Abstract
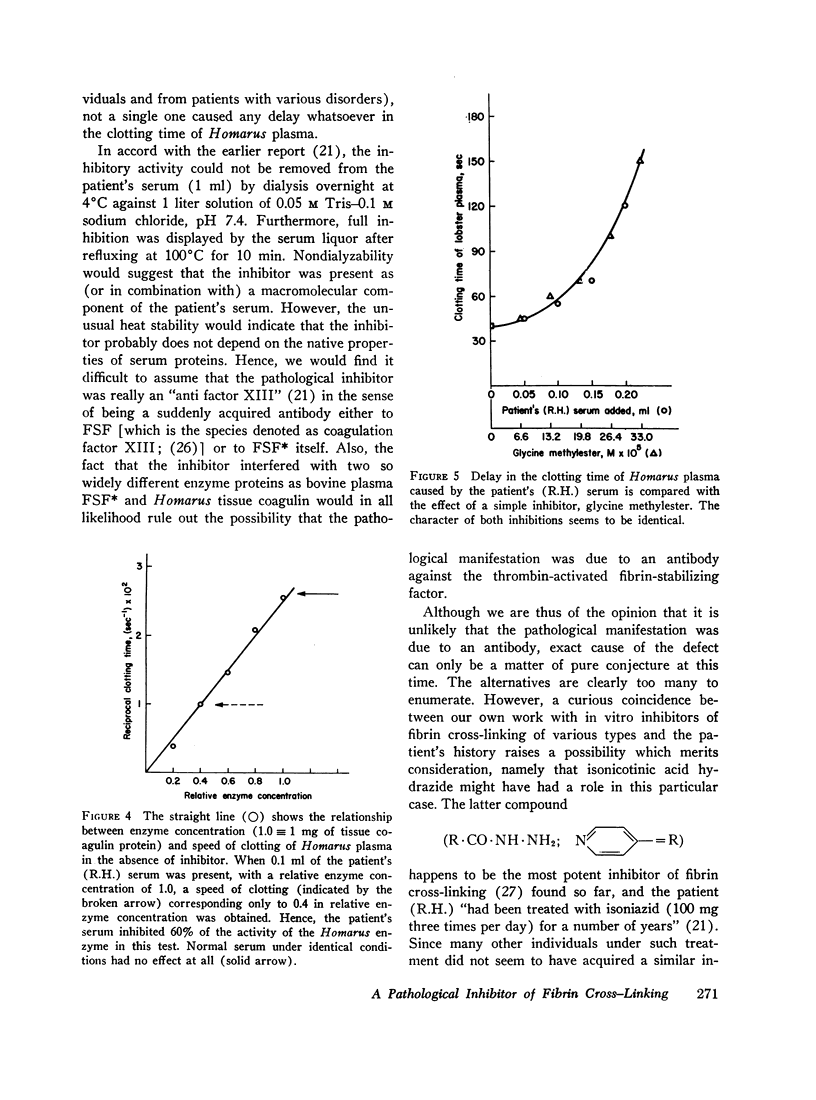
Lewis et al. recently reported on a patient who died of hemorrhages attributable to an acquired inhibitor of fibrin-stabilizing factor. They indicated that the inhibitor was associated with the immune globulins. Using the postmortem serum in the isolated fibrin cross-linking system, we have now further localized the site of inhibition in the scheme of blood coagulation. The interference occurs at the transpeptidation step catalyzed by the thrombin-activated fibrin-stabilizing factor. The patient's serum also uniquely delayed the clotting time of Homarus plasma, a test for specific inhibitors of transpeptidation. Since the inhibitor was effective in two such widely different systems, it probably is not an antibody, but falls into the category of cross-linking inhibitors which we have previously described (4, 5, 10, 12-17). While the exact nature of the inhibitor remains unknown, we raise the question whether some unusual metabolic transformation of isonicotinic acid hydrazide (with which the patient was treated and which itself we found to be a potent inhibitor fibrin cross-linking), in combination with a macromolecule, might not have given rise to an inhibitory compound.
Full text
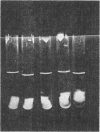
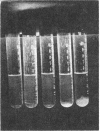
PDF
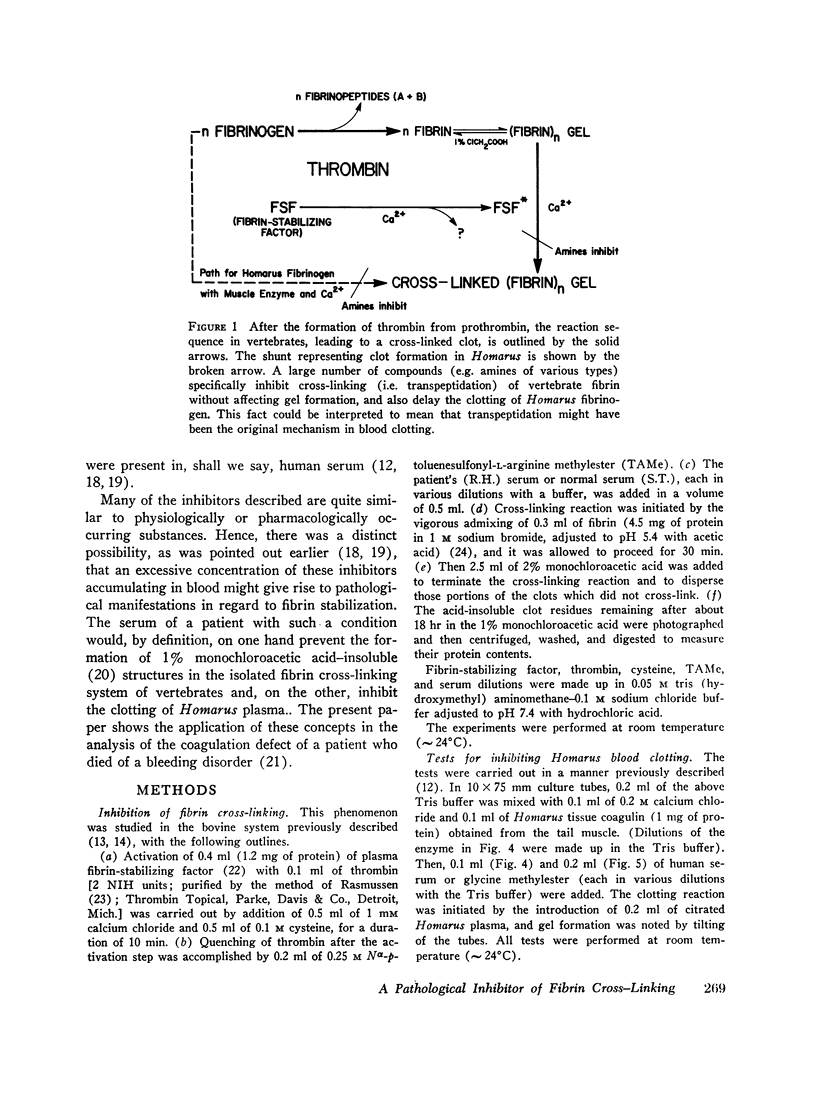
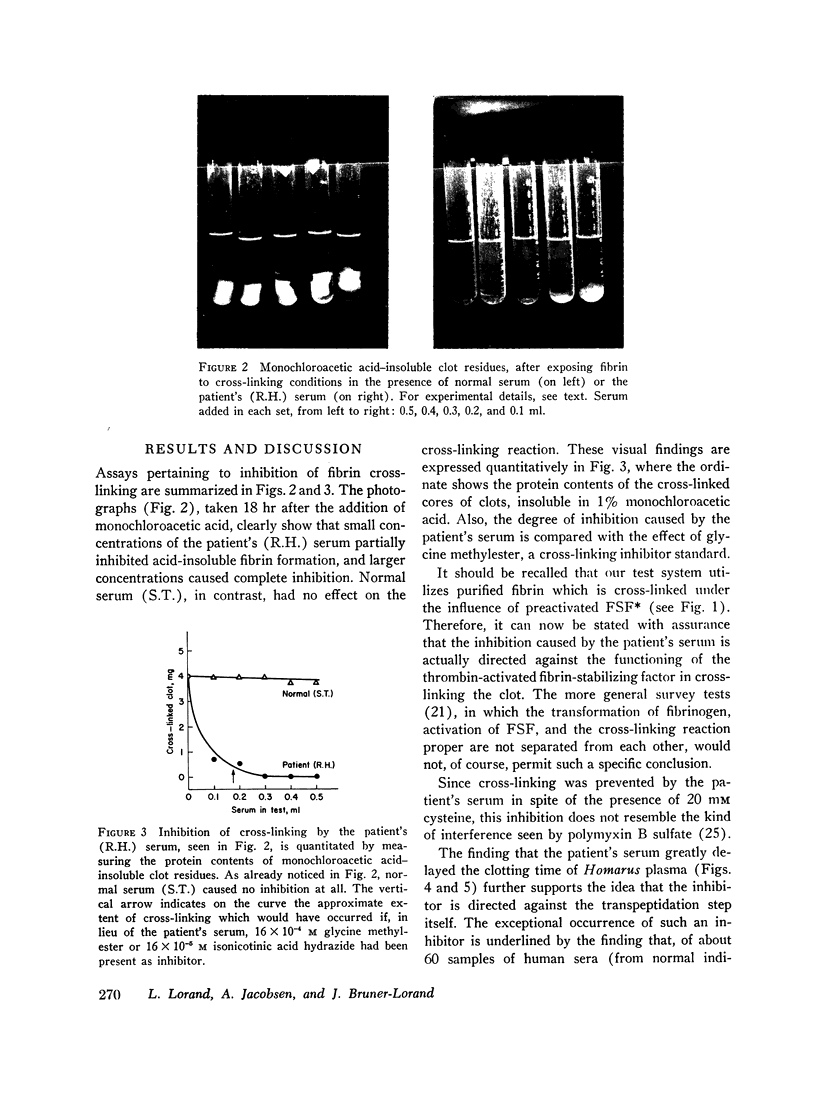


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETTELHEIM F. R., BAILEY K. The products of the action of thrombin on fibrinogen. Biochim Biophys Acta. 1952 Nov;9(5):578–579. doi: 10.1016/0006-3002(52)90213-8. [DOI] [PubMed] [Google Scholar]
- DONNELLY T. H., LASKOWSKI M., Jr, NOTLEY N., SCHERAGA H. A. Equilibria in the fibrinogen-fibrin conversion. II. Reversibility of the polymerization steps. Arch Biochem Biophys. 1955 Jun;56(2):369–387. doi: 10.1016/0003-9861(55)90258-7. [DOI] [PubMed] [Google Scholar]
- Konishi K., Lorand L. Separation of activated fibrin-stabilizing factor from thrombin. Biochim Biophys Acta. 1966 May 26;121(1):177–180. doi: 10.1016/0304-4165(66)90367-9. [DOI] [PubMed] [Google Scholar]
- LOEWY A. G., VENEZIALE C., FORMAN M. Purification of the factor involved in the formation of urea-insoluble fibrin. Biochim Biophys Acta. 1957 Dec;26(3):670–671. doi: 10.1016/0006-3002(57)90133-6. [DOI] [PubMed] [Google Scholar]
- LORAND L. 'Fibrino-peptide'; new aspects of the fibrinogen-fibrin transformation. Nature. 1951 Jun 16;167(4259):992–993. doi: 10.1038/167992a0. [DOI] [PubMed] [Google Scholar]
- LORAND L., DOOLITTLE R. F., KONISHI K., RIGGS S. K. A NEW CLASS OF BLOOD COAGULATION INHIBITORS. Arch Biochem Biophys. 1963 Aug;102:171–179. doi: 10.1016/0003-9861(63)90168-1. [DOI] [PubMed] [Google Scholar]
- LORAND L. Fibrin clots. Nature. 1950 Oct 21;166(4225):694–695. doi: 10.1038/166694a0. [DOI] [PubMed] [Google Scholar]
- LORAND L. Fibrino-peptide. Biochem J. 1952 Oct;52(2):200–203. doi: 10.1042/bj0520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAND L., JACOBSEN A. SPECIFIC INHIBITORS AND THE CHEMISTRY OF FIBRIN POLYMERIZATION. Biochemistry. 1964 Dec;3:1939–1943. doi: 10.1021/bi00900a026. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K. ACTIVATION OF THE FIBRIN STABILIZING FACTOR OF PLASMA BY THROMBIN. Arch Biochem Biophys. 1964 Apr;105:58–67. doi: 10.1016/0003-9861(64)90235-8. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K., JACOBSEN A. Transpeptidation mechanism in blood clotting. Nature. 1962 Jun 23;194:1148–1149. doi: 10.1038/1941148a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. H., Szeto I. L., Ellis L. D., Bayer W. L. An acquired inhibitor to coagulation factor 13. Johns Hopkins Med J. 1967 Jun;120(6):401–407. [PubMed] [Google Scholar]
- Lorand J. B., Pilkington T. R., Lorand L. Inhibitors of fibrin cross-linking: relevance for thrombolysis. Nature. 1966 Jun 18;210(5042):1273–1274. doi: 10.1038/2101273a0. [DOI] [PubMed] [Google Scholar]
- Lorand J. B., Urayama T., Lorand L. Transglutaminase as a blood clotting enzyme. Biochem Biophys Res Commun. 1966 Jun 21;23(6):828–834. doi: 10.1016/0006-291x(66)90562-6. [DOI] [PubMed] [Google Scholar]
- Lorand L., Jacobsen A. Isonicotinic acid hydrazide as an inhibitor of transpeptidation: relevance for blood coagulation. Nature. 1967 Nov 4;216(5114):508–509. doi: 10.1038/216508b0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Ong H. H. Labeling of amine-acceptor cross-linking sites of fibrin by transpeptidation. Biochemistry. 1966 May;5(5):1747–1753. doi: 10.1021/bi00869a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Ong H. H. Studies on fibrin crosslinking. Nature of the acceptor groups in transpeptidation. Biochem Biophys Res Commun. 1966 Apr 19;23(2):188–193. doi: 10.1016/0006-291x(66)90526-2. [DOI] [PubMed] [Google Scholar]
- Lorand L. Physiological roles of fibrinogen and fibrin. Fed Proc. 1965 Jul-Aug;24(4):784–793. [PubMed] [Google Scholar]
- Marshall F. N., Massad E. N. Interference with fibrin stabilization by polymyxin B sulfate. Proc Soc Exp Biol Med. 1967 Jun;125(2):420–423. doi: 10.3181/00379727-125-32109. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN P. S. Purification of thrombin by chromatography. Biochim Biophys Acta. 1955 Jan;16(1):157–158. doi: 10.1016/0006-3002(55)90194-3. [DOI] [PubMed] [Google Scholar]