Abstract
In comparison to the basal ganglia, prefrontal cortex, and medial temporal lobes, the cerebellum has been absent from recent research on the neural substrates of categorization and identification, two prominent tasks in the learning and memory literature. To investigate the contribution of the cerebellum to these tasks, we tested patients with cerebellar pathology (seven with bilateral degeneration, six with unilateral lesions, and two with midline damage) on rule-based and information-integration categorization tasks and an identification task. In rule-based tasks, it is assumed that participants learn the categories through an explicit reasoning process. In information-integration tasks, optimal performance requires the integration of information from multiple stimulus dimensions, and participants are typically unaware of the decision strategy. The identification task, in contrast, required participants to learn arbitrary, color-word associations. The cerebellar patients performed similar to matched controls on all three tasks and performance did not vary with the extent of cerebellar pathology. Although the interpretation of these null results requires caution, these data contribute to the current debate on cerebellar contributions to cognition by providing boundary conditions on understanding the neural substrates of categorization and identification, and help define the functional domain of the cerebellum in learning and memory.
Keywords: Discrimination learning, Classification, Paired associate learning, Memory disorders, Decision making, Association learning
INTRODUCTION
The past decade has seen a surge in research investigating the neural substrates of category learning, that is, the process of establishing the memory traces necessary to organize objects and events in the environment into separate classes. The basal ganglia, prefrontal cortex, and medial temporal lobes have been the primary focus of this work, with these studies informed by both theoretical models and empirical considerations of how these structures contribute to learning. One subcortical structure that is notably absent from this work, however, is the cerebellum. Given the extensive connectivity between the cerebellum and prefrontal cortex (Middleton & Strick, 2001), in addition to the well-established role of this structure in learning, it would seem imperative to explore if and how the cerebellum might contribute to the complex processes underlying category learning. These questions form the basis for the current study.
The role of the cerebellum in cognition has engendered considerable debate (Schmahmann, 1997). Several studies have examined whether damage to the cerebellum disrupts learning on cognitive tasks, similar to that observed in studies of motor learning. For example, Fiez et al. (1992) suggested that cerebellar damage impairs error-based learning on a range of tasks such as paired-associate learning and semantic retrieval (but see Helmuth et al., 1997).
With respect to category learning, however, studies have shown that patients with pathology restricted to the cerebellum perform similar to matched controls on category learning tasks (e.g., Maddox et al., 2005). These null results stand in contrast to the work of Canavan and colleagues who reported that patients with cerebellar pathology were impaired in learning arbitrary associations between six color stimuli and their unique response labels (Bracke-Tolkmitt et al., 1989; Canavan et al., 1994; see also Drepper et al., 1999). This task can be viewed as a form of an absolute identification task, where each stimulus defines a unique category. Researchers have long argued that identification and categorization involve a similar decision process (Ashby & Lee, 1991; Nosofsky, 1986; Shepard et al., 1961) given that the primary difference is in the nature of the stimulus-response mapping (many-to-one in category learning vs. one-to-one in identification). These tasks are also similar to discrimination learning in which several stimuli must be separated into categories containing just one member.
There are at least two reasons for these seemingly conflicting results. First, the contribution of the cerebellum to identification is controversial. Several studies have found impairments in associative learning tasks similar to the identification tasks described above (Timmann et al., 2002; Tucker et al., 1996). In contrast, lesions of the cerebellum in nonhuman primates do not affect performance in such tasks (Nixon & Passingham, 1999, 2000). While this discrepancy might reflect a nonhomologous role of the cerebellum across species, the results from the human studies are problematic. The sample size has been small (five and seven patients, in the Bracke-Tolkmitt et al. and Canavan et al. studies, respectively) with the impairment limited to only a subset of the individuals. Furthermore, interpretation of these data is complicated by below average IQ in the patient samples.
Second, a central idea in the category learning literature is that categorization is mediated by multiple learning systems (see Ashby & Maddox, 2005; Kéri, 2003). Although the specific nature and number of learning systems is controversial, many theorists hypothesize that one is a logical, hypothesis-testing system that is dependent on working memory and executive functions (e.g., Ashby et al., 1998; Erickson & Kruschke, 1998). This system is assumed to dominate in so-called rule-based category learning tasks in which the optimal rule that maximizes accuracy can easily be described verbally (Ashby et al., 1998).
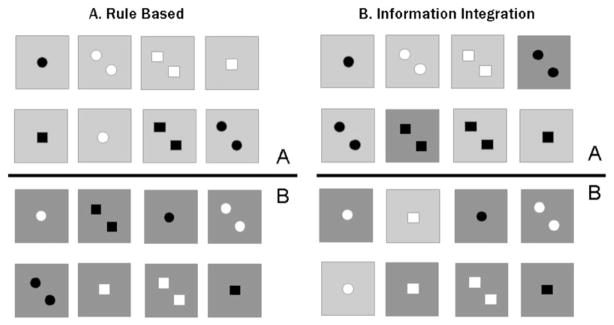
Consider a set of four-dimensional stimuli that vary in shape, numerosity, color, and background color (Figure 1a). A rule-based task here might require the subjects to learn to categorize the stimuli based on one of these dimensions (background color in the example), while ignoring variation on the other three dimensions. Thus, the participant’s task is to identify the relevant dimension and then map the different dimensional values to the relevant categories. Note that this task is very similar to the Wisconsin Card Sorting task (WCST; Grant & Berg, 1948), one of the standard tools for assessing executive function.
Fig. 1.
A: Category structure of a rule-based category-learning task. The optimal rule is: Respond A if the background color is blue (depicted as light gray), and respond B if the background color is yellow (depicted as dark gray). B: Category structure of an information-integration category-learning task. In this example, shape is irrelevant. For the three relevant dimensions, one level is arbitrarily assigned a numerical value of +1: symbol color of green (depicted as black), background color of blue (depicted as light gray), and numerosity of two. The other levels are assigned a numerical value of 0: symbol color of red (depicted as white), background color of yellow (depicted as dark gray), and numerosity of one. If the sum of the values on the relevant dimensions is greater than 1.5, the stimulus is assigned to Category A; if less than 1.5, the stimulus is assigned to Category B. Copyright © 2003 by the American Psychological Association. Reproduced with permission: Ashby, F.G., Noble, S., Filoteo, J.V., Waldron, E.M., & Ell, S.W. (2003). Category learning deficits in Parkinson’s disease. Neuropsychology, 17, 115–124. (The use of APA information does not suggest endorsement by APA.)
In contrast, an implicit, procedural-based system is assumed to dominate learning in information-integration category learning tasks in which accuracy is maximized when information from two or more dimensions is integrated at some predecisional stage (Ashby et al., 1998). For the task shown in Figure 1b, participants must evaluate stimulus information on three of the dimensions and ignore the value on the fourth, irrelevant dimension (shape in the example). Unlike rule-based tasks, participants have difficulty verbalizing the optimal decision strategy in information-integration tasks, despite being able to successfully learn the categories (Ashby et al., 1998).
Theoretical and empirical evidence suggests that qualitatively different systems are engaged during category learning in rule-based and information-integration tasks (Ashby & Maddox, 2005). At the neural level, the hypothesis-testing system thought to dominate learning in rule-based tasks has been associated with lateral prefrontal cortex, anterior cingulate, the basal ganglia (the head of the caudate nucleus and associated dopaminergic projections), and medial temporal lobes. The procedural-based system, in contrast, has been associated with high-level association regions (e.g., inferotemporal cortex in the case of visual stimuli), the basal ganglia (the body and tail of the caudate nucleus and associated dopaminergic projections), and premotor cortex (Ashby et al., 1998; Ashby & Valentin, 2005).
Although the cerebellum has not been central to theorizing about the neural substrates of category learning, it is in a position to influence learning in both rule-based (by connections with prefrontal cortex; Kelly & Strick, 2003; Ramnani, 2006) and information-integration tasks (by indirect projections to the basal ganglia via the pedunculopontine nucleus; Hazrati & Parent, 1992; Lavoie & Parent, 1993). Only a few studies have investigated the role of the cerebellum in category learning. Daum et al. (1993) investigated WCST performance in patients with cerebellar pathology. An impairment (relative to matched controls) was observed, but only for patients in which the damage extended into the brainstem (see also Fiez et al., 1992; Schmahmann, 1991). Maddox et al. (2005) investigated performance on a rule-based categorization task in a group of Parkinson’s disease patients and a group of patients with cerebellar pathology. Although Parkinson’s patients were impaired, the performance of patients with cerebellar pathology was comparable to matched controls.
The Daum et al. and Maddox et al. studies used rule-based tasks, however, the classification of a third study involving patients with cerebellar pathology is more problematic (Witt et al., 2002). Witt et al. investigated performance on the weather prediction task, comparing a group of patients with Parkinson’s disease and a group of patients with cerebellar pathology. Parkinson’s patients were impaired, but the patients with cerebellar pathology performed similarly to matched controls. While participants can achieve optimal accuracy in this task by integrating probabilistic cue-outcome relationships, near optimal performance can also be achieved with a variety of explicit strategies (e.g., memorization, rule-based strategies; see Ashby & Maddox, 2005; Gluck et al., 2002). Moreover, as noted, individuals with Parkinson’s disease have been shown to be impaired on the weather prediction task, but perform similarly to matched controls on the Figure 1b information-integration task (Ashby et al., 2003b).
As reviewed above, a few studies have investigated categorization and identification in patients with cerebellar pathology. This literature, however, lacks a systematic comparison in a single sample. Moreover, previous observations of impaired accuracy on identification tasks were obtained with small samples (e.g., Bracke-Tolkmitt et al., 1989). In the current study, we repeated this study in a larger sample. We also tested the same individuals on rule-based and information-integration categorization tasks. While previous work indicates that cerebellar pathology does not affect rule-based category learning (Maddox et al., 2005), it is important to test the generality of that null result. Moreover, computational models suggest distinct processes associated with rule-based and information-integration forms of category learning. As such, a direct comparison with a common set of stimuli allowed us to determine whether cerebellar pathology selectively affects one type of categorization task. To date, there have been no studies that have looked at the effect of cerebellar pathology on information-integration categorization, let alone directly compare these two forms of category learning.
The inconsistencies in the existing literature make it difficult to generate strong predictions; as such, this study is, in large part, exploratory. Given that the online maintenance and manipulation of information is important for both identification and rule-based tasks, an impairment on these tasks may be expected based on claims that cerebello-prefrontal pathways are part of a working memory circuit (Ben-Yehudah et al., 2007; Desmond et al., 2005). On the other hand, information-integration tasks are thought to depend upon a procedural learning system (Ashby et al., 2003a). If the cerebellar contribution to cognition were restricted to its role in working memory, then we would not expect impairment on the information-integration task. There is, however, a substantial literature implicating the cerebellum in various forms of procedural learning, at least with respect to motor tasks (Gomez-Beldarrain et al., 1998; Shin & Ivry, 2003; Torriero et al., 2004). If the cerebellum plays a general role in procedural learning, then we might also expect impairment on the information integration task.
METHOD
Participants and Design
Fifteen patients (three female) with damage to the cerebellum (CB) were either referred to the study by neurologists at an outpatient clinic at the VA Medical Center in Martinez, California, or recruited at meetings of ataxia support groups in the San Francisco Bay Area (Table 1). The CB group included eight patients with focal lesions due to tumor (n = 3) or stroke (n = 5). The pathology was restricted to one side in six of these patients and spanned the midline in two patients. Lesion reconstructions for unilateral patients are provided in Figure 2. We were unable to obtain access to scans for the two midline patients and, thus, relied on a review of their radiological records. The remaining seven patients had a diagnosis of cerebellar atrophy. The diagnosis for these patients was based on a combination of clinical evaluation, radiological records, and, when available, genetic testing (two patients had confirmed diagnosis). The degree of atrophy varied in these patients but was evident across the cerebellar hemispheres.
Table 1.
Participant demographic information and neuropsychological assessment
| ID | Age at Test | ED | MMSE | WAIS-III |
Years Post | Path | ICARS |
RB Errors | II Errors | ID Total Errors | ID Errors/Trial | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIQ | PIQ | WM | Posture/Gait | Ataxiaa | Speech | Occulomotor | ||||||||||
| Cerebellar Patients | ||||||||||||||||
| AC01 | 57 | 18 | 30 | 125 | 122 | 113 | 4 | ATRO | 11.3 | 5.7 | 4.3 | 2.3 | 3 | 39 | 6 | .3 |
| AC06 | 65 | 17 | 26 | 88 | 75 | 78 | 45 | ATRO | 12.8 | 11 | 5.5 | 2.5 | 25 | 107 | 221 | 1.5 |
| AC07 | 38 | 16 | 29 | 101 | 90 | 94 | 5 | SCA2 | 11.8 | 10 | 3.3 | 1.5 | 0 | 33 | 8 | .4 |
| AC08 | 51 | 14 | 29 | 104 | 92 | 94 | 9 | ATRO | 6.8 | 3 | 3.3 | 4.8 | 40 | 48 | 85 | 1.6 |
| AC09 | 65 | 20 | 27 | 101 | 110 | 94 | 4 | ATRO | 4 | 4 | 3.3 | 2.5 | 44 | 30 | 54 | .6 |
| AC10 | 74 | 12 | 29 | 90 | 94 | 92 | 44 | ATRO | 19.8 | 9.2 | 4.8 | 2.3 | 27 | 39 | 206 | 2.2 |
| AC11 | 43 | 16 | 30 | 89 | 80 | 73 | 14 | SCA6 | 20 | 8 | 4 | 3 | 18 | 4 | 9 | .4 |
| LC01 | 54 | 13 | 29 | 113 | 92 | 130 | 6 | CVA(L) | 1.8 | 6.8 | 1.5 | 0 | 2 | 43 | 32 | 1 |
| LC02 | 67 | 14 | 28 | 91 | 86 | 84 | 12 | CVA(R) | 1.3 | 1 | 1 | 1 | 0 | 94 | 68 | 1.2 |
| LC03 | 59 | 12 | 30 | 87 | 80 | 73 | 12 | TUM(L) | 7.5 | 6 | 5 | 3.8 | 90 | 16 | 167 | 1.3 |
| LC04 | 46 | 18 | 30 | 110 | 106 | 111 | 3 | CVA(R) | 3 | 5 | 0 | 3 | 3 | 12 | 3 | .2 |
| LC05 | 48 | 16 | 30 | 98 | 91 | 94 | 6 | TUM(R) | 10.5 | 15.3 | 3.5 | 3.5 | 4 | 10 | 31 | .8 |
| LC06 | 78 | 17 | 29 | 106 | 106 | 95 | 12 | CVA(R) | 17.5 | 10.5 | 3 | 3 | 78 | 76 | 37 | .5 |
| MC01 | 39 | 18 | 30 | 124 | 111 | 109 | 11 | TUM | — | — | — | — | 83 | 31 | 8 | .4 |
| MC03 | 49 | 18 | 30 | — | — | — | CVA | 12 | 2.5 | 1 | 2 | 0 | 69 | 37 | 1.2 | |
| Mean | 55.5 | 15.9 | 29.1 | 101.9 | 95.4 | 95.2 | 10.0 | 7.0 | 3.1 | 2.5 | 27.8 | 43.4 | 64.8 | .9 | ||
| SD | 12.3 | 2.4 | 1.2 | 12.7 | 13.7 | 16.1 | 6.3 | 3.9 | 1.7 | 1.2 | 32.4 | 30.7 | 73.6 | .6 | ||
| Control Participants | ||||||||||||||||
| MP03 | 54 | 14 | 29 | 119 | 105 | 117 | 3 | 12 | 39 | 1 | ||||||
| MP04 | 57 | 17 | 30 | 143 | 117 | 136 | 44 | 51 | 7 | .5 | ||||||
| MP10 | 45 | 12 | 28 | 72 | 76 | 90 | 4 | 43 | 94 | .9 | ||||||
| MP15 | 58 | 16 | 30 | 119 | 130 | 111 | 5 | 70 | 17 | .6 | ||||||
| MP21 | 43 | 12 | — | 98 | 105 | — | 81 | 74 | 15 | .4 | ||||||
| OP01 | 69 | 16 | 30 | 104 | 121 | 99 | 4 | 6 | 11 | .6 | ||||||
| OP08 | 61 | 20 | 30 | 118 | 85 | 87 | 2 | 66 | 59 | .6 | ||||||
| OP09 | 65 | 20 | 30 | 124 | 90 | 136 | 1 | 46 | 19 | .5 | ||||||
| OP11 | 63 | 16 | 30 | 133 | 136 | 150 | 22 | 53 | 16 | .6 | ||||||
| OP15 | 69 | 12 | 28 | 93 | 97 | — | 11 | 33 | 171 | 1.9 | ||||||
| OP26 | 77 | 20 | 29 | 116 | 110 | 119 | 5 | 70 | 91 | 1.3 | ||||||
| OP27 | 72 | 17 | 29 | 117 | 106 | 113 | 7 | 26 | 33 | 1.2 | ||||||
| Mean | 61.1 | 16.0 | 29.4 | 113.0 | 106.5 | 115.8 | 15.8 | 45.8 | 47.7 | .8 | ||||||
| SD | 10.4 | 3.0 | 0.8 | 18.9 | 17.9 | 20.6 | 23.9 | 22.9 | 49.1 | .4 | ||||||
| t | 1.25 | 0.06 | 0.70 | 1.78 | 1.80 | 2.74 | ||||||||||
| p | 0.22 | 0.95 | 0.49 | 0.09 | 0.08 | 0.01* | ||||||||||
Note. ID = participant identification code; AC = atrophy of the cerebellum; LC = lateral cerebellar damage; MC = midline cerebellar damage; MP = middle-aged participants; OP = older participants; ED = years of education; MMSE = Mini Mental State Examination; WAIS-III = Wechsler Adult Intelligence Scale III; VIQ = Verbal IQ; PIQ = Performance IQ; FSIQ = Full-Scale IQ; WM = Working Memory Index; Years Post = years post onset/lesion relative to the testing date; Path = pathology of the cerebellar damage (side of lesion is indicated in parentheses for unilateral patients); ATRO = atrophy of unknown origin; CVA = cerebrovascular accident; SCA = spinocerebellar ataxia (the genetic subtype is indicated in parentheses); TUM = tumor resection. The columns labeled Posture/Gait, Ataxia, Speech, and Occulomotor are ratings (higher scores indicate greater impairment) on subscales of the International Cooperative Ataxia Rating Scale (ICARS, Trouillas et al., 1997).
Ataxia ratings are either for the impaired limb (unilateral patients) or both limbs (bilateral patients). The average ataxia rating is presented for those participants with a difference between limbs: AC01 (left = 5.5, right = 5.8) and AC10 (left = 8.3, right = 10). All t-tests computed as Controls-Patients.
Significant difference between Cerebellar and Control groups (p < .05).
Fig. 2.
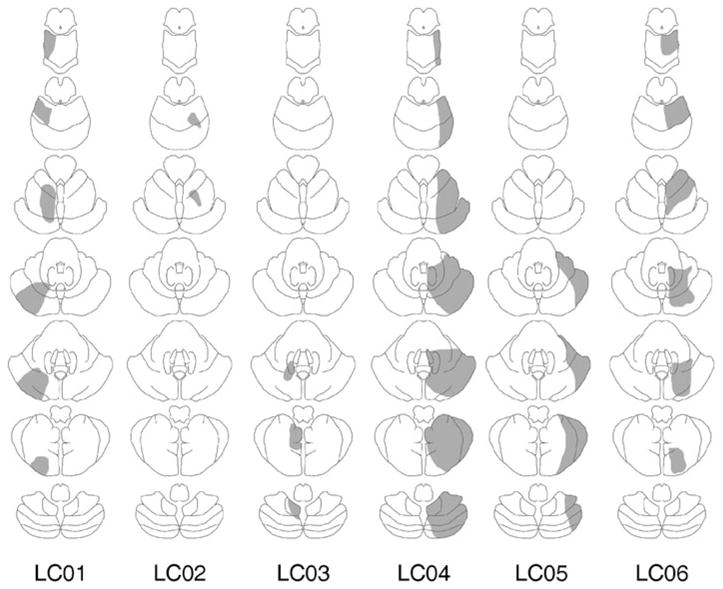
Lesion reconstructions (in gray) based upon computed tomography or magnetic resonance imaging for the patients with lateral cerebellar lesions. For each patient, the lesions are presented on a schematic of seven axial sections from superior (top) to inferior (bottom). LC, lateral cerebellar patients.
We did not include patients with more than one significant neurological event (focal group) or atrophy patients with clear evidence of extracerebellar symptomology or pathology. Patients with evidence of psychiatric impairment or current substance abuse were also excluded.
Twelve (five female) control participants (CO) were recruited from the Berkeley community (Table 1). The controls were screened for the presence of neurological disorders or a history of psychiatric illness and current substance abuse, and selected to span the range of the patients in terms of age and education. The CB and CO groups were reasonably matched on age and education. All participants reported 20/20 vision or vision corrected to 20/20 and normal color vision.
Participants were monetarily compensated. The study protocol was approved by the institutional review boards of the VA Medical Center in Martinez and University of California, Berkeley.
Neuropsychological Assessment
A battery of neuropsychological tests was used to assess different aspects of cognitive function (Table 1). The Mini-Mental State Examination was used to screen for dementia. Subtests of the Wechsler Adult Intelligence Scale (WAIS-III, Wechsler, 1997) were used to calculate verbal IQ, performance IQ, and full scale IQ. Standardized scores from the Vocabulary, Similarities, Arithmetic, Digit Span, and Information WAIS-III subtests generated a prorated verbal IQ. Standardized scores from the Picture Completion, Matrix Reasoning, Picture Arrangement, Symbol Search WAIS-III subtests generated a prorated performance IQ. Scores from the Digit Span, Arithmetic, and Letter-Number Sequencing subtests provided an index of working memory function. As assessed by the Beck Depression Inventory (2nd edition) (Beck et al., 1996), six of the patients were found to have mild (n = 4) or severe (n = 2) symptoms of clinical depression. None of the control participants were found to have symptoms of depression.
Stimuli and Stimulus Generation
Identification task
The stimuli were six rectangles that varied in color (black, blue, green, red, white, yellow). Each stimulus was mapped to a unique label (i.e., the letters A–F). To avoid a potential response bias, the stimulus-response label mappings were pseudorandomly designated for each participant with the constraint that the response label “B” was not mapped to the colors black or blue.
Categorization tasks
The stimuli and a representative category structure for the rule-based and information-integration tasks are presented in Figure 1. There were a total of 16 stimuli, formed by the factorial combination of four binary-valued dimensions: background color, symbol color, symbol shape, and symbol number. For the rule-based task, there were four possible category structures defined by the task-relevant dimension. For the information-integration task, there were four possible category structures defined by the task-irrelevant dimension. In both tasks, there were eight exemplars in category A and eight in category B.
In all tasks, each stimulus was presented on a black background and subtended a visual angle of 9.5 degrees at a viewing distance of approximately 60 cm. The stimuli were generated and presented using the Psychophysics Toolbox extensions for MATLAB (Brainard, 1997; Pelli, 1997). The stimuli were displayed on either a 15″ CRT with 1024 × 768 resolution in a dimly lit room or on a laptop LCD of the same resolution when patients were tested in their home.
Procedure
The participants were tested on the experimental tasks in a single session. Each session lasted approximately 2 hr, including an hour of neuropsychological testing. The order of the tasks was fixed with the exception that the order of the categorization tasks was counterbalanced across participants: categorization task (rule-based/information-integration), identification task, neuropsychological testing, categorization task (information-integration/rule-based). The placement of the identification task and neuropsychological testing was intended to minimize any potential interference effects between the two categorization tasks.
Identification task
At the beginning of the identification task, each participant was shown all stimuli. On each trial, a single stimulus was presented and the participant was instructed to verbally identify each stimulus using the letters A–F. The experimenter entered the participant’s response by pressing the appropriate key on the keyboard. We used verbal responses to minimize the motor demands of the task. The instructions emphasized accuracy and there was no response-time limit. After responding, the screen was blanked and auditory feedback was provided. Correct responses were indicated by the presentation of a 500 Hz tone; incorrect responses were indicated by a 200 Hz tone (for 1 s). Following feedback, the screen remained blank for 500 ms before the appearance of the next stimulus. Participants were given examples of the feedback at the beginning of the session and the experimenter did not proceed until it was evident that the participant understood the feedback.
Following the procedure of previous studies (e.g., Bracke-Tolkmitt et al., 1989), a trial was not complete until a correct response was made. Thus, when an incorrect response was made, following feedback, the stimulus was presented again; with this procedure repeating until the correct response was made. The identification task continued until the participant met a learning criterion (10 consecutive correct responses) or completed 150 trials. The presentation order of the stimuli was randomized separately for each participant with the constraints that the same stimulus was not presented on consecutive trials and that every stimulus was presented at least once within a window of 10 trials.
Categorization tasks
At the beginning of the categorization task, each participant was shown a series of sample stimuli and informed that the stimuli varied in terms of background color, background shape, symbol number, and symbol shape. On each trial, a single stimulus was presented and the participant was instructed to verbally classify each stimulus as belonging to category A or B. The category-response label mappings were counterbalanced across participants. The experimenter entered the participant’s response by pressing the appropriate key on the keyboard, again in an effort to minimize the motor demands of the task. The instructions emphasized accuracy and there was no response-time limit. After responding, the screen was blanked and feedback was provided in the same manner as for the identification task. Following feedback, the screen remained blank for 500 ms before the appearance of the next stimulus. The participant was told that there were two equally likely categories and informed that the best possible accuracy was 100%.
Each participant then completed five practice trials before beginning the experiment. The categorization task continued until the participant met a learning criterion (10 consecutive correct responses) or completed 200 trials. The presentation order of the stimuli was randomized (offline) separately for each participant with two constraints. First, the same stimulus could not be presented on consecutive trials. Second, in the information-integration task, the learning criterion of 10 consecutive correct trials could not be met by using a unidimensional strategy (e.g., respond according to background color only).1
Within each categorization task, the category structure was changed once the participant reached criterion or after 200 trials if the criterion was not met. This change involved the replacement of the current category structure with a different one (i.e., new relevant dimension in the rule-based task and new irrelevant dimension in the information-integration task). This change occurred without warning, although the participant was instructed at the beginning of the experiment that the rule would change at some point. Preliminary analyses revealed no difference in performance between the two category structures for either task on any of the dependent measures discussed below.2 Thus, all subsequent analyses are restricted to the first category structure.
RESULTS
Identification Task
As described above, a trial was not complete until a correct response was provided. Thus, it was possible for a participant to commit multiple errors within a single trial. For this reason, we analyzed both the number of total errors as well as the number of errors-per-trial. These data are plotted in the top and bottom panels of Figure 3, respectively. On both measures, we failed to observe a group difference: [number of errors: t(25) = .69; p = .5; d = .27; errors-per-trial: t(25) = .3; p = .77; d = .12].3 Given that this is a null result, it is important to consider if the failure to find an effect was related to the small sample size. A power analysis revealed that the observed difference between groups would be significant with a substantial increase in sample size (i.e., to 211/group for the number of errors).4
Fig. 3.
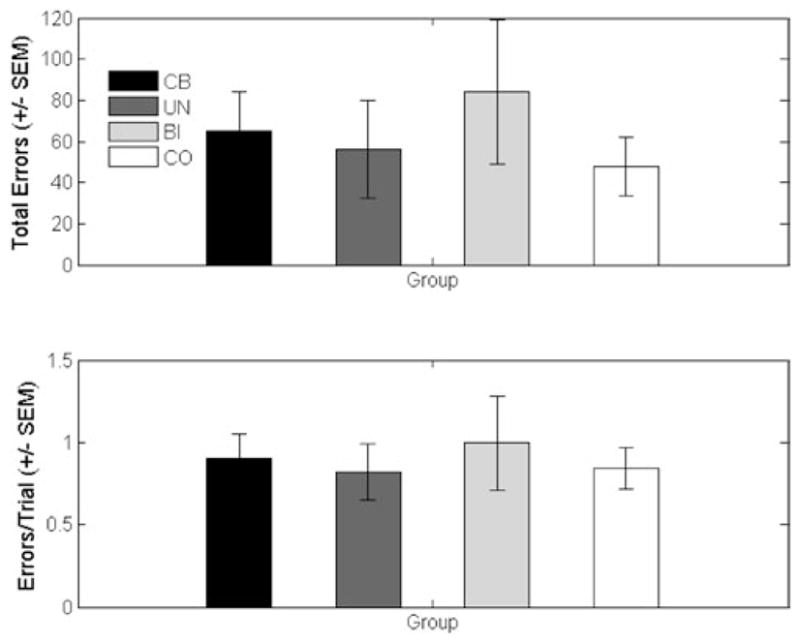
Mean data from the identification task for the cerebellar patients (CB) and control participants (CO). The patient data are further broken down into two subgroups, patients with unilateral (UN) or bilateral (BI) pathology.
As can be seen in the individual participant data, the mean difference in terms of the number of errors was driven by three poorly performing patients (AC06, AC10, and LC03; see Table 1), although even these data points fall within three standard deviations of the group mean, a convention frequently adopted to exclude outliers. These patients were the only participants who did not meet the learning criterion of ten consecutive correct responses. When these three patients were excluded from a secondary analysis, the mean number of errors was actually larger for the controls compared with the cerebellar group (CB: M = 41.9, SE = 12.6; CO: M = 47.7, SE = 14.2).
Categorization Tasks
Inspection of the mean number of errors in the categorization tasks indicates that the information-integration task was much more difficult for both groups (Figure 4). When analyzed in a group (controls vs. cerebellar) × task (rule-based vs. information-integration) ANOVA, the effect of task was significant, [F(1,25) = 9.44; p < .01; MSE = 737.17; ]. However, neither the effect of group, [F(1,25) = .94; p = .34; MSE = 479.5; ] nor the group × task interaction [F(1,25) = .95; p = .34; MSE = 737.17; ] were significant. Although the mean data would suggest a trend toward a group difference on the rule-based task, the control and patient groups were within 1 standard error of each other. A power analysis performed on these data indicated that 64 participants would have to be tested in each group for the interaction effect to become reliable.
Fig. 4.
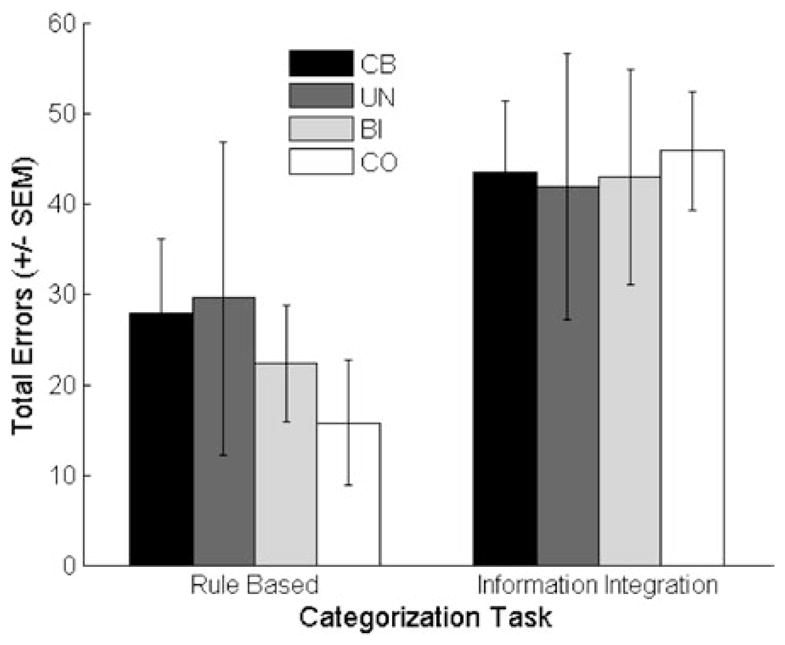
Mean data from the categorization tasks for the cerebellar patients (CB) and control participants (CO). The patient data are further divided into two subgroups, patients with unilateral (UN) or bilateral (BI) pathology.
Neuropsychological and Neuropathological Variables
Although the cerebellar patients were not impaired on the identification task, it is still important to ask whether IQ was predictive of performance in the current sample given the relationship between IQ and performance in previous work (Bracke-Tolkmitt et al., 1989). Verbal IQ was negatively correlated with identification errors for both groups [CB: r(14) = −.64; p = .01; CO: r(12) = −.57; p = .05], suggesting that low IQ is indeed predictive of increased errors on the identification task independent of the presence of cerebellar damage. The three patients who performed poorly on the identification task scored below average on the IQ indices.
Excluding the two patients with midline cerebellar damage, our sample was split between individuals with bilateral degeneration (n = 7) and unilateral lesions (n = 6). The error data for the three tasks are plotted in Figures 3 and 4. Inspection of these data suggests that the patients with bilateral damage performed more poorly on the identification task than did patients with unilateral damage. However, separate one-way ANOVAs conducted on the four dependent variables comparing the bilateral, unilateral, and control groups failed to reveal any group differences [rule-based: F(2,22) = .51, p = .61, MSE = 771.07, ; information-integration: F(2,22) = .05, p = .95, MSE = 824.88, ; identification total errors: F(2,22) = .69, p = .51, MSE = 4334.5, ; identification errors-per-trial: F(2,22) = .22, p = .8, MSE = .3, ]. A power analysis again revealed that these differences would require a significant increase in sample size (i.e., to approximately 52 participants/group for the largest effect) to be reliable.
GENERAL DISCUSSION
The study of the neural substrates of category learning is an area of intense research. This work has focused on the basal ganglia, medial temporal lobes, and prefrontal cortex, in large part because of the role of these regions in reinforcement-based learning and executive control. Given the prominent role of the cerebellum in learning and memory, at least within the domain of sensorimotor skills, the current study was designed to systemically examine the effects of cerebellar damage on a set of categorization and identification tasks. Patients with cerebellar pathology performed similarly to controls on rule-based and information-integration category learning tasks, as well as on an identification task.
We recognize that the main conclusion to be drawn here is a null result. While the interpretation of null results requires caution, three points should be noted. First, the observed effect sizes are in the small to moderate range (Cohen, 1977). Although it is possible that such small effects may be meaningful, it is important to note that, across all three tasks, the observed differences would only reach conventional significance levels if the sample size were increased to between 120 and 200 total participants. Thus, we do not expect that the null results can be easily attributed to a power problem related to our sample sizes. Moreover, our sample sizes meet or exceed those used in previous studies of cerebellar contributions to categorization and identification.
Second, the current results help provide an important boundary condition on understanding the neural substrates of learning and memory; that is, observations of impaired categorization in other patient groups are strengthened by the finding that these impairments are not a general feature of neural insult. In particular, the present data suggest that previous reports of impaired performance in the rule-based task may indeed be specific to fronto-striatal dysfunction due to Parkinson’s disease (e.g., Ashby et al., 2003b) rather than an inevitable consequence of neurological dysfunction. Furthermore, considered in conjunction with previous studies (Maddox et al., 2005; Witt et al., 2002), these data strengthen the claim that the cerebellum is not necessary for a variety of category learning tasks.
Our null results on the rule-based categorization task are consistent with previous research (e.g., Maddox et al., 2005). This study, however, provides the first report of the effects of cerebellar pathology on an information-integration task. Previous work has also observed that cerebellar patients perform similarly to controls in tasks that would seem similar to the Figure 1b task (e.g., the weather prediction task; Witt et al., 2002). It is unclear, however, whether the task used by Witt et al. is solved by using rule-based strategies (Ashby & Maddox, 2005; Gluck et al., 2002). Furthermore, individuals with Parkinson’s disease have been shown to be impaired on the weather prediction task (Knowlton et al., 1996), but performed similarly to matched controls on the Figure 1b information-integration task (Ashby et al., 2003b).
The spared performance on the information-integration task is of interest given that the cerebellum has frequently been associated with procedural learning (Gomez-Beldarrain et al., 1998; Shin & Ivry, 2003; Torriero et al., 2004). Taxonomic models of memory consistently emphasize a view in which procedural memory may take many forms that are associated with distinct neural systems (Squire et al., 1993). If we were to assume a general cerebellar contribution to procedural motor skill acquisition, then the current results would suggest that the processes involved in procedural cognitive learning involve distinct neural systems. On the other hand, the term procedural learning may be best viewed as a heuristic description, encompassing multiple forms of learning. Specifying the processes underlying these forms of learning will be essential for developing a more computational-based perspective. The current results help emphasize this point, suggesting that a procedural-declarative distinction is unlikely to prove fruitful in understanding if and how the cerebellum contributes to cognitive learning.
The null results on the identification task are perhaps the most surprising given previous work on this issue (Bracke-Tolkmitt et al., 1989; Canavan et al., 1994; see also Drepper et al., 1999); indeed, we used essentially the same task as that used in the work of Canavan and colleagues. Procedurally, the only difference was that we used letters as response labels instead of words (Bracke-Tolkmitt et al., 1989) or numbers (Drepper et al., 1999). It may be that color-letter associations are somehow easier to learn than color-word or color-number associations. If this was the case, however, the number of errors in our control group should be reduced relative to previous studies. In fact, this was not the case as the average number of errors committed by the control group was similar to previous work (M = 40.4; range = 9–96; Bracke-Tolkmitt et al., 1989).
It should be noted that the sample size was small in these previous studies of identification (five and seven patients, in the Bracke-Tolkmitt et al. and Canavan et al. studies, respectively). While our sample is larger, it is also quite heterogeneous, including individuals with bilateral degeneration and unilateral lesions. It is possible that subgroups within our cerebellar sample are impaired, but that this effect was obscured by averaging. A priori, posterior/inferior cerebellar regions that are reciprocally connected with prefrontal cortex would be expected to be important for the identification and rule-based tasks (Kelly & Strick, 2003; Ramnani, 2006). Similarly, processing in the deep cerebellar nuclei might be essential for feedback-related processing in the dopamine producing neurons of the substantia nigra (via the pedunculopontine nucleus; Hazrati & Parent, 1992; Lavoie & Parent, 1993) and pathology in these regions might predict learning impairments on our tasks. Our sample size of patients with focal lesions is limited for performing a subgroup analysis. We do note, though, that for five of six patients with focal lesions, the damage extends into posterior cerebellum and likely includes some parts of the dentate nucleus.
The spared performance for the atrophy patients also argues against the idea that our null results are related to the variability introduced by averaging across a heterogeneous patient sample. First, as expected from the diffuse atrophy found in these patients, the severity of the symptoms of cerebellar damage was equal to or more extreme than those observed in the patients with focal lesions. Second, we failed to find any differences between the focal and bilateral patients on the identification and categorization tasks (Figures 3 and 4). Even if the analyses were restricted to the patients with bilateral degeneration, individuals in whom the pathology is likely present across the cerebellum, we do not observe an impairment on any of the tasks.
Previous studies of identification observed mixed results with heterogenous samples of cerebellar patients (Bracke-Tolkmitt et al., 1989; Canavan et al., 1994). In Canavan et al., four of seven patients were impaired on an identification task: two of these patients had diffuse bilateral atrophy and two had unilateral damage due to tumor resection. Of these four patients, three had below normal verbal IQ. The three poorly performing patients in our study (two with bilateral atrophy and one with unilateral damage due to tumor resection) also had low verbal IQ scores. Verbal IQ was also negatively correlated with errors for our controls suggesting that identification performance, in general, is sensitive to verbal IQ. Taken together, these results suggest that previous reports of impairment on identification tasks following cerebellar damage may actually be related to group differences in IQ. We cannot say if the cerebellar pathology contributed to such differences because premorbid IQ data are not available.
One final noteworthy point is that the current results are of interest to the on-going debate about how best to characterize cerebellar contributions to cognition. Cerebellar pathology has been associated with a variety of nonmotor tasks, including those involving precise temporal discrimination (e.g., Ivry, 1996), working memory (e.g., Ravizza et al., 2006), and attention (Courchesne et al., 1994; Townsend et al., 1999; but see Ravizza & Ivry, 2001). Identifying such tasks is clearly important, and should prove essential in developing computational models of cerebellar function. However, a complete theory of cerebellar contributions to cognition cannot depend solely upon findings of impairment. Identifying tasks for which the integrity of the cerebellum is not essential can also prove useful in establishing the boundary conditions for cerebellar contributions to cognition.
Acknowledgments
This research was supported by grants NS047884, NS30256, and NS40813 from the National Institutes of Health. The authors thank Rebecca Spencer for assistance with the lesion reconstructions.
Footnotes
More specifically, following the randomization of the presentation order, models assuming that the participant attended to a single dimension (four total) were simulated. If any of the simulated models met the learning criterion, the presentation order was re-randomized. This procedure was repeated until the presentation-order constraint was satisfied.
For example, there was no difference in the number of errors committed in the two category structures of either the rule-based [t(14) = .43, p = .67; CO −t(11) = −.03, p = .98] or information-integration [t(14) = .76, p = .46; CO −t(11) = .21, p = .84] tasks.
A similar proportion of participants in both groups met the learning criterion (i.e., 10 consecutive correct responses) in both the rule-based (CB: 13/15; CO: 11/12) and information-integration (CB: 12/15; CO: 9/12) tasks. An analysis of the trials-to-criterion data for those participants that met the criterion mirrored the analysis of the error data.
All power analyses were performed at a criterion of 1 −β= .8 using G*power 3 (Faul et al., 2007).
References
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW, Waldron EM. Procedural learning in perceptual categorization. Memory & Cognition. 2003a;31:1114–1125. doi: 10.3758/bf03196132. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Lee WW. Predicting similarity and categorization from identification. Journal of Experimental Psychology: General. 1991;120:150–172. doi: 10.1037//0096-3445.120.2.150. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Noble S, Filoteo JV, Waldron EM, Ell SW. Category learning deficits in Parkinson’s disease. Neuropsychology. 2003b;17:115–124. [PubMed] [Google Scholar]
- Ashby FG, Valentin VV. Multiple systems of perceptual category learning: Theory and cognitive tests. In: Cohen H, Lefebvre C, editors. Categorization in Cognitive Science. New York: Elsevier; 2005. [Google Scholar]
- Beck AT, Steer R, Brown G. Beck Depression Inventory—Second Edition Manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: Beyond cognitive theory. The Cerebellum. 2007;6:193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Bracke-Tolkmitt R, Linden A, Canavan AGM, Rochstroh B, Scholz E, Wessel K, Diener HC. The cerebellum contributes to mental skills. Behavioral Neuroscience. 1989;103:442–446. [Google Scholar]
- Brainard DH. Psychophysics software for use with MAT-LAB. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Canavan AGM, Sprengelmeyer R, Diener HC, Homberg V. Conditional associative learning is impaired in cerebellar disease in humans. Behavioral Neuroscience. 1994;108:475–485. doi: 10.1037//0735-7044.108.3.475. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. San Diego, CA: Academic Press; 1977. [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, James HE, Haas RH, Schreibman L, Lau L. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Daum I, Ackermann H, Schugens MM, Reimold C, Dichgans J, Birbaumer N. The cerebellum and cognitive functions in humans. Behavioral Neuroscience. 1993;107:411–419. doi: 10.1037//0735-7044.107.3.411. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Annals of Neurology. 2005;58:553–560. doi: 10.1002/ana.20604. [DOI] [PubMed] [Google Scholar]
- Drepper J, Timmann D, Kolb FP, Diener HC. Non-motor associative learning in patients with isolated degenerative cerebellar disease. Brain. 1999;122:87–97. doi: 10.1093/brain/122.1.87. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rules and exemplars in category learning. Journal of Experimental Psychology: General. 1998;127:107–140. doi: 10.1037//0096-3445.127.2.107. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage: A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the “weather prediction” task?: Individual variability in strategies for probabilistic category learning. Learning & Memory. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Garcia-Monco JC, Rubio B, Pascual-Leone A. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Experimental Brain Research. 1998;120:25–30. doi: 10.1007/s002210050374. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA. Behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. Journal of Experimental Psychology. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Research. 1992;585:267–271. doi: 10.1016/0006-8993(92)91216-2. [DOI] [PubMed] [Google Scholar]
- Helmuth LI, Ivry RB, Shimizu N. Preserved performance by cerebellar patients on tests of word generation, discrimination learning, and attention. Learning & Memory. 1997;3:456–474. doi: 10.1101/lm.3.6.456. [DOI] [PubMed] [Google Scholar]
- Ivry R. The representation of temporal information in perception and motor control. Current Opinion in Neurobiology. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéri S. The cognitive neuroscience of category learning. Brain Research. Brain Research Reviews. 2003;43:85–109. doi: 10.1016/s0165-0173(03)00204-2. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: Projections to the basal ganglia as revealed by anterograde tract-tracing methods. The Journal of Comparative Neurology. 1993;344:210–231. doi: 10.1002/cne.903440204. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Aparicio P, Marchant NL, Ivry RB. Rule-based category learning is impaired in patients with Parkinson’s Disease but not patients with cerebellar disorders. Journal of Cognitive Neuroscience. 2005;17:707–723. doi: 10.1162/0898929053747630. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PD, Passingham RE. The cerebellum and cognition: Cerebellar lesions do not impair spatial working memory or visual associative learning in monkeys. The European Journal of Neuroscience. 1999;11:4070–4080. doi: 10.1046/j.1460-9568.1999.00825.x. [DOI] [PubMed] [Google Scholar]
- Nixon PD, Passingham RE. The cerebellum and cognition: Cerebellar lesions impair sequence learning but not conditional visuomotor learning in monkeys. Neuropsychologia. 2000;38:1054–1072. doi: 10.1016/s0028-3932(99)00138-4. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM. Attention, similarity, and the identification categorization relationship. Journal of Experimental Psychology: General. 1986;115:39–57. doi: 10.1037//0096-3445.115.1.39. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: Anatomy and function. Nature Reviews Neuroscience. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ivry RB. Comparison of the basal ganglia and cerebellum in shifting attention. Journal of Cognitive Neuroscience. 2001;13:285–297. doi: 10.1162/08989290151137340. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Archives of Neurology. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The Cerebellum and Cognition. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Shepard RN, Hovland CI, Jenkins HM. Learning and memorization of classifications. Psychological Monographs: General and Applied. 1961;75:1–42. [Google Scholar]
- Shin JC, Ivry RB. Spatial and temporal sequence learning in patients with Parkinson’s disease or cerebellar lesions. Journal of Cognitive Neuroscience. 2003;15:1232–1243. doi: 10.1162/089892903322598175. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton BJ, Musen G. The structure and organization of memory. Annual Review of Psychology. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Maschke M, Kolb FP, Boring D, Thilmann AF, Diener HC. Motor deficits cannot explain impaired cognitive associative learning in cerebellar patients. Neuropsychologia. 2002;40:788–800. doi: 10.1016/s0028-3932(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rTMS with procedural learning. Journal of Cognitive Neuroscience. 2004;16:1605–1611. doi: 10.1162/0898929042568488. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. Journal of Neuroscience. 1999;19:5632–5643. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. Journal of the Neurological Sciences. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Tucker J, Harding AE, Jahanshahi M, Nixon PD, Rush-worth M, Quinn NP, Thompson PD, Passingham RE. Associative learning in patients with cerebellar ataxia. Behavioral Neuroscience. 1996;110:1229–1234. doi: 10.1037//0735-7044.110.6.1229. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation Har-court Brace & Company; 1997. [Google Scholar]
- Witt K, Nuhsman A, Deuschl G. Dissociation of habit-learning in Parkinson’s and cerebellar disease. Journal of Cognitive Neuroscience. 2002;14:493–499. doi: 10.1162/089892902317362001. [DOI] [PubMed] [Google Scholar]