Abstract
The refinement of neural circuits during development depends upon a dynamic process of branching of axons and dendrites that leads to synapse formation and connectivity. The neurotrophin BDNF plays an essential role in the outgrowth and activity-dependent remodeling of axonal arbors in vivo. However, the mechanisms that translate extracellular signals into axonal branch formation are incompletely understood. Here we report that the MAP kinase phosphatase-1 (MKP-1) controls axon branching. MKP-1 expression induced by BDNF signaling exerts spatio-temporal deactivation of JNK, which negatively regulates the phosphorylation of JNK substrates that impinge upon microtubule destabilization. Indeed, neurons from mkp-1 null mice were unable to produce axon branches in response to BDNF. Our results indicate a heretofore-unknown signaling mechanism to regulate axonal branching and provide a framework for studying the molecular mechanism of innervation and axonal remodeling under normal and pathological conditions.
Introduction
Growth of neuronal processes into axons and dendrites, critical to the development of neuronal connectivity, is highly modulated during development by many extrinsic factors and cues. In addition to semaphorins, ephrins and netrins, neurotrophins such as BDNF (brain-derived neurotrophic factor) highly influence neurite branching1,2. For example, increased BDNF-TrkB signaling enhances axonal arborization and synaptic connectivity of retinal3 and trigeminal axons4 in the Xenopus tadpole, and of rat hippocampal explants5. Additionally, mice lacking TrkB show reduced axonal arborization in the hippocampus6. BDNF can elicit axon branching within two hours of treatment1, but the mechanisms that translate BDNF signaling into cytoskeletal rearrangements are not well understood. Rho family GTPases mediate actin reorganization, with Rac and Cdc42 promoting dendritic branching7. Other intracellular mechanisms of neurite arborization include activity-dependent calcium transients8 and the mitogen-activated protein kinases (MAPKs) Erk1/29.
The MAPK family integrates signals from many extracellular factors, such as neurotrophins, to create specific outcomes, including differentiation, survival and synaptic plasticity. The strength of MAPK signaling frequently determines not only the duration of biological responses, but also provides distinctive physiological results10,11.
Because the magnitude and duration of a MAPK signal is a critical determinant of different cellular outcomes, the ability to negatively regulate MAPK activities enables finely tuned responses of individual family members, including Erk, P38 and JNK. Dual specificity MAPK phosphatases (DUSP or MKP) inactivate specific MAPKs through dephosphorylation at threonine and tyrosine residues (TXY motifs)12. Phosphorylation at both residues is required for full MAPK activity. Eleven different MKP enzymes have been identified, and many are expressed in the nervous system12. Some MKP members are highly regulated and are induced as immediate early genes after stimulation with growth factors, oxidative stress or heat shock13.
Multiple tissues express the phosphatase MKP-1, and in the brain challenges such as long-term potentiation and visual cues robustly induce mkp-1 mRNA14,15. While MKP-1 activity has been well studied in the context of cell cycle and immune responses, its physiological functions in the central nervous system are unknown. Here we report that ectopic expression of MKP-1 leads to aberrant axon branching through a mechanism that involves negative regulation of JNK and microtubule destabilization. Interestingly, the MKP-1 protein is highly regulated by BDNF in a spatio-temporal fashion. In the absence of MKP-1, there is an inability to produce axon branches in response to BDNF and neuronal activity. These findings reveal a critical control mechanism that lies between signaling by extracellular factors and a specific intrinsic mechanism to control axon branching events and ultimately, neuronal connectivity.
Results
MKP-1 expression alters neuronal morphology in vivo
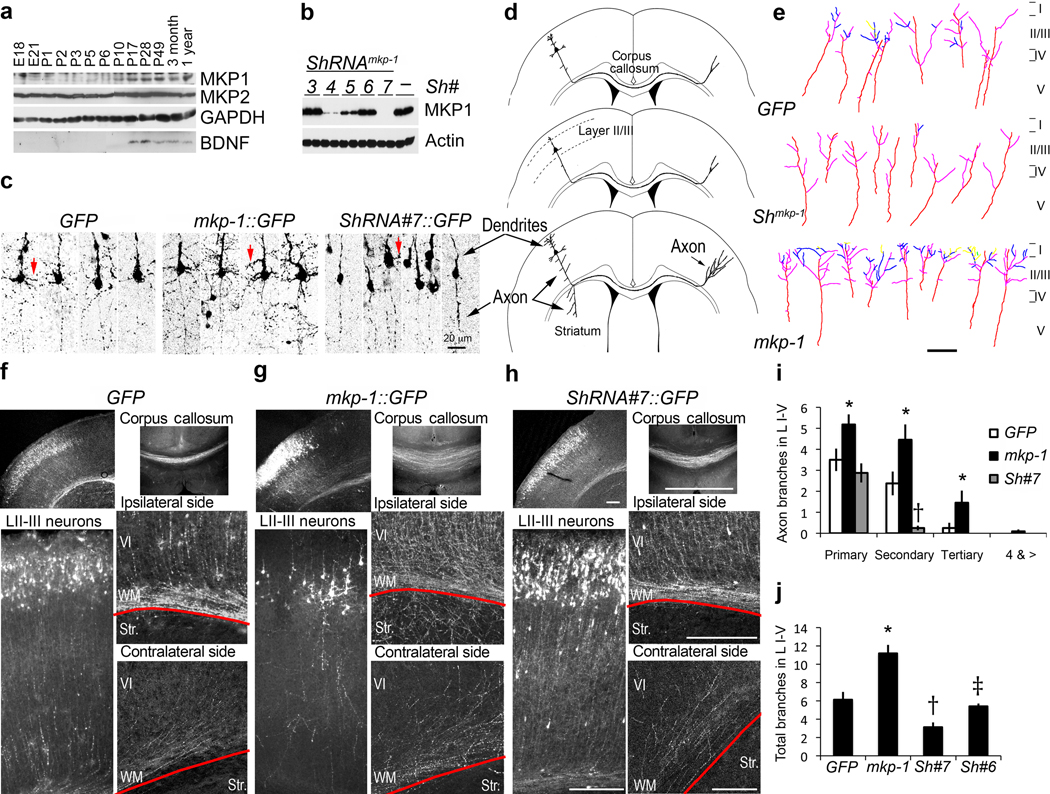
Both bdnf and mkp-1 mRNA are detectable in the central nervous system throughout embryonic development16,17. To test the hypothesis that MKP-1 downstream of BDNF can influence neuronal morphology, we first established its developmental protein expression pattern in the rat brain in vivo. MKP-1 protein level was low during embryogenesis and early postnatal days, but increased gradually from postnatal day 5 (P5) onward in the prefrontal cortex (Fig. 1a) as well as the hippocampus and striatum (not shown). In contrast, expression of another family member, MKP-2, remained unchanged (Fig. 1a).
Figure 1. Gain- and loss-of-function of MKP-1 in vivo affect neural development.
(a) Developmental expression of endogenous MKP-1 in the cortex. (b) Knockdown efficiency of short hairpin RNAs (shRNA#3-7; –, empty vector) against MKP-1. (c) Representative images of individual cortical layer II–III neurons from in utero electroporated brains expressing control (GFP), mkp-1::IRES-GFP (mkp-1::GFP) or shRNA#7 (shRNA#7::GFP) plasmids. Arrows highlight differences in dendritic development. (d) Summary of the morphological effects of MKP-1 gain and loss-of-function on layer II/III cortical neurons. (e) Traces of axon terminals within the contralateral cortical layers of transfected brains. Primary (purple), secondary (blue), tertiary (yellow) and quartenary (green) branches from axons (red) are highlighted. (f–h) Representative images of sections from P7 brains electroporated at E15.5 with control (GFP) (f), mkp-1::IRES-GFP (mkp-1::GFP) (g) or shRNA #7 (shRNA#7::GFP) (h) plasmids. Red lines indicate the boundary between white matter (WM) and lateral striatum (str). Data from MKP-1 (n=23), GFP (n=14) and shRNA#7 (n=11) animals were collected from at least 3 independent experiments. Scale bars, 200 µm. (i,j) Number of axonal branches within the contralateral cortical layers were expressed as mean ± s.e.m.. Data were collected from individual axons expressing GFP (n=8), mkp-1::GFP (n=11), shRNA#7::GFP (n=16), shRNA#6::GFP (n=10) from at least 5 different brains and at least 3 independent electroporations per condition. Significant differences are indicated as follows: p<0.05, t-test *, MKP1 vs. GFP; †, GFP vs. shRNA#7; ‡, shRNA#7 vs. shRNA#6.
To determine the functional significance of MKP-1 expression in the developing cortex, we modified MKP-1 levels during neural development in vivo. Using in utero electroporation at embryonic day 15, we targeted constructs to the neuronal lineage of the cortical plate that specifies future layer II–III excitatory neurons, and allowed the embryos to be born and develop to P7 before analysis. Expression (MKP1::GFP) and downregulation (shRNA#7::GFP; efficiency was verified in transfected HEK cells, Fig. 1b) constructs carried a GFP reporter cassette to monitor neuronal morphology in brain sections. At P7, when neuronal migration through the cortical plate is completed18, transfected LII–III neurons showed a polarized morphology with developing dendritic arbors and thin, straight axons. Ectopic expression of MKP-1 increased morphological complexity of dendritic and axonal arbors, whereas downregulation of MKP-1 resulted in less dendritic arborization compared to GFP-transfected cells (Fig. 1c–h). Axons from MKP-1 overexpressing cells entered the white matter (WM) with aberrant branching and innervation into the dorso-lateral striatum (str) and contralateral hemisphere when compared to the GFP and shRNA expressing cells (Fig. 1d–h). The complexity of axon terminals was increased by ectopic MKP-1 expression but decreased by MKP-1 knockdown, with notably less secondary branches (Fig. 1i). The number of total axonal branches within the contralateral cortical layers I–V was increased or decreased, respectively by upregulation or downregulation of MKP-1 (Fig. 1j). ShRNA incapable of silencing MKP-1 (shRNA#6; Fig. 1b,j), or overexpression of MKP-6, a related MKP family member (not shown), did not alter the neuronal morphology of transfected layer II–III neurons compared to the GFP control. Therefore, changing MKP-1 expression levels during neural development produced specific alterations of cortical neuron cytoarchitecture.
JNK is the target of MKP-1
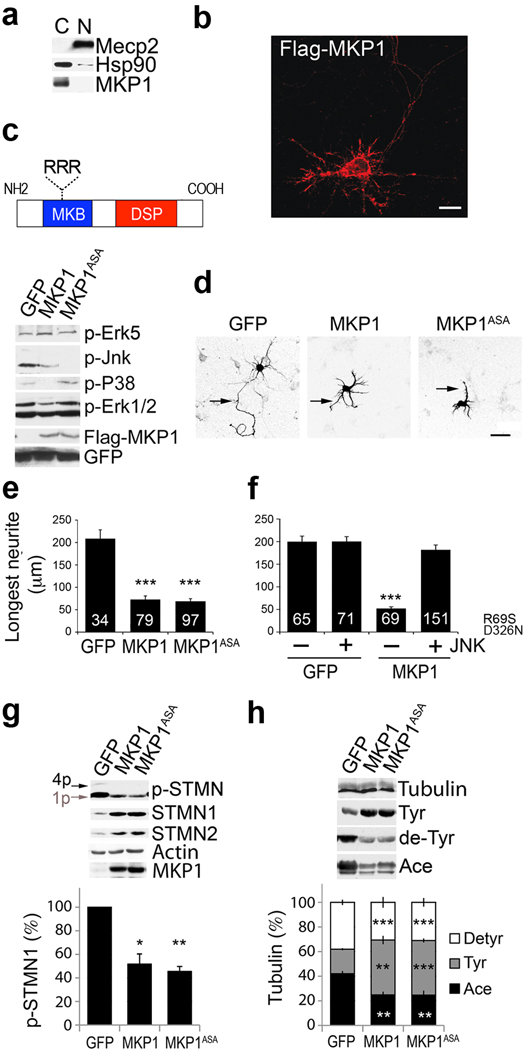
MKP-1 dephosphorylates and inactivates MAPKs with preference to P38 and JNK, and to a lesser extent, Erk1/219. To dissect the mechanism by which MKP-1 alters neuronal morphology, we used cortical neurons in culture. Both endogenous and exogenously expressed MKP-1 were detected throughout the cytoplasm, as indicated by the presence of the cytosolic protein Hsp90 and the absence of the nuclear protein MeCP2 after fractionation (Fig. 2a, endogenous MKP-1; b, transfected MKP-1). We found that MKP-1 expression in primary cortical neurons dephosphorylated JNK, P38 and Erk1/2. In contrast, Erk5 (Fig. 2c) and the parallel signaling pathways Akt, PLCγ and GSK3 (not shown) remained unchanged.
Figure 2. JNK is the MKP-1 substrate altering axonal morphology.
(a,b) Localization of MKP-1 in primary cortical neurons. (a) Cytosolic (C) and nuclear (N) extracts were analyzed for endogenous MKP-1. (b) Transfected Flag-MKP-1. Scale bar, 20 µm. (c) Cortical neurons were infected with wildtype MKP-1, MKP-1ASA or GFP between DIV1–5 and analyzed by Western blot. (d) Representative images of MKP-1, MKP-1ASA or GFP expressing neurons. Arrows indicate the longest neurite. Scale bar, 40 µm. (e) Length of the longest neurite per cell shown as mean ± s.e.m. Numbers in bars indicate the number of cells analyzed. ***, t-test p<0.0001, n=3 independent experiments. (f) Rescue of axon growth by a MKP1-insensitive JNK mutant (JNKR69S/D326N) when co-transfected with MKP-1. ***, t-test, p<0.001, n=4 independent experiments. (g) MKP-1 overexpression from DIV1–5 in cortical neurons affected the phosphorylation of stathmins, expressed as percentile of control GFP cells (mean ± s.e.m.; *, p<0.015, MKP1 vs. GFP and ***, p<0.001, MKP1ASA vs. GFP using t-test; n=3 independent experiments). Mono (1p) and multi (4p) phospho-isoforms are active and inactive, respectively. (h) Tubulin tyrosination and acetylation were analyzed to assess microtubule stability. Results are expressed as percentile of control (mean ± s.e.m., n=3 independent experiments). Significant differences are as follows: MKP-1 vs. GFP (**, tyr, p=0.03; **, ace, p=0.006; ***, de-tyr, p=0.0001) and MKP-1ASA vs. GFP (***, tyr, p=0.001; **, ace, p=0.004; ***, de-tyr, p<0.0001) using t-test.
Axon outgrowth of cultured cortical neurons decreased upon prolonged MKP-1 overexpression from DIV1–3 and concomitant dephosphorylation of pErk1/2, pP38 and pJNK (Fig. 2d,e). Among the three targets of MKP-1, JNK is constitutively phosphorylated along the axon20, while P38 and Erk1/2 are activated transiently in response to a stimulus (not shown). To test whether JNK is the MKP-1 substrate that controls axon outgrowth and therefore neuronal morphology, we used two independent approaches.
First, we used a MKP-1 mutant that targeted only the dephosphorylation of JNK. When a conserved RRR motif within the MAPK-binding domain of MKP-1, which is required for selective binding of Erk1/2 and P38, is mutated to ASA (Fig. 2c), the resulting MKP-1ASA mutant no longer binds to Erk1/2 and P38, but is capable of retaining normal binding to JNK21. Indeed, expression of MKP-1ASA in cortical neurons selectively dephosphorylated JNK, without affecting Erk1/2 or P38 (Fig. 2c). Similar to wild-type MKP-1, this mutant restricted axon outgrowth in culture (Fig. 2d,e).
Second, we engineered a JNK mutant (JNKR695S/D326N) by analogy to a previously published constitutively-active Erk1/2 mutant22,23, which displayed decreased sensitivity to MKP-1 (Supplementary Fig. 1). When co-expressed with MKP-1, JNKR69S/D326N reversed the negative effect of MKP-1 on axon outgrowth (Fig. 2f). Therefore, prolonged dephosphorylation of JNK by MKP-1 expression perturbed axon outgrowth, and restoration of JNK signaling was sufficient to rescue the observed axonal defects. Together, the effects observed with the MKP-1ASA and JNKR69S/D326N mutants suggest that JNK is the major neuronal MAPK substrate of MKP-1 that controls axonal shape.
JNK inactivation leads to microtubule destabilization
JNKs have diverse substrates, including transcription factors and cytoskeletal proteins24. Since expression of MKP-1 led to defects in axon outgrowth, we tested the effects of the prolonged JNK inactivation on cytoskeletal substrates that maintain microtubule integrity25. Among thesesubstrates, the stathmins (STMN) are structural modifiers involved in neurite branching and growth cone remodeling26,27. Activation of STMNs by dephosphorylation decreases microtubule stability through sequestration of free tubulin. Both MKP-1 and MKP-1ASA possessed similar abilities to suppress phosphorylation of STMN1 (Fig. 2g).
Because prolonged dephosphorylation of JNK through MKP-1 overexpression enhanced STMN activity, we also monitored microtubule stability by assaying post-translational modifications of tubulin. Expression of either MKP-1 or MKP-1ASA led to an increase of tyrosinated microtubules and a concomitant decrease of acetylated microtubules (Fig. 2h), changes that are associated with overall microtubule destabilization25. Therefore, MKP-1 expression leads to JNK dephosphorylation, and ultimately to decreased microtubule stability in cortical neurons.
BDNF controls MKP-1 expression
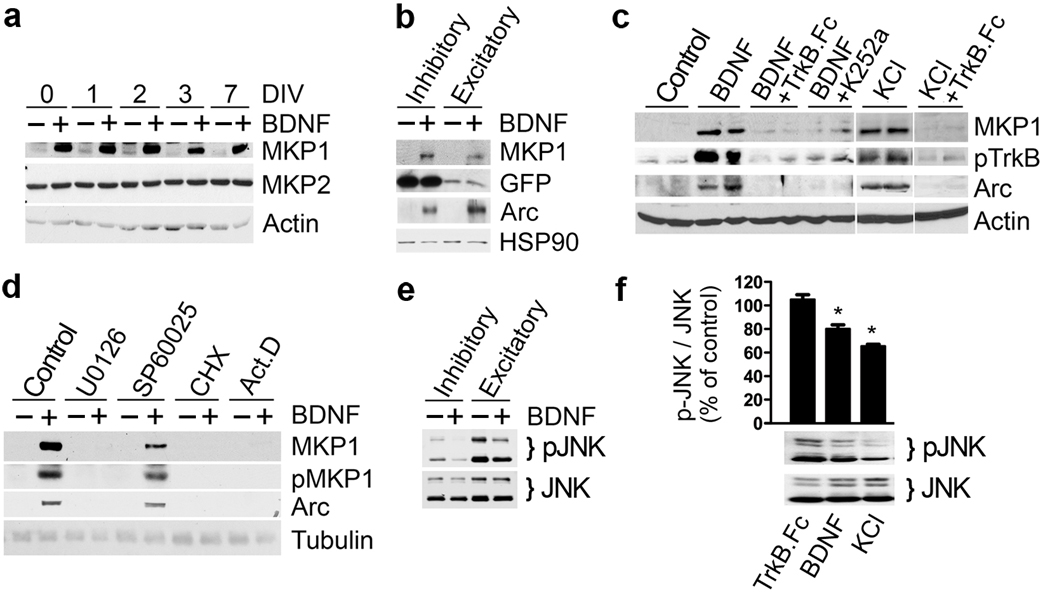
Previous reports showed that MKP-1 expression is highly inducible by a variety of stimuli. Moreover, mkp-1 mRNA is downregulated in the prefrontal cortex of conditional bdnf−/− mice28. To explore these observations further, we investigated whether BDNF acts upstream of MKP-1 expression in cultured cortical neurons. Three hours of treatment with BDNF robustly induced MKP-1 protein expression from a low basal level at different developmental stages in vitro, while MKP-2 levels remained unchanged (Fig. 3a). NT-3 and NT-4 also induced MKP-1 expression without altering MKP-2 or MKP-5 levels (Supplementary Fig. 2a). We focused our study on BDNF as it is widely expressed within the CNS.
Figure 3. Induction of MKP-1 expression by BDNF.
Dissociated cortical neurons were cultured for 7 days or the indicated DIV before pharmacological treatments. MKP-1 protein is highly inducible by BDNF (50 ng/ml) at any stage in culture (a) both in FACS sorted GFP-positive interneurons and GFP-negative excitatory neurons (b), as well as by depolarization (c) using 25 mM KCl for 3 hours. (c,d) Induction was prevented when TrkB signaling (TrkB.Fc, 100 ng/ml; K252a, 100 nM), the Erk pathway (U0126, 10 µM) or gene expression (CHX, 20 µg/ml; ActD, 5 µM) were pharmacologically blocked. (e,f) Treatment of inhibitory or excitatory neurons FACS sorted from transgenic mice expressing GFP in all cortical interneurons (e) and mixed cortical neuron cultures (f) with BDNF (50 ng/ml) or 45 mM KCl for 2 h leads to a decrease in pJNK immunoreactivity. Results from 4 independent experiments were quantified, normalized to JNK and expressed as percentile of control GFP cells (mean ± s.e.m., *, t-test p=0.004).
Primary cortical cultures contain various neuronal subtypes, including approximately 11% GABAergic interneurons (125 GAD65 positive cells/ 1115 neurons counted). Since both inhibitory and excitatory neurons express the BDNF receptor TrkB29, we asked if both cell types expressed MKP-1. To this end, we isolated GFP expressing cortical interneurons (Dlx5/6 positive30) using fluorescence-activated cell sorting, and subsequently cultured GFP–positive (inhibitory) and –negative (excitatory) cell populations. Treatment of these cells with BDNF induced MKP-1 in both excitatory and inhibitory populations (Fig. 3b). BDNF-dependent MKP-1 expression was blocked by pre-incubation with either the BDNF scavenger TrkB.Fc or the TrkB inhibitor K252a (Fig. 3c). Because BDNF is secreted in an activity-dependent manner31, we tested whether depolarization of cortical neurons with KCl also promoted MKP-1 expression. Like BDNF treatment, an increase in KCl was capable of upregulating MKP-1 protein levels, which was abrogated by TrkB.Fc (Fig. 3c) or K252a (not shown). Thus, an increase in neuronal activity with KCl resulted in an induction of MKP-1 that depended upon endogenous BDNF release and TrkB signaling. Additionally, the MEK1/2 inhibitor U0126 blocked the expression of MKP-1, whereas the JNK inhibitor SP60025 had no effect, and the protein and RNA synthesis inhibitors, cycloheximide (CHX) and actinomycin D (act D), both prevented MKP-1 induction (Fig. 3d).
Therefore, mkp-1 behaved as an immediate early gene in response to BDNF, similar to Arc, a well-characterized immediate early gene downstream of BDNF (Fig. 3b–d). In addition, we observed that prolonged (3h) KCl or BDNF treatments led to a decrease of total pJNK in cortical neurons, both in the inhibitory and excitatory populations (Fig. 3e,f). Consistently, ectopic expression of MKP-1ASA both in the inhibitory and excitatory populations decreased pJNK, pSTMN1 and increased tubulin tyrosination (Supplementary Fig. 3), further supporting our idea that activity-dependent BDNF signaling via MKP-1 negatively influences JNK. The effect of KCl on pJNK was only partially blocked by TrkB.Fc, indicating that additional, BDNF-independent pathways also influence pJNK in response to neuronal activity.
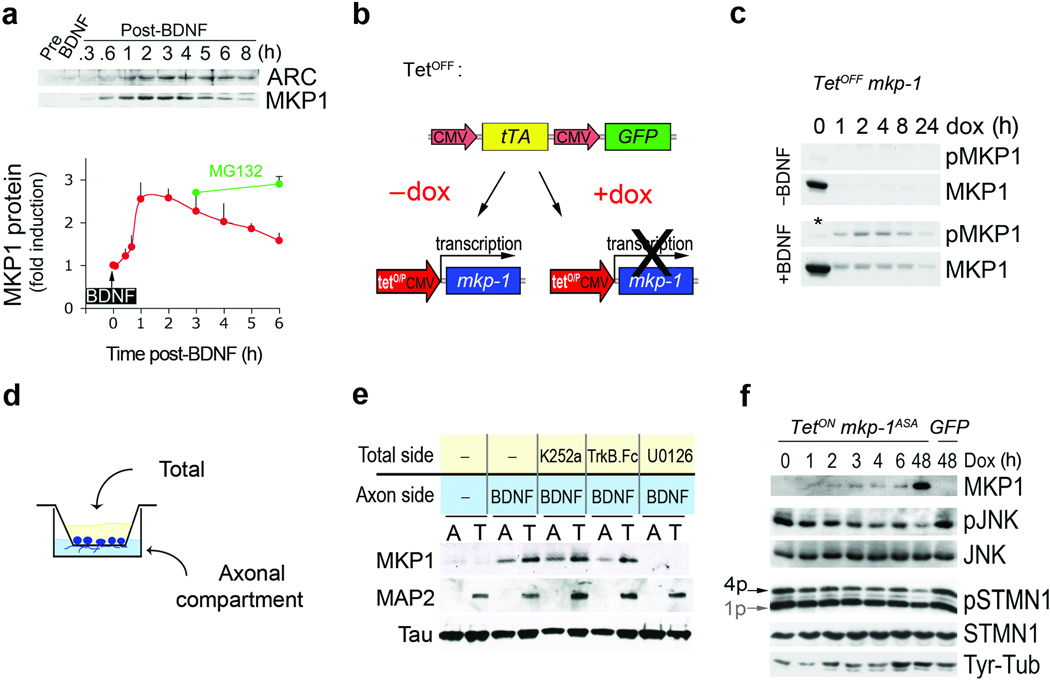
BDNF has well-established positive effects on axonal growth and branching1 that are at variance with the axonal defects observed upon a prolonged, global expression of MKP-1 (Fig. 2d,e). Therefore, we rationalized that BDNF effects must be transduced by MKP-1 in a tightly controlled spatio-temporal manner. In fact, BDNF elicited only transient expression of MKP-1 protein that peaked within the first 3 hours of treatment and subsequently declined (Fig. 4a). Previous reports described a molecular switch between phosphorylation and ubiquitination as a mechanism for prolonged MKP-1 protein stability32,33. Indeed, MKP-1 degradation was overcome by inhibition of the proteasome with MG132 (Fig. 4a; supplementary Fig. 4a,c). To address whether BDNF also affects MKP-1 phosphorylation and protein stability, we expressed MKP-1 in 293-TrkB cells using an inducible tetOFF system (Fig. 4b). In absence of doxycyclin (DOX), MKP-1 was expressed but no phosphorylation was observed (Fig. 4c). Addition of DOX to prevent MKP-1 expression led to a rapid loss of MKP-1 protein within 1 h (Fig. 4c, upper panel). However, if BDNF was added together with DOX, MKP-1 was phosphorylated and the turnover of the protein was delayed (Fig. 4c, lower panel; supplementary Fig. 5b). Further experiments showed that phosphorylation of some sites (S359, S364) mediated MKP-1 stability, whereas phosphorylation at other sites (S296, S323) enhanced turnover32,33. Mutation of all four residues (S296D, S323D, S359A, S364A) was required to render MKP-1 turnover insensitive to BDNF (supplementary Fig. 4b,5b). Therefore, MKP-1 ubiquitination and phosphorylation are not mutually exclusive (supplementary Fig. 4d). Taken together, BDNF controls MKP-1 expression by mediating both induction and stabilization of the protein.
Figure 4. BDNF controls MKP-1 expression in a spatio-temporal manner.
(a) A 5-min pulse treatment with 50 ng/ml BDNF induces transient expression of MKP-1. Arc is shown for comparison. MKP-1 protein levels were expressed as fold induction compared to pre-stimulation (mean + s.d., n=3). MG132 was added just after the BDNF pulse for the indicated times. (b) Scheme of the tetOFF system composed of 2 plasmids used to uncouple MKP-1 expression from BDNF signaling. (c) MKP-1 protein turnover was rapid but prolonged by BDNF-induced phosphorylation in 293-TrkB cells when MKP-1 induction was uncoupled from BDNF signaling. Addition of doxycyclin (DOX) turned off MKP-1 expression. The * indicates that the sample was not treated with BDNF. (d) Schematic representation of the setup used to physically separate axons from the somatodendritic compartment. (e) Representative Western blots of the material scraped from both sides of the filter membrane. Pre-treatment of the total side with K252a, TrkB.Fc or U0126 exerted distinct effects on retrograde BDNF signaling and MKP-1 induction. Tau and MAP2 are markers of the axons and somatodendritic compartments, respectively. (f) To uncouple MKP-1ASA induction from BDNF signaling we used a tetON system where DOX induced MKP-1 in cortical neurons infected at DIV1–5. 1p and 4p indicate the mono- and multi-phosphorylated forms of STMN1, respectively.
To address the spatial regulation of MKP-1 expression, we investigated whether distal BDNF signaling from the axon is sufficient to induce MKP-1 expression in neurons. To this end, we grew cortical neurons on porous membranes (Fig. 4d) to physically separate axons that cross through the pores to the lower side of the chamber (“axons”) from cell bodies, dendrites and proximal axons, that grow in the top compartment of the setup (“total”)34. To confirm the purity of the axonal preparation, we validated that the axonal marker tau was found in both compartments, while the dendritic marker MAP2 was restricted to the total compartment (Fig. 4e).
Administration of BDNF to the axonal compartment was sufficient to induce MKP-1 expression in both compartments (Fig. 4e). Neither the addition of TrkB.Fc nor of K252a to the total compartment abolished MKP-1 induction upon distal BDNF stimulation. In contrast, MKP-1 protein expression was blocked by pretreatment of the total compartment with U0126, suggesting that the signal relayed from the distal axon to the soma in order to upregulate MKP-1 protein involves pErk. Therefore, BDNF stimulation of axons induced MKP-1 expression throughout the neuron. Continuous distal BDNF signaling might locally stabilize MKP-1 through specific phosphorylation. Indeed, stabilization of neuronal MKP-1 protein strongly correlated with its phosphorylation, which was also sensitive to U0126 (supplementary Fig. 4a). Thus, BDNF can regulate MKP-1 in a spatio-temporal manner by controlling the induction of the protein and subsequently its local stabilization at the sites of BDNF signaling.
To test whether transient induction of MKP-1 is sufficient to inactivate JNK signaling and alter microtubule stability in cultured cortical neurons, we used an inducible tetON mkp-1ASA construct. We found that treatment with DOX produced transient induction of MKP-1ASA, which resulted in dephosphorylation of JNK and stathmin, and tyrosination of microtubules (Fig. 4f), confirming that temporally controlled MKP-1 activity was sufficient to destabilize cortical microtubules. However, transient (≤ 6h) and prolonged (48h) expression of MKP-1ASA differed in the amplitude of changes, which is reflected in the morphological outcome and therefore may reconcile the fact that BDNF induces both MKP-1 and axonal growth.
MKP-1 is required for BDNF-dependent axonal branching
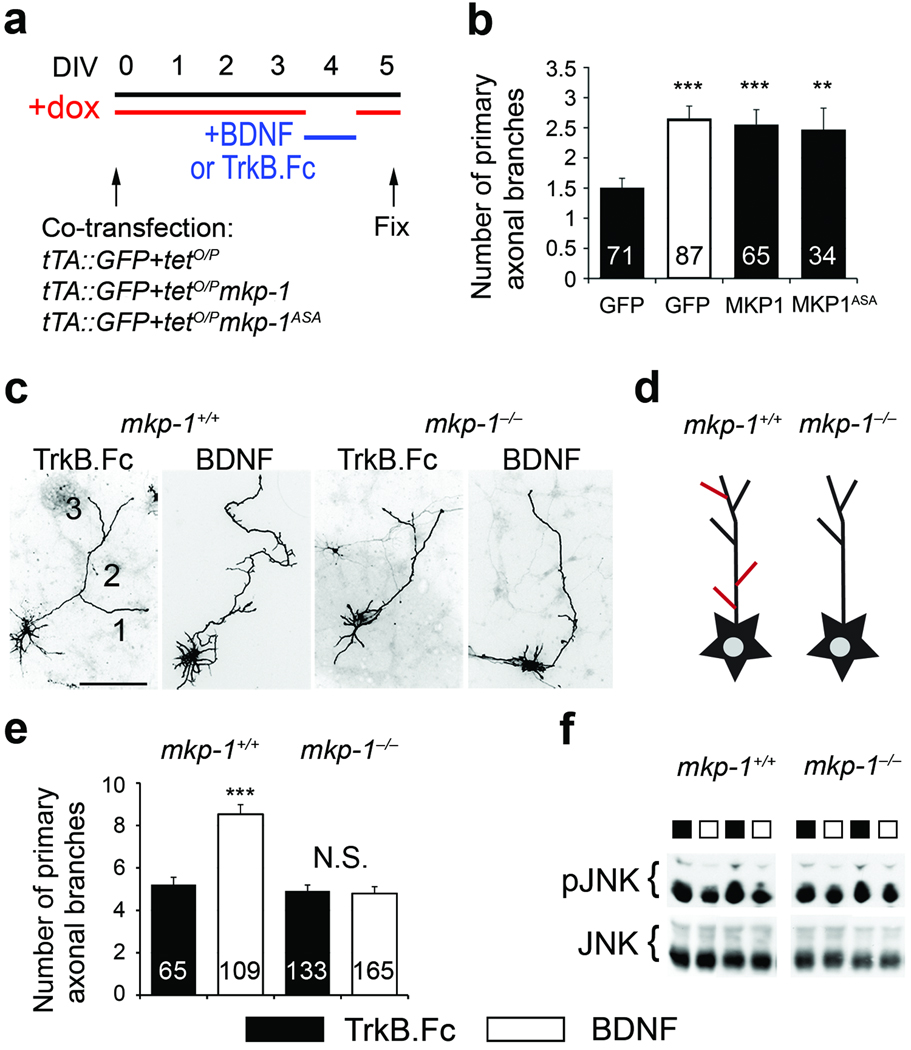
Microtubule destabilization has been implicated in axonal branching25. To test whether transient MKP-1 activity downstream of BDNF induces axonal arborization, we uncoupled MKP-1 activity from BDNF signaling by using a tetOFF system that allowed inducible MKP-1 expression, while constitutive GFP expression was used as a reporter (Fig. 4b). We electroporated cortical neurons with plasmids encoding mkp-1, mkp-1ASA or GFP and cultured them with DOX from DIV0 onward. MKP-1 expression was induced at DIV4 by withdrawal of DOX overnight and subsequent re-addition. During the time of MKP-1 expression, TrkB.Fc was applied to quench any endogenous BDNF. For the GFP control cells, BDNF was applied during the DOX washout to induce BDNF signaling. Finally, cells were grown for an extra day to consolidate newly formed branches (Fig. 5a). These conditions mimicked the transient nature of MKP-1 expression when induced by BDNF, and allowed us to compare BDNF signaling with brief MKP-1 expression independent of BDNF. The experimental timeline is consistent with previous studies investigating growth factor-induced axonal branch formation1,9. We found that activation of BDNF signaling increased the number of primary axonal branches when compared to the TrkB.Fc control (Fig. 5b). This is consistent with previous findings, where BDNF induced axon branching in the optic tectum in vivo1. Moreover, we observed that transient MKP-1 induction increased axonal branching to a similar extent as BDNF (Fig. 5b), despite the total loss of BDNF signaling by application of TrkB.Fc (Fig. 3c). This suggests that MKP-1 acts downstream of BDNF in axon branching. The MKP-1ASA mutant phenocopied the effect of wild-type MKP-1 (Fig. 5b), suggesting that axon arborization is mediated by deactivation of JNK signaling. To address the role of BDNF-dependent stabilization of MKP-1, we coupled BDNF treatment with the ectopic expression of wild type MKP-1 and mutant MKP-1DDAA, which lacks BDNF-dependent stabilization (supplementary Fig. 5b). Stimulation with BDNF increased axon branching on top of MKP-1 ectopic expression, an effect lost with the MKP-1DDAA mutant (supplementary Fig. 5c). Therefore, both the induction and stabilization of MKP-1 activity play a key role in shaping axon arbors and instructing branch formation.
Figure 5. MKP-1 mediates BDNF-induced axon branching.
(a) Timeline of the experimental procedure used to manipulate axonal branching in vitro. (b) Number of primary axonal branches expressed as mean ± s.e.m. GFP/TrkB.Fc is significantly different from MKP-1 (***, p=0.0004, n=6 experiments), MKP-1ASA (**, p=0.0046, n=3 experiments) and GFP/BDNF (***, p<0.0001, n=5 experiments) using a t-test. (c,e) Images (c) and quantification (e) of the number of primary branches in GFP-transfected cortical neurons derived from mkp-1−/− mice and their wildtype littermates. Results from 3 mkp-1+/+ and 6 mkp-1−/− embryos were expressed as mean±s.e.m. The number of cells analyzed is indicated in the bars. BDNF induces branching in wildtype neurons only (wildtype, TrkB.Fc vs BDNF: p=0.0036; mkp-1−/−, N.S, not significant, TrkB.Fc vs BDNF: p=0.547 using a t-test). Scale bar, 50 µm. (d) Scheme summarizing the effects on branching. Cultured neurons grow axons and branch (black outline). This branching can be enhanced by BDNF or KCl (red lines) in wildtype neurons, however, mkp-1 null neurons fail to increase arborization in response to stimulation. (f) While incubation with BDNF for 3 h decreases pJNK levels in wildtype neurons (see also Fig. 3e,f), it fails to do so in mkp-1−/− cells.
To ensure MKP-1 acts downstream of BDNF in axon branching instead of in a parallel pathway, we prepared cortical neurons from mkp-1−/− embryos35. We transfected these neurons with GFP, added KCl, BDNF or TrkB.Fc overnight (see Fig. 5a), and analyzed the number of primary axonal branches. In the presence of TrkB.Fc, there was no difference in axonal branching between cortical neurons derived from mkp-1−/− mice and those derived from their wildtype littermates (Fig. 5c,e). However, in contrast to wildtype neurons, mkp-1-deficient neurons showed no increase in primary branch formation in response to BDNF (Fig. 5c–e) or KCl (not shown). Moreover, unlike neurons derived from wildtype embryos, prolonged exposure to BDNF did not decrease pJNK in mkp-1−/− cells (Fig. 5f), further corroborating that MKP-1 inactivates JNK downstream of BDNF. Together, these results confirm that MKP-1 is a specific downstream target of BDNF signaling that is required for axonal branching decisions.
Discussion
In this study, we describe evidence for an intracellular mechanism of BDNF-dependent axonal branch formation (supplementary Fig. 6a,b). Our results indicate that BDNF, through TrkB-Erk signaling, induces the expression of the immediate early gene mkp-1. Distal stimulation of the axonal compartment of cortical neurons with BDNF was sufficient to induce MKP-1 in both the soma and axon. The ability of neurotrophins to signal over long distances is well established in peripheral neurons. Both NGF and BDNF are capable of inducing nuclear responses via long-range retrograde signaling. Ample evidence suggests that this signal is conveyed in the form of a signaling endosome, containing the activated ligand-receptor complex as well as associated signaling molecules such as MAPK36. Both retrograde and anterograde neurotrophin transport have been described for central neurons37, with anterograde BDNF transport from the cortex to the striatum being critical for maintaining striatal integrity38. Here we show that in addition to its anterograde function, a retrograde axonal BDNF signal in cortical neurons, involving pErk1/2, induces a nuclear response in form of immediate early gene expression.
Following induction, MKP-1 protein accumulates in the neuronal cytosol, but undergoes rapid turnover and degradation. However, sustained levels of MKP-1 exist at the site of BDNF-MAPK signaling by increasing its half-life. With no basal expression, high inducibility, rapid turnover (< 1h) and opportunity for stabilization, MKP-1 displays the necessary attributes to fine-tune the duration and amplitude of MAPK signaling at defined locations.
MKP-1 is a well-characterized phosphatase that can deactivate the three major classes of MAPK19. Here we show that the morphological and cytoskeletal effects of MKP-1 in neurons, which are phenocopied by the MKP-1ASA mutant, are mediated by dephosphorylation of JNK. The unique polarized localization of activated JNK along the axon despite its expression in every neuronal compartment20 makes it an attractive target for dynamic regulation through phosphatases. JNK signaling controls many features of the nervous system including apoptosis, regeneration and neuronal architecture39. Thus, dephosphorylation of axonal JNK represents a master molecular switch for many pleiotropic effects. The decision of where, when and for how long to deactivate JNK may provide functional specificity. Suppression of JNK activity by pharmacological inhibitors, dominant negatives or genetic ablation in various models suggests that JNK controls the formation and the maintenance of axons20,39,40. For example, axon outgrowth is impaired following inactivation of JNK by the reversible inhibitor SP600125, an effect that is annulled upon washout20. Therefore, the transient nature of MKP-1 induction ensures temporally controlled deactivation of JNK signaling that is compatible with BDNF-induced axonal remodeling.
BDNF induces axon branching and outgrowth, which are supported by stable microtubules. However, formation of nascent axon branches relies upon transient destabilization of microtubules. Several lines of evidence suggest that spatio-temporal regulation of a select group of JNK substrates, including stathmins and neurofilaments, can produce cytoskeletal remodeling. First, jnk1 knockout impairs the integrity of microtubules40. Second, stathmins are direct substrates of JNK27. Third, dephosphorylation of stathmins acts as an activation switch to destabilize microtubules41. Fourth, local dephosphorylation of SCG10, a stathmin family member, in the axon was observed in response to the morphogens ephrin B and laminin42. Fifth, SCLIP, another stathmin family member, regulates axon branching26. The transient induction of MKP-1 opens a signaling window during which BDNF trophic effects converge to controlled microtubule destabilization, and ultimately, axonal branch initiation (supplementary Fig. 6).
Ectopic expression of MKP-1 in vivo produced aberrant axon branching and innervation into the dorso-lateral striatum. Pyramidal LII–III neurons from the sensory cortex normally innervate the contra-lateral cortical areas43, and MKP-1 overexpressing neurons displayed a gain of collateral branches. BDNF levels in the cortex are low during embryonic and early postnatal development (Fig. 1a), nevertheless, TrkB loss-of-function during late embryogenesis impairs cortical progenitor function in vivo44. Therefore, we speculate that limited amounts of ectopic MKP-1 protein are stabilized during this time in vivo, which accounts for increased axonal branching rather than the drastic outgrowth defects observed in vitro.
Both endogenous BDNF and MKP-1 expression are upregulated in the brain at later stages of development, when neuronal networks are refined in an activity-dependent manner. The formation and maturation of axonal arbors is tightly coupled with increased synaptic connectivity, and both processes are positively modulated by BDNF45. Furthermore, plasticity of neuronal networks is relayed by MAPK signaling46 and neuronal activity can induce MKP-1 expression. Therefore, MKP-1 downstream of BDNF may be an important regulator of synaptogenesis, and misregulation of this phosphatase may have deleterious effects on plasticity-dependent processes such as learning and memory. Altogether, our results provide a framework for studying the molecular mechanisms of cytoskeletal remodeling under normal and pathological conditions.
Materials and Methods
Animals
Time-pregnant Sprague-Dawley rats, CD1 mice (Charles River Laboratories), Dlx5/6-Flpe and RCE:FRT transgenic mice30 and mkp-1−/− mice47 were allowed ad libitum access to food and water, and maintained on a 12h light/dark cycle. Genotyping was performed as previously described47. All protocols complied with the National Institute of Health guide for the Care and Use of Laboratory Animals.
Reagents
Trophic factors were obtained from PeproTech. Small molecule inhibitors were purchased from: U0126 (Cell Signaling), K252a and SP600125 (Calbiochem), cycloheximide and MG132 (Sigma). Doxycycline and TrkB.Fc were from Clontech and R&D Systems, respectively. Antibodies used were as follows: Arc (H300), MKP-1 (M18), Erk 1/2, JNK, STMN1 and pSTMN1 (S16-P or S25-P or S63-P) were from Santa Cruz Biotechnology; pMKP-1 (S359/S364-P), pJNK (T183/Y185-P), pERK (T202/Y204-P), pP38 (T180/Y182-P), were from Cell Signaling; Flag, actin, tubulin, pErk5 (T218/Y220-P), tyrosinated-tubulin, acetylated-tubulin were from Sigma–Aldrich; GFP and pMKP-1 (S296-P) were from Abcam; HSP90, MKP2, GAD65 and Tau1 were from BD Bioscience; MeCP2 and detyrosinated-tubulin were from Millipore; MAP2 was from Covance; GAPDH was from Biodesign; and pTrkB antibodies were described elsewhere48.
Plasmids
The coding sequences for the mouse MKP-1 (obtained from Y.S. Lee) was fused to a N-terminal 3×Flag tag. Flag-MKP-1 was further subcloned into FCIV1, a lentiviral plasmid with a GFP reporter. For the TetOff system, the plasmid ratio for co-transfection was 1:10 (pUHD15.1:pTRE2, from Addgene). The pUHD15.1 was modified by inserting a CMV-GFP reporter cassette (tTA::GFP) and the Flag-MKP-1 sequence was subcloned into the pTRE2 vector (tetO/PMKP-1). For the TetON system, the MKP-1 sequence was first subcloned into the pENTRY and further recombined into the pSLIK-venus plasmid according to manufacturer’s instructions (ATCC). Flag-JNK isoforms were purchased from Addgene. Two mutations (R65S and D319N) previously described23 in Erk1/2 as providing insensitivity to MKP-3 were introduced into the JNK sequence (R69S and D326N). To restrict MKP-1 substrate specificity, the RRR motif in the MAPK binding domain of MKP-1 was replaced by ASA triple mutation21. Alanine residues were introduced in place of Ser359 and Ser364 whereas glutamate residues were inserted in place of Ser296 and Ser323 to produce phospho-mimicking mutations. Site-directed mutagenesis was performed using the QuickChange kit from Stratagene. All constructs were verified by sequencing.
Short hairpin (ShRNA) sequences were inserted into the pLentiLox3.7 vector (ATCC) using 5’-ttcaagaga-3’ as a loop sequence. Although ShRNA #6 and ShRNA #7 anneals to a similar region in the mkp-1 gene, only the ShRNA #7 produced knockdown. Therefore, we used ShRNA #6 as a control. The ShRNA sequences used are as following: Sh#3, 5’-gctggtccttatttattta-3’; Sh#4, 5’-gctccactcaagtcttctt-3’; Sh#5, 5’-ccaattgtcctaaccactt-3’; Sh#6, 5’-gtcttcttcctccaaggagga-3’; Sh#7, 5’-gctccactcaagtcttcttcc-3’.
Electroporation
In utero electroporation methodology has been previously described49. Briefly, DNA was injected in the ventricles of E15–E15.5 CD1 mouse embryos and the mice developed to P7, when neuronal migration in the cortical plate is accomplished. In vitro electroporation of the tetOFF plasmids tetO/P MKP-1:tTA::GFP with a 10:1 ratio was achieved with the AMAXA system according to manufacturer’s instructions.
Immunohistochemistry
Following transcardiac perfusion with 4% ice cold PFA, the brains were post-fixed for 24h and equilibrated in 30% sucrose solution for another day. Free-floating coronal sections rinsed in PBS were blocked in 5% normal goat serum /5% normal horse serum/ PBS/ 0.1% TritonX-100 for 1h at 25°C. Primary antibodies (anti-GFP, 1:2000) were incubated for 48h at 4°C with shaking. Alexa Fluor-conjugated secondary antibodies (Molecular Probes) were incubated for 1h at 25°C. Sections were imaged using a LSM510 laser-scanning confocal microscope equipped with 4×, 10× and 20× objectives (Carl Zeiss Microimaging). Images were processed using LSM510 software (Zeiss) and ImageJ (NIH).
Cell culture
Primary cortical neurons from embryonic day 18 rats or E15–E18 mice were prepared from timed-pregnant Sprague–Dawley rats and transgenic mice as described previously48. GFP-positive interneurons were separated from GFP-negative excitatory neurons using fluorescence-activated cell sorting, and both populations were cultured separately as described below. There were 11% of GFP-positive cells within a standard preparation of E18 primary cortical neurons. Primary neurons were cultured on glass coverslips coated with poly-D-lysine, maintained in Neurobasal media containing B27 supplement, 0.5 mM l-glutamine, 5-fluoro-uridine, and uridine (10 µM each). 293FT and 293-TrkB cells (Invitrogen) were grown in DMEM containing 10% FBS plus 200 µg/ml G418.
Transfection and infection
Neurons were transfected at DIV1 using Lipofectamine2000 as described previously48. For all biochemical analysis, cells were infected with lentivirus. Viruses were produced by transfecting packaging plasmids into 293FT cells. The set of plasmids used were Δ8.9, pCMV-VSVG, and FCIV1. Media were collected after 48h and diluted 1:4 with regular culture medium to infect cells at DIV0.
Cell lysis
Neurons were lysed in 10mM Tris-HCl, pH 8.0/ 150mM NaCl/ 1mM EDTA/ 10% glycerol/ 1% NP40/ 0.1% SDS complemented with proteases inhibitors, 1 mM Na3VO4, and 10mM NaF. Cleared lysates (14,000 rpm 10 min) were analyzed by Western blot. To purify nuclear extracts, neurons were rinsed in ice cold PBS and incubated for 15 min on ice with buffer A (10mM HEPES-KOH, pH 7.9, 10mM KCl, 0.1mM EDTA, 0.1mM EGTA, 1mM DTT with protease and phosphatase inhibitors) before harvesting. 0.5% NP40 was added to the cells for 3 min on ice and lysates were centrifuged for 1 min at 13000 rpm. The resulting supernatant was stored as the cytoplasmic fraction and the pellet was further rinsed in 1 ml of buffer A. The pellet was vortexed for 20 min in 50ml buffer B (20mM HEPES-NaOH, pH 7.9, 0.4mM NaCl, 1mM EDTA, 1mM EGTA with protease and phosphatase inhibitors) and cleared by centrifugation for 10 min at 13000 rpm. The resulting supernatant was collected as the nuclear fraction.
Immunofluorescence and microscopy
Cells were fixed at indicated days in vitro (DIV) in 4% PFA, 20% sucrose in PBS for 10 min at room temperature (RT). Fixative was quenched with 50mM NH4Cl in PBS, cells were permeabilized with 0.1% TritonX-100 in PBS for 3 min, blocked with 10% goat serum, 2% BSA, 0.25 % fish skin gelatin in TBS for 30 min and then incubated with the relevant antibodies (anti-FLAG, 1:3000; anti-GFP, 1:3000; secondary antibodies, 1:1000) for 30 min at RT in blocking solution. Cells were washed in TBS, 0.25% fish skin gelatin and mounted in Mowiol488. Images were taken on an EclipseE800 microscope equipped with a 20×, Plan Apo NA 0.75 Phase 2 objective (both Nikon Instruments), FITC and TRITC filters (HQ96170M and HQ96171M, Chroma Technology Corp) and an Axiocam HR camera using Axiovision software (both Carl Zeiss Vision GmBH).
Images stacks were processed using ImageJ (NIH). Neurite length was quantified and axon branches traced using the NeuronJ plugin50, and axonal arborization was measured by counting the number of primary branches extending from the longest neurite. All image analysis was performed blinded to the condition.
Statistics
Statistical analysis (GraphPad Prism software, version 4.0) was carried out using one-way analysis of variance with paired Student t-test. Statistical significance was defined at P<0.05.
Supplementary Material
Acknowledgements
This research was supported by NARSAD (FJ and GM), the Human Frontier Science Program Organization (KD), and grants from the NIH to MVC (NS21072 and HD23315) and AMB (R01 AR46504).
Abbreviations
- MAPK
Mitogen-activated protein kinase
- MKP
MAPK phosphatase
- BDNF
brain-derived neurotrophic factor
- Trk
tropomyosin related kinase
- tet
tetracycline
- ERK
extracellular related kinase
- Arc
activity-regulated cytoskeleton-associated protein
- STMN
stathmin
- JNK
c-jun N-terminal kinase
Footnotes
Author contributions
F.J. and K.D. designed, performed and analyzed experiments. F.J., K.D. and M.V.C. wrote the manuscript. G.M. helped with the in utero electroporations. G.M. and A.M.B. provided mice and reagents.
References
- 1.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 2.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 3.Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang JK, Dorey K, Ishibashi S, Amaya E. BDNF promotes target innervation of Xenopus mandibular trigeminal axons in vivo. BMC Dev Biol. 2007;7:59. doi: 10.1186/1471-213X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danzer SC, Crooks KR, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci. 2002;22:9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez A, et al. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Van Aelst L, Cline HT. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat Neurosci. 2000;3:217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- 8.Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J Neurosci. 2008;28:143–153. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 11.Chao MV., q Growth factor signaling: where is the specificity? Cell. 1992;68:995–997. doi: 10.1016/0092-8674(92)90068-n. [DOI] [PubMed] [Google Scholar]
- 12.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 13.Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992;359:644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- 14.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi M, et al. Light-inducible and clock-controlled expression of MAP kinase phosphatase 1 in mouse central pacemaker neurons. J Biol Rhythms. 2007;22:127–139. doi: 10.1177/0748730406298332. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco D, Bravo R. Expression of the nontransmembrane tyrosine phosphatase gene erp during mouse organogenesis. Cell Growth Differ. 1993;4:849–859. [PubMed] [Google Scholar]
- 17.Maisonpierre PC, et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 18.Langevin LM, et al. Validating in utero electroporation for the rapid analysis of gene regulatory elements in the murine telencephalon. Dev Dyn. 2007;236:1273–1286. doi: 10.1002/dvdy.21126. [DOI] [PubMed] [Google Scholar]
- 19.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 20.Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci. 2006;26:9462–9470. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem. 2001;276:16491–16500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- 22.Camps M, et al. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science. 1998;280:1262–1265. doi: 10.1126/science.280.5367.1262. [DOI] [PubMed] [Google Scholar]
- 23.Levin-Salomon V, Kogan K, Ahn NG, Livnah O, Engelberg D. Isolation of intrinsically active (MEK-independent) variants of the ERK family of mitogen-activated protein (MAP) kinases. J Biol Chem. 2008;283:34500–34510. doi: 10.1074/jbc.M806443200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 26.Poulain FE, Sobel A. The "SCG10-LIke Protein" SCLIP is a novel regulator of axonal branching in hippocampal neurons, unlike SCG10. Mol Cell Neurosci. 2007;34:137–146. doi: 10.1016/j.mcn.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Tararuk T, et al. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J Cell Biol. 2006;173:265–277. doi: 10.1083/jcb.200511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glorioso C, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- 29.Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi G, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 33.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–926. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 34.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorfman K, et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 36.Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 37.DiStefano PS, et al. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 38.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waetzig V, Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol Sci. 2005;26:455–461. doi: 10.1016/j.tips.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 41.Curmi PA, et al. Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin. Cell Struct Funct. 1999;24:345–357. doi: 10.1247/csf.24.345. [DOI] [PubMed] [Google Scholar]
- 42.Suh LH, Oster SF, Soehrman SS, Grenningloh G, Sretavan DW. L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10. J Neurosci. 2004;24:1976–1986. doi: 10.1523/JNEUROSCI.1670-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise SP, Jones EG. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J Comp Neurol. 1976;168:313–343. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- 44.Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134:4369–4380. doi: 10.1242/dev.008227. [DOI] [PubMed] [Google Scholar]
- 45.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 46.Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- 47.Wu JJ, et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006;4:61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci U S A. 2008;105:4862–4867. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- 50.Meijering E, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.