Abstract
Neuropeptide Y (NPY) is a 36-amino-acid neuromodulator that is distributed throughout the central nervous system and has been implicated in a wide range of neurobiological responses including the integration of emotional behavior. The anxiolytic properties of NPY are modulated by NPY signaling in the hippocampus and in the central (CeA) and basolateral (BLA) nuclei of the amygdala. Recently, the neurotoxin saporin, when conjugated to NPY (NPY-SAP), was shown to selectively kill NPY receptor-expressing neurons and has been used as a tool to study the central NPY neurocircuitry involved with feeding behaviors. Here we determined if NPY-SAP can be used as a tool to study the central NPY neurocircuitry that modulates anxiety-like behaviors. BALB/cJ mice were given injection of either NPY-SAP or a control blank saproin (B-SAP) into the CeA or the basomedial hypothalamus (BMH) as a control injection site. The elevated zero maze test was used to assess anxiety-like behavior and NPY-SAP-induced lesions were verified using NPY Y1 receptor (Y1R) immunoreactivity (IR). Results showed that injection of NPY-SAP into the CeA site-specifically blunted Y1R IR in the CeA which was associated with a significant increase in anxiety-like behavior. Injection of NPY-SAP into the BMH, while locally blunting Y1R IR, promoted a compensatory increase of Y1R IR in the BLA and the CA3 region of the hippocampus which was associated with a significant reduction of anxiety-like behavior. The present set of experiments suggest that the NPY-SAP neurotoxin may be a useful tool for studying the NPY neurocircuitry that modulates anxiety-like behaviors.
Keywords: Neuropeptide Y, Anxiety-like Behavior, Amygdala, Hippocampus, Saporin, BALB/cJ
1. Introduction
Neuropeptide Y (NPY) is a 36-amino-acid neuromodulator that is widely distributed throughout the nervous system [7]. NPY entails anxiolytic properties, first revealed by the observation that central infusion of NPY attenuated anxiety-like behavior in rodents [13]. This was followed by a study demonstrating that site-directed infusion of NPY into the central nucleus of the amygdala (CeA) blunted anxiety-like behavior in rats without altering food intake [12]. NPY signaling in the hypothalamus, on the other hand, modulates feeding behaviors (e.g., [26, 28]). More recently, NPY signaling in the basolateral nucleus of the amygdala (BLA) and the hippocampus have been shown to also reduce anxiety-like behaviors [17, 23, 27, 29]. Mutant mice lacking the NPY Y1 receptor (Y1R) show elevated anxiety-like behavior [14], and blockade of Y1R in the amygdala increased anxiety-like behaviors [21], implicating the Y1R in the modulation of anxiety.
In the present experiment, we further explored the role of NPY signaling in the modulation of anxiety-like behavior in BALB/cJ mice, a strain that has been shown to be highly reactive to the effects of stress and exhibits high levels of anxiety-like behavior [2, 4]. Our main goal was to determine if the neurotoxin saporin, when conjugated to NPY (NPY-SAP), could be used as a tool to help define the NPY neurocircuitry that modulates anxiety-like behavior. Saporin is a type 1 ribosomal inactivating protein [9] which kills specific populations of neurons by conjugation with proteins that are selectively internalized by the targeted cells [24, 30]. NPY-SAP has been shown to selectively kill NPY receptor-expressing neurons (e.g., [3, 5]) and has been used as a tool to study the central NPY neurocircuitry involved with feeding and foraging behaviors [3, 5, 16, 22]. The usefulness of NPY-SAP as a tool to study the role of central NPY in anxiety-like behavior has not been established. In Experiment 1, we injected NPY-SAP into the CeA, a region in which NPY injection has been shown to produce anxiolytic effects in rodents [12], and subsequently measured behavior on an elevated zero maze, a procedure that is analogous to the elevated plus maze and which is commonly used to assess anxiety-like behavior in rodents [8]. The elevated zero maze has been validated as a test of anxiety from observations that anxiolytic drugs increase open area time in the maze, while anxiogenic drugs reduce open area time [25]. To determine the possibility the treatment with NPY-SAP produced non-specific effects on motor behavior, consummatory behavior and open-field activity were assessed. Lesions were verified by subsequent assessment of Y1R immunoreactivity (IR). As a control for site-specificity, in Experiment 2 a second set of mice were injected with NPY-SAP into the basomedial hypothalamus (BMH), an area in which NPY signaling has been linked to feeding behaviors [3].
2. Methods
2.1. Animals and housing conditions
Male BALB/cJ mice (Jackson Laboratory, Bar Harbor ME) were used in all experiments. Mice were 6–8 weeks old, weighed between 25–30 g at the start of the experiments, and were single housed in polypropylene cages with corncob bedding and ad libitum access to food and water. Standard rodent chow (Teklad, Madison, WI) and water were available at all times except where noted during experimental procedures. The animal facility was maintained at a temperature of 22° C with a 12-hour/12-hour light-dark cycle with lights out at 6:00 p.m. All experimental procedures were approved by the University of North Carolina Animal Care and Use Committee (IACUC) and complied with the NIH Guide for Care and Use of Laboratory Animals (National Research Council, 1996).
2.2. Surgery
Mice were anesthetized with an intraperitoneal (i.p.) injection of a ketamine and xylazine mixture (100 mg/ml and 20 mg/ml, respectively). Using a 33-gauge injection needle, mice received bilateral infusions of NPY-SAP (48 ng/500 nl per side over a 5 minute injection) into the CeA (Experiment 1) or BMH (Experiment 2). Injection dose was based on previous work which showed that this dose significantly reduced Y1R IR in the BMH [3]. Control mice were given injection of blank saporin (B-SAP) in the same dose and volume. B-SAP is a control conjugate of saporin with a non-targeted peptide with no known binding site or biological function, and has the same molecular weight as NPY-SAP. NPY-SAP and B-SAP were obtained from Advanced Targeting Systems, San Diego, CA. The stereotaxic coordinates that were used for the CeA were 1.5 mm posterior to bregma, ± 2.8 mm lateral to midline, and 4.4 mm ventral to skull surface. The stereotaxic coordinates that were used for the BMH were 1.5 mm posterior to bregma, ± 0.4 mm lateral to midline, and 5.5 mm ventral to skull surface. Mice were given 10 days of recovery and to allow time for the saporin to induce lesions.
2.3. Elevated zero maze testing
Ten days after surgery, mice were transported from their vivarium to a room immediately adjacent to the testing room and allowed to habituate for at least 30 minutes before testing began. The elevated zero maze (Hamilton-Kinder, Poway, CA) was positioned in the center of a room below a ceiling-mounted lamp fitted with a single 25-watt red light bulb which provided the only light for the room. Each mouse was individually removed from its home cage and immediately placed just inside a closed area of the elevated zero maze with its nose pointing into the closed area section. The 5 minute test session was video recorded with a tripod-mounted camcorder to eliminate the need for an investigator’s presence in the testing room. Sessions were scored by treatment-blind investigators for time spent in open or closed areas (sec), and the number of open and closed area entries. An animal was considered to have entered the open area if all four paws had left the closed area. Open area time was considered terminated once all 4 paws were placed back into the closed area.
2.4. Assessment of consummatory behavior and open-field testing
To determine the potential effects of NPY-SAP treatment on general motor activity, consummatory behavior (food, water, and 10% (v/v) ethanol intake) and open-field locomotor activity were analyzed in a subset of mice that were used in the elevated zero maze test (the remaining mice were used in procedures that involved stress exposure subsequent to elevated zero maze testing and thus were not included in the present analysis). Consummatory measures were collected over 6 weeks. This was followed by open-field testing in which mice were transported to the testing room and allowed to habituate for at least 30 minutes. Mice were placed into the center of an open-field arena that automatically recorded activity via photo beam breaks (Harvard Apparatus, Inc., Holliston, MA). The open field arena measured 40.64 cm by 40.64 cm by 30.48 cm and was made of clear Plexiglas. Testing sessions were 60 minutes in duration and the chambers were cleaned with isopropyl ethanol wipes after each session. Total distance traveled (cm) was measured over the course of the session.
2.5. Immunohistochemistry (IHC) procedures
Upon completion of the study (approximately two months after receiving infusion of NPY-SAP or B-SAP), mice received an i.p. injection of a ketamine/xylazine mixture (100 mg/ml and 20 mg/ml, respectively) and were then perfused within 10 minutes transcardially with 0.1mM phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde in phosphate buffer. Mice were perfused in pairs and counterbalanced by group. Brains were collected and postfixed in paraformaldehyde for 48 hours at 4°C, at which point they were transferred to PBS. Using a vibrotome, mouse brains were sliced into 40 μm sections and stored in PBS until IHC procedures. The sections were then rinsed in PBS 3 times (10 minutes each). Sections were blocked in 10% goat serum and 0.1% triton-X-100 in PBS for 1 hour. Sections were then transferred to fresh PBS containing primary rabbit Y1 receptor antibody (1:25,000) for 72 hours at 4°C. Antibody 96106 raised against NPY Y1R was provided by CURE/Digestive Disease Research Center, Antibody/RIA Core, NIH Grant #DK41301 (Los Angeles, CA). After primary incubation, brain sections were rinsed 3 times with PBS and processed with Vectastain Elite Kits (Vector Labs, Burlingame, CA) as per manufacturer’s instructions. Sections were visualized by a reaction with 3,3′-diamino-benzidine (DAB, Polysciences Inc, Warrington, PA) in a reaction solution containing 0.05% DAB, 0.005% cobalt, 0.007% nickel ammonium sulfate, and 0.006% hydrogen peroxide. Sections were mounted on glass slides, air-dried, and cover slipped.
Digital images of Y1R IR were taken in candidate brain regions using a Nikon E400 microscope with a Nikon Digital Sight DS-U1 digital camera run with Nikon provided software. For analysis, great care was taken to match sections through the same region of brain and the same level using anatomical landmarks with the aid of a mouse stereotaxic atlas [10]. Densitometric procedures were used to assess protein levels. Flat-field corrected digital pictures (8-bit grayscale) were taken and density of staining was analyzed using Image J software (Image J, National Institute of Health, Bethesda, MD) by calculating the percent of the total area examined that showed signal (cell bodies and processes) relative to a subthreshold background. The size of the areas that were analyzed were the same between animals and groups. The subthreshold level for the images was set in such a way that any area without an experimenter defined level of staining (determined by terminal- and/or soma-positive regions) was given a value of zero. Within each region, the same subthreshold level was used for each slice that was scored. Data from each brain region in an animal were calculated by taking the average counts from the left and right sides of the brain at the specific brain region of interest. For each brain region, photographs were taken at approximately the medial area of the structure (with respect to the rostral-caudal axis). In all cases, quantification of immunohistochemistry data was conducted by an experimenter that was blinded to group identity. For some brain regions, representative slices were not available for 1–2 mice, which is reflected in the degrees of freedom in analysis described below.
2.6. Data analysis
All data is presented as mean ± SEM. For elevated zero maze and open-field locomotor activity data, differences between groups were analyzed using independent-sample t-tests. Consummatory data were analyzed using 2 × 6 (saporin treatment × average daily consumption blocked by week) repeated-measures analysis of variance (ANOVA). In all cases, p < 0.05 (two-tailed) was used to indicate statistical significance.
3. Results
3.1. Experiment 1: CeA infusion of NPY-SAP
3.1.1. CeA infusion of NPY-SAP is associated with increased anxiety-like behavior
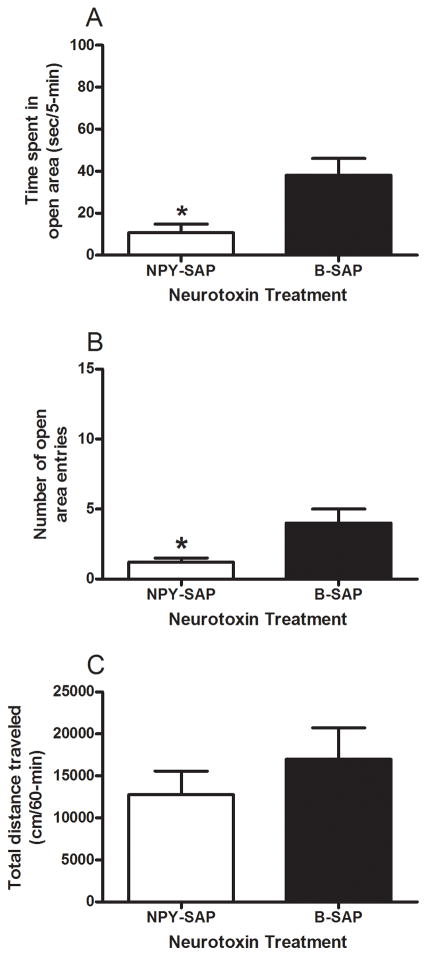
Results from the elevated zero maze test and open-field locomotor activity test in mice treated with CeA infusion of NPY-SAP or B-SAP are presented in Fig. 1. Mice treated with NPY-SAP (n = 23) exhibited increased anxiety-like behavior relative to mice treated with B-SAP (n = 27), evidenced by a significant reduction of time spent in the open area of the elevated zero maze (Fig. 1A) and a significant reduction in open area entries (Fig. 1B). T-tests performed on open area time [t(48) = 2.9, p = 0.006] and open area entries [t(48) = 2.366, p = 0.022] data were both statistically significant, confirming the above conclusions. On the other hand, mice treated with NPY-SAP (n = 12) failed to show alterations in locomotor activity relative to mice treated with B-SAP (n = 15), suggesting that alterations of elevated zero maze behavior in NPY-SAP-treated mice were not likely related to overall alterations of motor behavior (Fig. 1C). A t-test performed on locomotor activity data failed to achieve statistical significance [t(25) = 0.855, p = 0.400]. Consummatory measures provide further evidence that the NPY-SAP treatment did not impact motor behavior or overall health of the mice as there were no significant differences between NPY-SAP (n = 12) and B-SAP (n = 15) groups in terms of average food intake (305.47 ± 15.41 versus 299.47 ± 13.78 g/kg/day, respectively), water drinking (196.58 ± 10.33 versus 215.64 ± 9.24 ml/kg/day, respectively), or ethanol intake (1.14 ± 0.32 versus 1.21 ± 0.28 g/kg/day, respectively). Repeated-measures ANOVAs performed on food and water intake data revealed significant main effects of week [F(5, 125) = 24.728, p = 0.001; F(5, 25) = 4.456, p = 0.001, respectively], but no other effects were statistically significant. A repeated-measures ANOVA performed on ethanol intake data showed no significant effects.
Fig. 1.
Results from elevated zero maze and open-field testing in BALB/cJ mice given NPY-SAP or B-SAP injection into the CeA. Relative to B-SAP treated mice, time spent in the open area (A) and number of open area entries (B) were significantly reduced in mice treated with NPY-SAP. There were no significant differences between the NPY-SAP and B-SAP groups in open-field locomotor activity (C). *p < 0.05.
3.1.2. CeA infusion of NPY-SAP is associated with a significant reduction of Y1R IR in the CeA
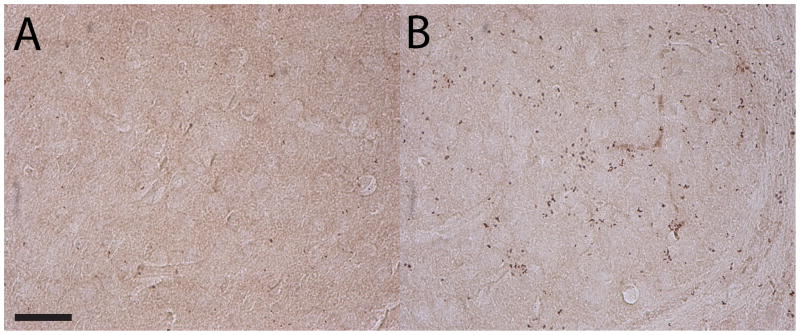
Data representing Y1R IR from mice treated with CeA infusion of NPY-SAP or B-SAP are presented in Table 1. Of the 10 regions that were assessed, the only region that showed a significant reduction of Y1R IR in mice treated with NPY-SAP was the CeA (see Fig. 2 for representative photomicrographs through the CeA). A t-test performed in Y1R IR data collected from the CeA was statistically significant [t(42) = 2.963, p = 0.005] confirming the above conclusion. These observations suggest that the NPY-SAP treatment site-specifically killed cells expressing Y1R in the region in which the neurotoxin was injected.
Table 1.
Y1R IR from mice given neurotoxin injection into the CeA
| Y1 Immunoreactivity (% Area) | |||
|---|---|---|---|
| Brain Region | NPY-SAP Treatment | B-SAP Treatment | p value |
| Basomedial Hypothalamus | 0.189 ± 0.026 | 0.210 ± 0.038 | 0.648 |
| Paraventricular Nucleus of Hypothalamus | 0.812 ± 0.114 | 0.919 ± 0.079 | 0.432 |
| Basolateral Amygdala | 0.097 ± 0.019 | 0.164 ± 0.032 | 0.110 |
| Central Nucleus of the Amygdala | 0.083 ± 0.016 | 0.262 ± 0.049 | 0.005* |
| Medial Amygdala | 0.176 ± 0.032 | 0.259 ± 0.052 | 0.237 |
| Bed Nucleus of Stria Terminalis | 0.222 ± 0.050 | 0.249 ± 0.045 | 0.691 |
| CA1 | 0.286 ± 0.028 | 0.362± 0.065 | 0.304 |
| CA2 | 0.574 ± 0.033 | 0.547 ± 0.050 | 0.667 |
| CA3 | 0.314 ± 0.029 | 0.391 ± 0.038 | 0.202 |
| Dentate Gyrus | 0.184 ± 0.016 | 0.243 ± 0.026 | 0.067 |
p < 0.05 (two-tailed). Data are presented as Mean ± SEM.
Fig. 2.
Representative photomicrographs of 40 μm coronal sections through the CeA showing Y1R IR in mice injected with NPY-SAP (A) or B-SAP (B) into the CeA. Images were photographed and quantified at a magnification of 40x. Scale bar = 50 μm.
3.2. Experiment 2: BMH infusion of NPY-SAP
3.2.1. BMH infusion of NPY-SAP is associated with decreased anxiety-like behavior
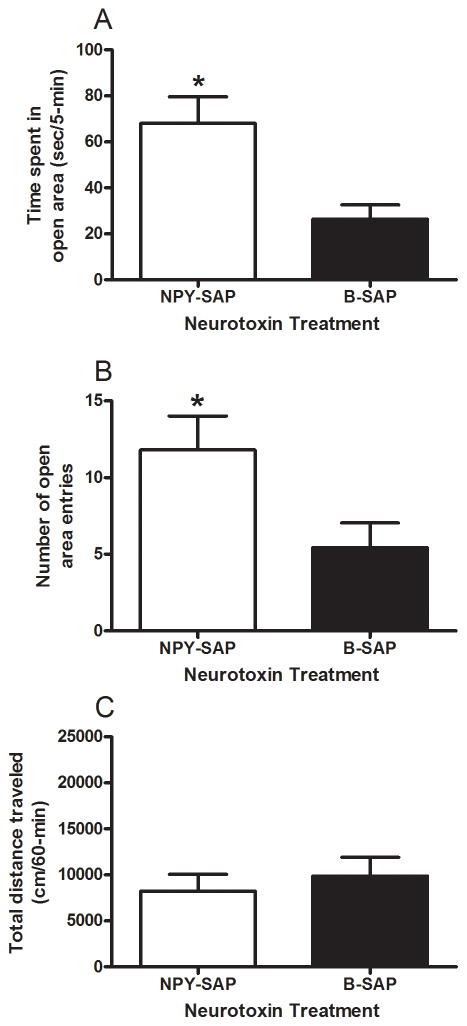
Results from the elevated zero maze and open-field locomotor activity tests in mice treated with BMH infusion of NPY-SAP or B-SAP are presented in Fig. 3. Relative to mice treated with B-SAP (n = 27), mice given BMH infusion of NPY-SAP (n = 21) showed a significant reduction of anxiety-like behavior, evidenced by significant increases in open area time (Fig. 3A) and number of open area entries (Fig. 3B). T-tests performed on open area time [t(46) = 3.353, p = 0.002] and open area entries [t(46) = 2.404, p = 0.02] data both achieved statistical significance. As shown in Fig. 3C, mice treated with BMH infusion of NPY-SAP (n = 13) did not show alterations in open-field locomotor activity relative to mice treated with B-SAP (n = 14), confirmed by a non-significant t-test performed in this data set [t(25) = 0.587, p = 0.563]. As above, consummatory measures provide further evidence that NPY-SAP treatment did not impact motor behavior or the health of the mice as there were no significant differences between NPY-SAP (n = 13) and B-SAP (n = 14) groups in terms of average food intake (328.75 ± 12.24 versus 338.36 ± 11.79 g/kg/day, respectively), water drinking (186.41 ± 9.22 versus 207 ± 8.88 ml/kg/day, respectively), or ethanol intake (1.14 ± 0.39 versus 1.14 ± 0.38 g/kg/day, respectively). A Repeated-measures ANOVA performed on food intake data revealed a significant main effect of week [F(5, 125) = 14.735, p = 0.001], but no other effects were statistically significant. Repeated-measures ANOVA performed on water and ethanol intake data showed no significant effects.
Fig. 3.
Results from elevated zero maze and open-field testing in BALB/cJ mice given NPY-SAP or B-SAP injection into the BMH. Relative to B-SAP treated mice, time spent in the open area (A) and number of open area entries (B) were significantly increased in mice treated with NPY-SAP. There were no significant differences between the NPY-SAP and B-SAP groups in open-field locomotor activity (C). *p < 0.05.
3.2.2. BMH infusion of NPY-SAP is associated with a significant reduction of Y1R IR in the BMH, but a significant increase in Y1R IR in the BLA and CA3 region if the hippocampus
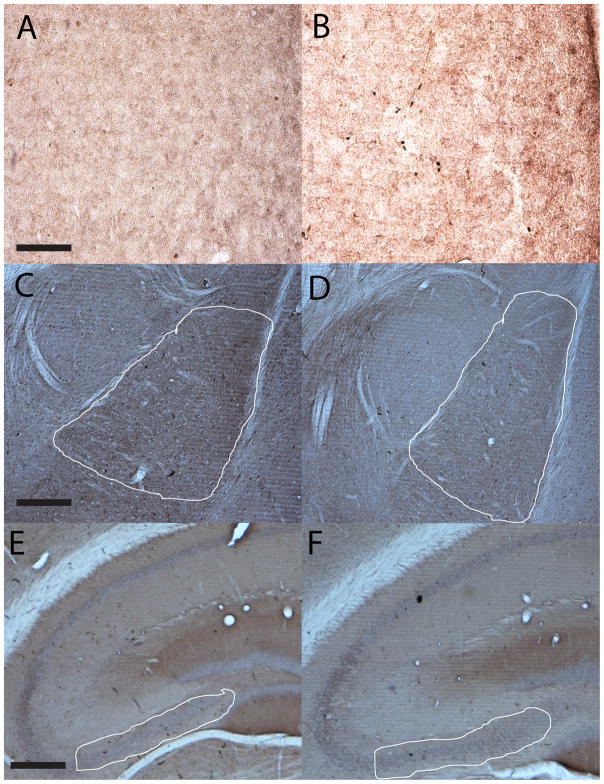
Data representing Y1R IR from mice treated with BMH infusion of NPY-SAP or B-SAP are presented in Table 2. Of the 10 regions that were assessed, significant alterations of Y1R IR were noted in the BMH, the BLA, and the CA3 region of the hippocampus (see Fig. 4 for representative photomicrographs). A t-test performed in Y1R IR data collected from the BMH was statistically significant [t(51) = 4.553, p = 0.001], reflecting the significant reduction of Y1R IR in NPY-SAP-treated mice. As above, these observations suggest that the NPY-SAP treatment was successful in killing cells expressing the Y1R in the region in which the neurotoxin was injected. Surprisingly, relative to mice treated with B-SAP, mice treated with BMH infusion of NPY-SAP showed a significant compensatory increase in Y1R IR in the BLA [t(46) = 2.322, p = 0.025] and the CA3 region of the hippocampus [t(50) = 2.156, p = 0.036].
Table 2.
Y1R IR from mice given neurotoxin injection into the BMH
| Y1 Immunoreactivity (% Area) | |||
|---|---|---|---|
| Brain Region | NPY-SAP Treatment | B-SAP Treatment | p value |
| Basomedial Hypothalamus | 0.031 ± 0.005 | 0.076 ± 0.008 | 0.05* |
| Paraventricular Nucleus of Hypothalamus | 1.018 ± 0.11 | 0.919 ± 0.089 | 0.451 |
| Basolateral Amygdala | 0.42 ± 0.053 | 0.287 ± 0.026 | 0.023* |
| Central Nucleus of Amygdala | 0.27 ± 0.035 | 0.236 ± 0.018 | 0.384 |
| Medial Amygdala | 0.119 ± 0.007 | 0.119 ±0.007 | 0.940 |
| Bed Nucleus of Stria Terminalis | 0.241 ± 0.021 | 0.247 ± 0.026 | 0.765 |
| CA1 | 0.59 ± 0.05 | 0.647 ± 0.059 | 0.493 |
| CA2 | 1.094 ± 0.078 | 1.026 ± 0.096 | 0.557 |
| CA3 | 0.666 ± 0.051 | 0.535 ± 0.034 | 0.032* |
| Dentate Gyrus | 1.365 ± 0.174 | 0.967 ± 0.0128 | 0.051 |
p < 0.05 (two-tailed). Data are presented as Mean ± SEM.
Fig. 4.
Representative photomicrographs of 40 μm coronal sections through the BMH (A and B), the BLA (C and D), and CA3 region of the hippocampus (E and F) showing Y1R IR in mice injected with NPY-SAP (A, C, and E) or B-SAP (B, D, and F) into the BMH. Images of the BMH were photographed and quantified at a magnification of 40x (scale bar = 50 μm), while images of the BLA and CA3 were photographed and quantified at a magnification of 10x (scale bar = 200 μm; solid white lines depict the regions that were selected for quantification).
3.3. Between study comparison of elevated zero maze behavior
We assessed the similarity of anxiety-like behavior in control subjects from the studies involving neurotoxin injection into the CeA versus the BMH. T-test performed to compare B-SAP groups from each study in terms of open area time [t(52) = 1.145, p = 0.258] and open area entries [t(52) = 0.672, p = 0.505] failed to achieve statistical significance, suggesting that the control groups between the studies exhibited similar anxiety-like behavior on the elevated zero maze test. Additionally, when the B-SAP group from the BMH infusion study was compared with the NPY-SAP group from the CeA infusion study, there were significant group differences in terms of open area time [t(48) = 2.049, p = 0.005] and open area entries [t(48) = 2.382, p = 0.021], confirming that mice given NPY-SAP injection into the CeA exhibited behavior consistent with increased anxiety. Finally, when the B-SAP group from the CeA infusion study was compared with the NPY-SAP group from the BMH infusion study, there were significant differences between groups in open area time [t(46) = 2.202, p = 0.033] and open area entries [t(46) = 3.340, p = 0.002], confirming that mice given NPY-SAP injection into the BMH exhibited behavior consistent with blunted anxiety.
4. Discussion
Here we show that there was a significant increase in anxiety-like behavior in BALB/cJ mice injected with the NPY-SAP neurotoxin into the CeA relative to mice treated with the control B-SAP. Thus, CeA NPY-SAP-treated mice spent significantly less time in the open area of the elevated zero maze and made significantly less open arm entries relative to B-SAP-treated mice. Reduced open area activity was not likely related to a general reduction of motor behavior or compromised heath status as there were no significant differences between mice treated with CeA infusion of NPY-SAP or B-SAP in terms of open-field locomotor activity or in measures of consummatory behaviors. Increased anxiety-like behavior was likely the result of blunted NPY signaling in the region of the CeA, as mice treated with NPY-SAP showed a significant reduction of Y1R IR in the CeA relative to B-SAP treated animals. The Y1R IR results reinforce the conclusion that the NPY-SAP toxin successfully lesioned cells in the CeA that express Y1R. CeA-infusion of NPY-SAP site-specifically attenuated Y1R IR in the CeA, and did not significantly alter Y1R IR in nearby regions including the BLA, medial amygdala, and bed nucleus of the stria terminalis. Thus, NPY-SAP appears to be a tool that will allow very precise definition of the NPY neurocircuitry involved in modulating anxiety-like behavior. It is noteworthy that since NPY-SAP binds to all NPY receptors, the NPY-SAP treatment would have also killed Y2- and Y5-expression cells, though we did not quantify these changes in the present report. The observed increase of anxiety-like behavior following CeA injection of NPY-SAP are consistent with results obtained using other tools to blunt NPY signaling such as NPY receptor antagonist and NPY antisense [6, 15, 20, 21]. It should be noted that while NPY signaling in the CeA has been shown to modulate ethanol consumption in rodents [11, 20], the lack of an effect of CeA-infused NPY-SAP on ethanol intake here is likely the result of an almost complete avoidance of ethanol in the BALB/cJ mice that were used (only 1.14 to 1.21 g/kg/day).
As a control, we injected NPY-SAP into the BMH and assessed subsequent anxiety-like behavior. We chose the BMH because, to our knowledge, there is no known link to NPY signaling in this region to the modulation of anxiety-like behavior, and NPY signaling in the hypothalamus appears to be primarily involved in feeding behaviors [3, 26, 28]. Unexpectedly, mice given BMH injection of NPY-SAP showed reduced anxiety-like behavior relative to B-SAP treated mice, evidenced by increased open area time and open area entries with no associated alterations of open-field locomotor activity or consummatory behaviors. While BMH infusion of NPY-SAP was associated with a significant reduction of Y1R IR in the BMH (confirming the lesioning of Y1R-expressing cells), this treatment also caused a significant increase in Y1R IR in the BLA and the CA3 region of the hippocampus when compared to B-SAP-treated mice. Increased Y1R signaling stemming from an upregulation of Y1R IR in the BLA and/or CA3 region may account for the paradoxical decrease in anxiety-like behavior in mice given NPY-SAP injection into the BMH. If it is assumed that increased Y1R IR translates into increased NPY signaling in these regions, such increased NPY signaling may have promoted anxiolysis which led to the observed reduction of anxiety-like behavior in the present work. In fact, accumulating evidence indicates that NPY signaling in the BLA and hippocampus protect against anxiety-like behaviors in rodents [17, 23, 27, 29], reinforcing the idea that an upregulation of NPY signaling in these regions accounts for the reduced anxiety-like behavior in mice given BMH infusion of NPY-SAP.
While it is unclear how destruction of Y1R-expressing cells in the BMH confers increases of Y1R IR in other brain regions, it is interesting to note that a previous study that used NPY-SAP to induce lesions of NPY receptor-expression cells in the arcuate nucleus of the hypothalamus observed a significant increase in Y1R IR in other regions including the paraventricular nucleus of the hypothalamus and the perifornical area. Analogous to the present findings, there was a paradoxical increase in feeding behavior in NPY-SAP-treated animals, hypothesized to stem from the compensatory increase in Y1R signaling on other brain regions [5]. Similarly, area postrema lesions were found to be associated with a significant reduction of anxiety-like behavior in rats, an effect that was hypothesized to be related to compensatory increases of NPY mRNA levels in other brain regions that included the amygdala [18]. We suggest that the observed compensatory increase in Y1R IR in the BLA and/or CA3 likely explains the reduced anxiety-like behavior in mice treated with BMH injection of NPY-SAP. Importantly, there are connections between the area of the BMH and the BLA [19] as well as the medial hypothalamus and the hippocampus [1], and these pathways may be involved with the compensatory increases of Y1R IR in the regions outside of the lesion site.
In conclusion, the present set of experiments suggest that the NPY-SAP neurotoxin may be a useful tool for studying the NPY neurocircuitry that modulates anxiety-like behaviors. Consistent with previous work, blunted NPY receptor signaling in the CeA following local injection of NPY-SAP was associated with increased anxiety-like behavior, reinforcing the critical role of NPY signaling in the CeA in the integration of emotional responses. The unexpected reduction of anxiety-like behavior following BMH injection of NPY-SAP may be related to the compensatory increase in Y1R IR in the BLA and/or CA3. This latter observation raises the important point that while NPY-SAP may be a useful tool, caution is necessary when drawing conclusions regarding the role of NPY signaling at the specific lesion site. Characterization of the NPY system in brain regions beyond the site of NPY-SAP injection may be necessary to gain a more accurate picture of the system involved.
Research Highlight.
Saporin, when conjugated to NPY (NPY-SAP), kills cells expression NPY receptors.
Injecting NPY-SAP into the central amygdala (CeA) reduces local Y1 receptor levels.
Injecting NPY-SAP into the CeA increases anxiety-like behavior.
NPY-SAP may be a useful tool to study the central NPY circuitry modulating anxiety.
Acknowledgments
This work was supported by NIH grants AA013573, AA015148, and AA017818, and Department of Defense grants W81XWH-06-1-0158 and W81XWH-09-1-0293.
Footnotes
Disclosure Statement
Both authors disclose that there are no actual or potential financial, personal, or other conflicts of interested related to the work reported in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anschel S, Alexander M, Perachio AA. Multiple connections of medial hypothalamic neurons in the rat. Exp Brain Res. 1982;46:383–92. doi: 10.1007/BF00238633. [DOI] [PubMed] [Google Scholar]
- 2.Brinks V, van der Mark M, de Kloet R, Oitzl M. Emotion and cognition in high and low stress sensitive mouse strains: a combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front Behav Neurosci. 2007;1:8. doi: 10.3389/neuro.08.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–91. doi: 10.1210/en.2004-1166. [DOI] [PubMed] [Google Scholar]
- 4.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 5.Dailey MJ, Bartness TJ. Arcuate nucleus destruction does not block food deprivation-induced increases in food foraging and hoarding. Brain Res. 2010;1323:94–108. doi: 10.1016/j.brainres.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deo GS, Dandekar MP, Upadhya MA, Kokare DM, Subhedar NK. Neuropeptide Y Y1 receptors in the central nucleus of amygdala mediate the anxiolytic-like effect of allopregnanolone in mice: Behavioral and immunocytochemical evidences. Brain Res. 2010;1318:77–86. doi: 10.1016/j.brainres.2009.12.088. [DOI] [PubMed] [Google Scholar]
- 7.Dumont Y, Martel JC, Fournier A, St-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol. 1992;38:125–67. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 8.Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption by RIIbeta knockout mice: Assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–68. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreras JM, Barbieri L, Girbes T, Battelli MG, Rojo MA, Arias FJ, et al. Distribution and properties of major ribosome-inactivating proteins (28 S rRNA N-glycosidases) of the plant Saponaria officinalis L. (Caryophyllaceae) Biochim Biophys Acta. 1993;1216:31–42. doi: 10.1016/0167-4781(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 10.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 11.Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–80. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, et al. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–63. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- 13.Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic- like effects in animal anxiety models. Psychopharmacology. 1989;98:524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- 14.Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Kokare DM, Dandekar MP, Chopde CT, Subhedar N. Interaction between neuropeptide Y and alpha-melanocyte stimulating hormone in amygdala regulates anxiety in rats. Brain Res. 2005;1043:107–14. doi: 10.1016/j.brainres.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Li AJ, Dinh TT, Ritter S. Hyperphagia and obesity produced by arcuate injection of NPY-saporin do not require upregulation of lateral hypothalamic orexigenic peptide genes. Peptides. 2008;29:1732–9. doi: 10.1016/j.peptides.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin EJ, Lin S, Aljanova A, During MJ, Herzog H. Adult-onset hippocampal-specific neuropeptide Y overexpression confers mild anxiolytic effect in mice. Eur Neuropsychopharmacol. 2010;20:164–75. doi: 10.1016/j.euroneuro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Miller CC, Holmes PV, Edwards GL. Area postrema lesions elevate NPY levels and decrease anxiety-related behavior in rats. Physiol Behav. 2002;77:135–40. doi: 10.1016/s0031-9384(02)00847-8. [DOI] [PubMed] [Google Scholar]
- 19.Ono T, Luiten PG, Nishijo H, Fukuda M, Nishino H. Topographic organization of projections from the amygdala to the hypothalamus of the rat. Neurosci Res. 1985;2:221–38. doi: 10.1016/0168-0102(85)90002-1. [DOI] [PubMed] [Google Scholar]
- 20.Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 21.Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–97. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- 22.Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–72. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 24.Santanche S, Bellelli A, Brunori M. The unusual stability of saporin, a candidate for the synthesis of immunotoxins. Biochem Biophys Res Commun. 1997;234:129–32. doi: 10.1006/bbrc.1997.6597. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 26.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–42. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 27.Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–90. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorsell A, Caberlotto L, Rimondini R, Heilig M. Leptin suppression of hypothalamic NPY expression and feeding, but not amygdala NPY expression and experimental anxiety. Pharmacology, Biochemistry & Behavior. 2002;71:425–30. doi: 10.1016/s0091-3057(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 29.Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–7. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiley RG, Kline IR. Neuronal lesioning with axonally transported toxins. J Neurosci Methods. 2000;103:73–82. doi: 10.1016/s0165-0270(00)00297-1. [DOI] [PubMed] [Google Scholar]