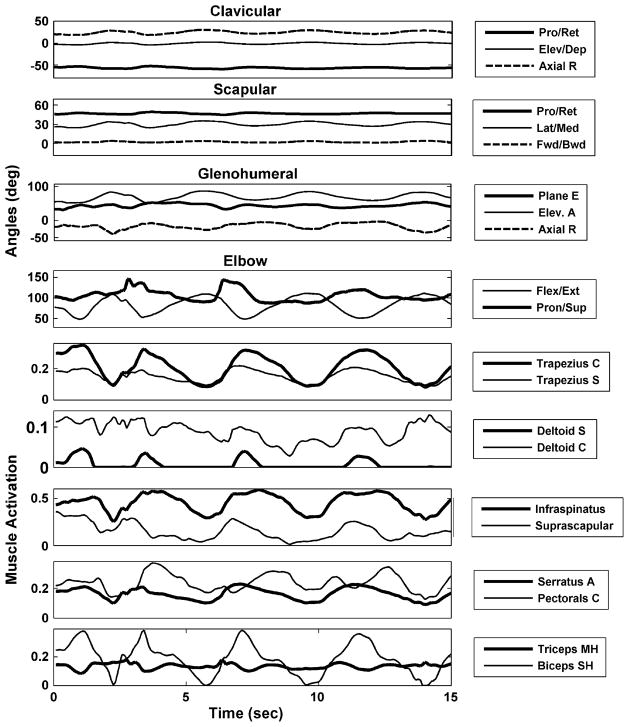
Movement kinematics were obtained from four human subjects as described in Section II. Fig. 2 shows an example of a typical trial corresponding to fifteen seconds of an activity of daily living where a human subject was making movements as if they were eating with a spoon. The top four panels show the various Euler angles derived from the motion analysis procedures that describe the orientations of the clavicle (3 DOF), scapula (3 DOF), glenohumeral joint (3 DOF), and elbow joint (2 DOF). In the illustrated movement, the scapula rotates laterally as the humerus elevates, as expected given a typical scapulohumeral rhythm. The elbow also flexes and extends bringing the spoon to the mouth. The lower six panels of Fig. 2 show the model-predicted relative forces of representative muscles during this movement. As can be seen from the amplitude of the patterns during the movement, the trapezius and deltoids play a major role in elevation of the arm, while the rotator cuff muscles (Infraspinatus and Supraspinatus) preserve the stability of the glenohumeral joint by contracting during almost any action of the arm. The serratus anterior is also rather active to keep the scapula pressed toward the thorax during arm elevation. Finally, pectoralis major, biceps, and triceps are activated in different patterns to locate the arm in the workspace by providing horizontal flexion of the arm and flexion and extension at the elbow. Even though the relative muscle forces presented in the figure are an average over all the elements of the muscle, notice that most of them are under 0.5. However, these were sufficient (and necessary) to generate the joint moments needed for this inverse simulation to be successful.
Fig. 2.
Input kinematics and output relative muscle forces from the inverse simulations with the musculoskeletal model of the shoulder and elbow during an activity of daily living where the subject is eating with a spoon. Top four panels show the Euler angles describing the orientation of the clavicle, scapula, humerus and forearm. Each angle plot shows lines corresponding to the three degrees of freedom at the clavicle (Pro/Ret: protraction/retraction, Elev/Dep: elevation/depression, Axial R: axial rotation), scapula (Pro/Ret: protraction/retraction, Lat/Med: lateral/medial rotation, Fwd/Bwd: forward/backward rotation) and glenohumeral joint (Plane E: plane of elevation, Elev A: elevation angle, Axial R: axial rotation), and the two DOFs at the elbow (Flex/Ext: flexion/extension, Pron/Sup: pronation/supination). The lower six panels show the model-predicted relative forces of representative muscles (trapezius clavicular, trapezius scapular, deltoid clavicular, deltoid scapular, infraspinatus, supraspinatus, serratus anterior, pectoralis clavicular, triceps medial head, and biceps short head) during this movement.
B. Muscle Selection
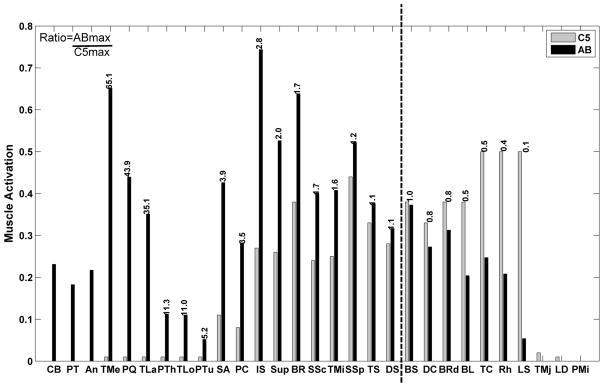
As described in the Section II, we chose the initial set of “paralyzed” muscles to consider for FES based on the forces needed during “able-bodied” simulations compared to the likely maximum forces in an individual with C5 SCI. Fig. 3 shows the maximum available relative muscle forces in the C5 model, as well as the maximum relative forces required from the able-bodied model across all simulated movements (using the kinematics of one representative able-bodied subject) without the added mass at the endpoint. The ratio between able-bodied maximum relative forces and C5 maximum relative forces without the mass was calculated, and the muscles were ranked from left to right according to this ratio. The ratio is written above the bars for muscles of interest. In general, a ratio above 1.0 (i.e., for all muscles to the left of dotted line) indicates that the movement required this muscle but that a typical individual with a C5 SCI will not have sufficient voluntary strength. Several muscles that would be completely paralyzed in C5 SCI are clearly important contributors to the targeted movements: coracobrachialis, pectoralis major, anconeus, the three heads of the triceps, pronator teres, pronator quadratus, serratus anterior and infraspinatus all have ratios greater than 1.0 and thus have a deficit that could potentially be overcome via electrical stimulation. Several muscles on the far right of Fig. 3 (teres major, latissimus dorsi, and pectoralis minor) will typically be paralyzed in C5 SCI, but they were not included in the candidate FES muscle set because they were not required by the specific movements evaluated (i.e., the maximum able-bodied relative forces required for the simulated movements were 0). The candidate muscle set was further reduced because of practical reasons or redundancy. Anconeus was not considered because of its very low force generating capability relative to triceps. Only the medial and lateral heads of triceps were included because the shoulder adduction also produced by the triceps long head has been problematic in previous FES systems where it interferes with the retained voluntary capability of elevating the arm. We chose to include pronator quadratus but not pronator teres because only one is necessary and the pronator quadratus is less likely to have denervation due to its spinal innervation pattern. Thus, six paralyzed muscle groups: coracobrachialis, pectoralis major, triceps, pronators, serratus anterior, and infraspinatus were chosen as candidates for including in an FES system for C5 SCI.
Fig. 3.
Maximum available relative forces for the C5 SCI adjusted model (light grey)and able bodied maximum relative forces achieved over all simulated movements without weight (black). Muscles have been ranked from left to right according to this ratio. The numerical value of the ratio is written above the bars for muscles of interest. A ratio above 1 (all muscles to the left of the vertical dotted line) indicates that the movements examined require higher muscle forces than can be generated by a typical individual with C5 SCI. (Key for muscle abbreviation taken from Table I).
Simulations were then performed with the various combinations of included muscles (detailed in the methods): “able-bodied,” “C5 SCI,” “C5 SCI+6FES,” and “C5 SCI+6FES-XXX.” Experimentally recorded motions were simulated with the various modified models, and the percentage of failure was calculated as the number of timesteps where the simulation failed over the total number of timesteps of the trial. The percentage of failure for different muscle sets and different movement types is summarized in Table II for each muscle set simulated, both with and without the added endpoint mass. The leftmost column shows the types of movements recorded and the remaining columns show the percentages of failure for each condition. The failure rates for the C5 SCI (no stimulation) case were quite large (78.8% and 100% with and without the added mass, respectively), but this was significantly reduced (0.2% and 39.1% with and without the added mass, respectively) in the “C5+6FES” case (i.e., with all of the candidate FES muscles included). The relative importance of each muscle in the candidate FES set can be seen by the increase in failure rate above the “C5+6FES” case. In particular, it is clear that the serratus anterior is critical for these movements—the failure rate was quite high (66.6% and: 99.8% with and without the added mass, respectively) when this muscle was not included in the “stimulated” set. Serratus anterior was therefore chosen as an “essential muscle” that must be included in the FES set. The infraspinatus was also chosen as an essential muscle because 1) removing it resulted in a 64.5% failure rate in the “added endpoint mass” trials, 2) it was the only rotator cuff muscle in the candidate set to assure glenohumeral stability, and 3) since it is under partial voluntary control, the functional effect of selecting it should be equal or better than the outcome of the simulations. Triceps (medial and lateral heads) was also included as an essential muscle. Removing it produced failure rates of 12.2% and 46.5% (with and without the added mass, respectively), and it was especially important for the key ADL tasks of eating (failure rates of 17.8% and 87.8% with and without the added mass, respectively), combing hair (92.8% and 95%), and for high reach movements (36.1% and 67.2%). Pectoralis major, pronator quadratus, and coracobrachialis were not essential (at least for the specific movements simulated) because the failure rates accompanying the deletion of these muscles did not significantly increase the failure rates of the simulations. It is important to note that for all of these simulations, failure was always due to lack of muscle force to balance the joint moments and never due to glenohumeral stability.
Table II.
Summary of Simulation Failure Rates (Defined as Failed # Timesteps/Total # Timesteps) for Different Types of Movements and Different Sets of Muscles Included in the Simulation
| Movement | Able Bodied | C5 SCI | C5 + 6 FES | C5 + 6 - Serr.Ant. | C5 + 6 - InfraSpin | C5 + 6 - Triceps | C5 + 6 - CoracoBr. | C5 + 6 Pronator Q | C5 + 6 -PecMajor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | No Wt | w/Wt | |
| humeral elevation in the coronal plane | 0.0 | 0.0 | 100.0 | 100.0 | 0.6 | 70.7 | 100.0 | 100.0 | 0.0 | 70.7 | 0.6 | 70.7 | 0.0 | 70.7 | 0.0 | 70.7 | 0.0 | 81.5 |
| humeral elevation in the scapular plane | 0.0 | 0.0 | 97.6 | 100.0 | 0.0 | 63.3 | 97.6 | 100.0 | 0.0 | 79.3 | 0.2 | 63.3 | 0.0 | 76.1 | 0.0 | 63.5 | 0.0 | 72.6 |
| humeral elevation in the sagittal plane | 0.0 | 1.3 | 75.8 | 100.0 | 1.5 | 7.1 | 75.5 | 100.0 | 1.5 | 54.6 | 2.0 | 7.1 | 1.5 | 27.7 | 2.0 | 12.1 | 1.5 | 13.1 |
| horizontal flexion-extension | 0.0 | 0.0 | 100.0 | 100.0 | 0.0 | 90.8 | 100.0 | 100.0 | 0.0 | 100.0 | 0.0 | 98.0 | 0.0 | 94.1 | 0.0 | 90.8 | 0.0 | 91.1 |
| internal/external rotation | 0.0 | 0.0 | 100.0 | 100.0 | 0.0 | 56.9 | 100.0 | 100.0 | 0.0 | 100.0 | 0.8 | 70.8 | 7.8 | 70.3 | 0.6 | 57.2 | 0.0 | 56.9 |
| elbow flexion/extension | 0.0 | 0.0 | 38.0 | 100.0 | 0.6 | 1.3 | 38.0 | 100.0 | 0.6 | 1.3 | 0.6 | 1.3 | 0.6 | 1.3 | 0.6 | 1.3 | 0.6 | 1.3 |
| elbow pronation/supination | 0.0 | 0.0 | 54.6 | 100.0 | 0.0 | 0.0 | 44.6 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 20.6 | 30.7 | 0.2 | 15.2 |
| reaching knee level | 0.0 | 0.0 | 53.3 | 100.0 | 0.0 | 22.0 | 53.0 | 100.0 | 0.0 | 33.0 | 0.4 | 0.0 | 0.0 | 22.1 | 0.0 | 22.1 | 0.1 | 27.8 |
| reaching mid level | 0.0 | 0.3 | 73.7 | 100.0 | 0.3 | 37.7 | 70.4 | 100.0 | 0.1 | 65.2 | 3.5 | 1.3 | 0.1 | 39.0 | 1.3 | 37.8 | 0.1 | 43.7 |
| reaching scapular shoulder level | 0.0 | 0.0 | 89.2 | 100.0 | 0.0 | 9.6 | 74.1 | 100.0 | 0.0 | 75.9 | 15.1 | 45.2 | 0.0 | 14.5 | 0.0 | 9.6 | 0.0 | 17.5 |
| combing hair | 0.0 | 0.0 | 100.0 | 100.0 | 0.0 | 62.2 | 11.1 | 97.2 | 0.0 | 88.9 | 92.8 | 95.0 | 0.0 | 88.9 | 20.6 | 72.2 | 0.0 | 62.8 |
| eating with spoon | 0.0 | 0.0 | 92.2 | 100.0 | 0.0 | 66.1 | 75.0 | 100.0 | 0.0 | 100.0 | 17.8 | 87.8 | 0.0 | 88.9 | 0.0 | 66.1 | 0.0 | 65.6 |
| wash axilla | 0.0 | 0.0 | 31.5 | 100.0 | 0.0 | 42.7 | 25.0 | 100.0 | 2.4 | 51.6 | 1.6 | 42.7 | 0.8 | 45.2 | 1.6 | 42.7 | 0.8 | 46.8 |
| high level reaching | 0.0 | 0.0 | 97.2 | 100.0 | 0.0 | 16.7 | 68.3 | 100.0 | 0.0 | 82.2 | 36.1 | 67.2 | 0.0 | 33.3 | 0.0 | 16.7 | 0.0 | 21.7 |
| Average | 0.0 | 0.1 | 78.8 | 100.0 | 0.2 | 39.1 | 66.6 | 99.8 | 0.3 | 64.5 | 12.2 | 46.5 | 0.8 | 48.0 | 3.4 | 42.4 | 0.2 | 44.1 |
Some movements are averaged from 2–3 trials where the same movement was repeated. The “able-bodied” columns shows failure rates for simulations with no muscle reduction, the “C5 SCI” for C5 muscle force reductions but no FES, and the “C5+6 FES” column for C5 SCI muscle force reductions plus FES (0.5 maximum relative force) of the 6 selected muscles: serratus anterior, infraspinatus, triceps (medial and lateral heads), coracobrachialis, pronator quadratus and pectoralis major (clavicular head). The remaining columns show the failure rates that occurred when one muscle at a time was removed from the “C5+6FES” muscle set.
Note that the above analyses were based on the kinematic measurements from a single (representative) human subject to avoid artifacts due to averaging motions from subjects who may perform the tasks differently. The above muscle selection process was repeated in three additional subjects, however, with largely consistent results. Table III shows the maximum relative forces for the generic C5 SCI case and the model-predicted maximum relative forces required for the various tasks as performed by the four different subjects (S1 corresponds to the subject used for the detailed analyses above). The same muscles (e.g., serratus anterior, triceps, pectoralis major, pronator quadratus, infraspinatus, and coracobrachialis) chosen for evaluation for S1 also show a considerable deficit for the other three subjects, indicating that these are indeed key muscles despite kinematic variations in task performance by different able-bodied subjects.
Table III.
Maximum Relative Muscle Forces Available for the C5 SCI Adjusted Model (Left Column) and the Model-Predicted Maximum Relative Muscle Forces Needed to Achieve the Targeted Movements in Four Able-Bodied Subjects (Rightmost Four Columns)
| Maximum Relative Muscle Force | Able-bodied simulations |
||||
|---|---|---|---|---|---|
| C5 SCI | s1* | s2 | s3 | s4 | |
| Triceps (Medial) | 0.01 | 0.65 | 0.64 | 0.87 | 0.61 |
| Serratus Anterior | 0.11 | 0.43 | 0.81 | 0.59 | 0.45 |
| Pectoralis M. (clav.) | 0.08 | 0.28 | 0.30 | 0.31 | 0.20 |
| Pronator Q | 0.01 | 0.44 | 0.15 | 0.47 | 0.10 |
| Infraspinatus | 0.27 | 0.74 | 0.55 | 0.67 | 0.51 |
| Coracobrachialis | 0 | 0.23 | 0.18 | 0.45 | 0.12 |
Subject 1 (S1) corresponds to the subject whose kinematics were chosen for the detailed muscle selection process described previously.
IV. Discussion
In this study, a musculoskeletal model of the upper extremity was used to sufficiently replicate the condition of an individual with a C5 spinal cord injury so that the impact of a neuroprosthesis to restore elbow and shoulder function could be evaluated prior to its actual deployment in human subjects. Specifically we evaluated the likely functional impact of electrically stimulating a number of muscles that are typically paralyzed in this population using inverse-dynamic simulations with the musculoskeletal model that were driven by the upper extremity kinematics of able-bodied subjects. Although the degree of function restored will almost certainly increase as the number of stimulated muscles included increases, we found that three muscles (serratus anterior, triceps lateral and medial heads, and infraspinatus) were “essential” for upper extremity neuroprostheses in that important functional tasks could not be performed if their mechanical contributions were not included. The proposed approach could be used with minor modification for C6 SCI and for other targeted movements, and could be customized for a particular individual with cervical SCI if specific maximum muscle forces can be estimated.
A. Musculoskeletal Model of the Shoulder and Elbow
We performed extensive inverse dynamic simulations of the effects of various FES configurations, various arm movements, and two loading conditions that would have been impractical to test in human experiments. Avoiding the invasiveness of implanting electrodes in the targeted muscles and using objective techniques rather than trial and error to define the magnitude and temporal properties of stimulation patterns is also likely to make this approach an invaluable tool in the ongoing development of advanced upper extremity neuroprostheses.
For the purposes of this study, we simulated a generic C5 SCI and selected a number of functionally important but specific movements to restore. Both of these choices will clearly affect the muscles needed for stimulation because the strengths of paralyzed, denervated, and voluntarily controlled muscles vary and the functional objectives (e.g., reaching and manipulation tasks versus weight shifts) vary across individuals. Thus, our results are specific to the conditions assumed. Although the maximum relative forces assumed for each muscle are just an approximation, it is unlikely that the muscle selection (primary goal of this study) will change by modest changes in these maxima since failure rate was mainly affected by the inclusion or exclusion of muscles. We believe that our results will have broad implications because we used conservative estimates of muscle strength and because of the general nature of many of the movements targeted. Furthermore, the approach described here can be easily customized by simulating different movements and/or by supplying specific values for maximum muscle forces (both voluntary and stimulated). For example, voluntary maximum muscle forces can be estimated for specific individuals using manual muscle test results or by estimates based on MRI measurements of muscle volume [26]. Furthermore, these simulations could be used to determine if including alternative or additional muscles, or using exercise to strengthen these muscles, would substantially improve functional outcome.
The model we used represents many muscles using several elements to account for wide origins and insertions. We constrained all the elements of each “stimulated” muscle to have the same relative force level, thus reflecting the most likely gross actions of FES, with one exception. The serratus anterior, originating on the upper eight ribs and inserting on the medial border of the scapula, is defined in the model by 12 elements and it is usually paralyzed in the C5 SCI population. Our able-bodied simulations showed that this was a key muscle for performing almost any movement. However, when all 12 elements of this muscle were simultaneously activated, the various “C5+FES” simulations were not successful because the relative force levels needed to insure scapular stability (i.e. a compressive force between the scapula and the thorax that prevents scapular winging) also produced secondary “off axis” moments that required forces from antagonistic muscles not included in the FES system. However, we found that stimulating only the lower portion of the serratus anterior resolved this issue. Fortunately, surgical access to the lower part of the serratus anterior (e.g., via a cuff electrode on the long thoracic nerve) is much better than for the upper part of the muscle. We, therefore, simulated FES for only the lower portions of the serratus anterior.
B. Muscle Selection
We selected a set of paralyzed muscles that could be electrically stimulated as part of a neuroprosthesis to restore upper extremity function, The muscles selected are all essential contributors to the functions that we are interested in restoring, i.e., reaching tasks and simple ADL tasks like feeding or lifting objects. Serratus anterior, as mentioned above, is essential for maintaining scapular stability during reaching movements. Triceps is the principal extensor of the elbow and it is required for overhead reaching tasks. Infraspinatus is a rotator cuff muscle that helps maintain glenohumeral stability. We believe that this set of muscles will be enough to provide an increase of the workspace for this population. However, we also recognize that this set of muscles can be augmented or varied according to the level of denervation of the individual or to the number of stimulation channels available. For example, in the candidate muscle set chosen for this study, Pectoralis Major is the main horizontal flexor of the arm and it would be important to increase the workspace near the midline of the body. Pronator Quadratus would help provide precise orientation of the hand when reaching and grabbing an object in the workspace. Our approach would identify these (and other) essential muscles simply by using different targeted motions for the inverse simulations.
We also recognize that individuals with SCI (with or without FES) can learn to perform functional tasks in a perfectly acceptable way that is significantly different from the movement strategies used by able-bodied subjects. In such a case, the optimal muscles to include in an FES system might vary considerably. Choosing optimal movement trajectories subject to muscle strength limitations is a fascinating question that is intractable with existing optimization techniques and computer power. However, we chose rather simple (yet very important) tasks that must be performed in a way that at least approximates able-bodied strategies in order to be functional. Thus, we believe that our choice of essential muscles is likely to be rather general across different individuals with C5 SCI.
Our choice of essential muscles was based largely on a simple, very general index (percentage failure) that summarized behavior across a number of movements. Such a general summary variable inevitably oversimplifies the real situation. For example, if a simulation had three repetitive reaching movements in the upper workspace and it only failed at the extreme of the movement’s range-of-motion, the percentage of failure might be large but the movement might actually be feasible if range of motion was reduced by a trivial amount that would not affect functional completion of the task. Another example is a movement that failed for just a few timesteps at the beginning of the trial such that the rest of the movement (which would otherwise be feasible) could not be performed. Such occurrences were very rare in this study, however, and had no real impact on our basic conclusions.
C. Relevance and Future Work
We used a musculoskeletal model of the shoulder and elbow to replicate the condition of an individual with C5 SCI and showed that it was a useful tool in selecting muscles for stimulation and for predicting the likely functional impact of an implanted neuroprosthesis. The methodology developed here could be used by clinicians, surgeons, and engineers to make decisions about neuroprosthesis implementations and rehabilitation strategies. The model can be customized according to the condition of a specific individual with SCI and then used to study the effects of the different interventions available so that an optimal neuroprosthesis can be specified. We hope that deployment of such neuroprostheses will lead to a significant improvement in movement strength, speed, and coordination that will enhance the function of the recipients in activities of daily living.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke (NIH NINDS) under Contract N01-NS-1-2333 and Contract N01-NS-5-2365.
Biographies

Juan Gabriel Hincapie received the B.Sc. degree in electronics engineering from Universidad de los Andes, Bogotá, Colombia, in 2002 and the M.Sc. in biomedical engineering from Case Western Reserve University, Cleveland, OH, in 2005 where he is currently working toward the Ph.D. degree.
His research interests include rehabilitation strategies such as functional electrical stimulation (FES) for restoring function in individuals with neurological disorders, EMG-based control for upper extremity neuroprostheses, neuromuscular control, biomechanics, and musculoskeletal modeling.

Dimitra Blana received the B.S. degree in electrical and computer engineering from the National Technical University of Athens, Athens, Greece, in 2001 and the M.S. degree in biomedical engineering from Case Western Reserve University, Cleveland, OH, in 2003, where she is currently working toward the Ph.D. degree.
Her research focuses on biomechanical modeling and controller design for upper extremity neuroprostheses.

Edward K. Chadwick received the B.Eng. degree in mechanical engineering from The University of Nottingham, Nottingham, U.K., in 1993 and the Ph.D. degree in bioengineering by the University of Strathclyde, Glasgow, U.K., in 1999.
He is currently employed as a Senior Research Associate in the Biomedical Engineering Department, Case Western Reserve University, Cleveland, OH. His main areas of research are biomechanical modelling of the upper limb, human movement analysis, and motor control, applied to the field of rehabilitation engineering.

Robert F. Kirsch (M’82) received the B.S. degree in electrical engineering from the University of Cincinnati, Cincinnati, OH, in 1982, and the M.S. and Ph.D. degrees in biomedical engineering from Northwestern University, in 1986 and 1990, respectively.
He was a postdoctoral fellow in the Department of Biomedical Engineering, McGill University, Montréal, QC, Canada, from 1990 to 1993. He is currently an Associate Professor of Biomedical Engineering at Case Western Reserve University, Cleveland, OH, and Associate Director for Research in the Cleveland VA FES Center. His research focuses on restoring movement to disabled individuals using functional electrical stimulation (FES) and controlling FES actions via natural neural commands. Computer-based models of the human upper extremity are used to develop new FES approaches. FES user interfaces, including ones based on brain recordings, are being developed to provide FES users with the ability to command movements of their own arm.
Contributor Information
Juan Gabriel Hincapie, Email: juan.hincapie@case.edu, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA and with the VA Cleveland Functional Electrical Stimulation Center, Cleveland, OH 44106 USA.
Dimitra Blana, Email: dimitra.blana@case.edu, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA and with the VA Cleveland Functional Electrical Stimulation Center, Cleveland, OH 44106 USA.
Edward K. Chadwick, Email: edward.chadwick@case.edu, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA and with the VA Cleveland Functional Electrical Stimulation Center, Cleveland, OH 44106 USA
Robert F. Kirsch, Email: robert.kirsch@case.edu, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA and also with the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, Cleveland, OH 44702 USA.
References
- 1.Spinal Cord Injury Statist. Center; Birmingham, AL: 2005. Annual statistical report for the model spinal cord injury care systems Natl; p. 2005. [Google Scholar]
- 2.Kirsch RF, Acosta AM, van der Helm FCT, Rotteveel RJJ, Cash LA. Model-based development of neuroprostheses for restoring proximal arm function. J Rehab Res Develop. 2001 Nov;38(6):619–626. [PubMed] [Google Scholar]
- 3.Keith MW, Peckham PH, Thrope GB, Buckett JR, Stroh KC, Menger V. Functional neuromuscular stimulation neuroprostheses for the tetraplegic hand. Clin Orthop Related Res. 1988:25–33. [PubMed] [Google Scholar]
- 4.Kilgore KL, Peckham PH, Thrope GB, Keith MW, Gallaher-Stone KA. Synthesis of hand grasp using functional neuromuscular stimulation. IEEE Trans Biomed Eng. 1989 Jul;36(7):761–770. doi: 10.1109/10.32109. [DOI] [PubMed] [Google Scholar]
- 5.Hoshimiya N, Naito A, Yajima M, Handa Y. A multichannel FES system for the restoration of motor functions in high spinal cord injury patients: A respiration-controlled system for multijoint upper extremity. IEEE Trans Biomed Eng. 1989 Jul;36(7):754–760. doi: 10.1109/10.32108. [DOI] [PubMed] [Google Scholar]
- 6.Nathan RH, Ohry A. Upper limb functions regained in quadriplegia: A hybrid computerized neuromuscular stimulation system. Arch Phys Med Rehabil. 1990;71:415–421. [PubMed] [Google Scholar]
- 7.Smith BT, Mulcahey MJ, Betz RR. Development of an upper extremity FES system for individuals with C4 tetraplegia. IEEE Trans Rehabil Eng. 1996 Dec;4:264–270. doi: 10.1109/86.547926. [DOI] [PubMed] [Google Scholar]
- 8.Mulcahey MJ, Smith BT, Betz RR. Evaluation of the lower motor neuron integrity of upper extremity muscles in high level spinal cord injury. Spinal Cord. 1999;37:585–591. doi: 10.1038/sj.sc.3100889. [DOI] [PubMed] [Google Scholar]
- 9.Crago PE, Memberg WD, Usey MK, Keith MW, Kirsch RF, Chapman GJ, Katorgi MA, Perreault EJ. An elbow extension neuroprosthesis for individuals with tetraplegia. IEEE Trans Rehabil Eng. 1998 Mar;6(1):1–6. doi: 10.1109/86.662614. [DOI] [PubMed] [Google Scholar]
- 10.Perry J. Biomechanics and functional anatomy of the shoulder. In: Chapman MW, editor. Operative Orthopaedics. 2. Philadelphia, PA: Lippincott; 1993. [Google Scholar]
- 11.Veeger HE, van der Helm FC, Chadwick EK, Magermans D. Toward standardized procedures for recording and describing 3D shoulder movements. Behav Res Methods Instrum Comput. 2003;35:440–446. doi: 10.3758/bf03195521. [DOI] [PubMed] [Google Scholar]
- 12.Johnson GR, Stuart PR, Mitchell S. A method for the measurement of three-dimensional scapular movement. Clin Biomech. 1993;8:269–273. doi: 10.1016/0268-0033(93)90037-I. [DOI] [PubMed] [Google Scholar]
- 13.de Groot JH, Brand R. A three-dimensional regression model of the shoulder rhythm. Clin Biomech. 2001;16:735–743. doi: 10.1016/s0268-0033(01)00065-1. [DOI] [PubMed] [Google Scholar]
- 14.Meskers CG, van der Helm FC, Rozendaal LA, Rozing PM. In vivo estimation of the glenohumeral joint rotation center from scapular bony landmarks by linear regression. J Biomech. 1998;31:93–96. doi: 10.1016/s0021-9290(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—part II: Shoulder, elbow, wrist and hand. J Biomech. 2005;38:981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Acosta AM, Kirsch RF, van der Helm FC. Three-dimensional shoulder kinematics in individuals with C5-C6 spinal cord injury. Proc Inst Mech Eng. 2001;215:299–307. doi: 10.1243/0954411011535894. [DOI] [PubMed] [Google Scholar]
- 17.Hebert LJ, Moffet H, McFadyen BJ, St-Vincent G. A method of measuring three-dimensional scapular attitudes using the optotrak probing system. Clin Biomech. 2000;15:1–8. doi: 10.1016/s0268-0033(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 18.de Groot JH. The variability of shoulder motions recorded by means of palpation. Clin Biomech. 1997;12:461–472. doi: 10.1016/s0268-0033(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 19.van der Helm FC. A finite element musculoskeletal model of the shoulder mechanism. J Biomech. 1994;27:551–569. doi: 10.1016/0021-9290(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 20.Klein Breteler MD, Spoor CW, Van der Helm FC. Measuring muscle and joint geometry parameters of a shoulder for modeling purposes. J Biomech. 1999;32:1191–1197. doi: 10.1016/s0021-9290(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 21.Chadwick EK, van Noort A, van der Helm FC. Biomechanical analysis of scapular neck malunion—A simulation study. Clin Biomech. 2004;19:906–912. doi: 10.1016/j.clinbiomech.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Magermans DJ, Chadwick EK, Veeger HE, Rozing PM, van der Helm FC. Effectiveness of tendon transfers for massive rotator cuff tears: A simulation study. Clin Biomech. 2004;19:116–122. doi: 10.1016/j.clinbiomech.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Veeger HE, Rozendaal LA, van der Helm FC. Load on the shoulder in low intensity wheelchair propulsion. Proc Clin Biomech. 2002;17:211–218. doi: 10.1016/s0268-0033(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 24.Praagman M, Chadwick EK, van der Helm FC, Veeger HE. The relationship between two different mechanical cost functions and muscle oxygen consumption. J Biomech. 2006;39:758–765. doi: 10.1016/j.jbiomech.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Kobetic R, Marsolais EB. Synthesis of paraplegic gait with multichannel funcional neuromuscular stimulation. IEEE Trans Biomed Eng. 1994 Jun;2:66–79. [Google Scholar]
- 26.Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Day MK, Lee PL, Kwong-Fu H, Edgerton VR. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992;10:928–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]