Abstract
According to current concepts, the liver and gastrointestinal tract are considered to be the major, if not the sole, sources of circulating serum cholesterol. While several mechanisms have been described which control the rate of hepatic cholesterogenesis, only biliary diversion is known to alter the rate of sterol synthesis in the intestine. The present study was designed to identify the inhibitory constituent of bile and to define its anatomic and biochemical sites of action.
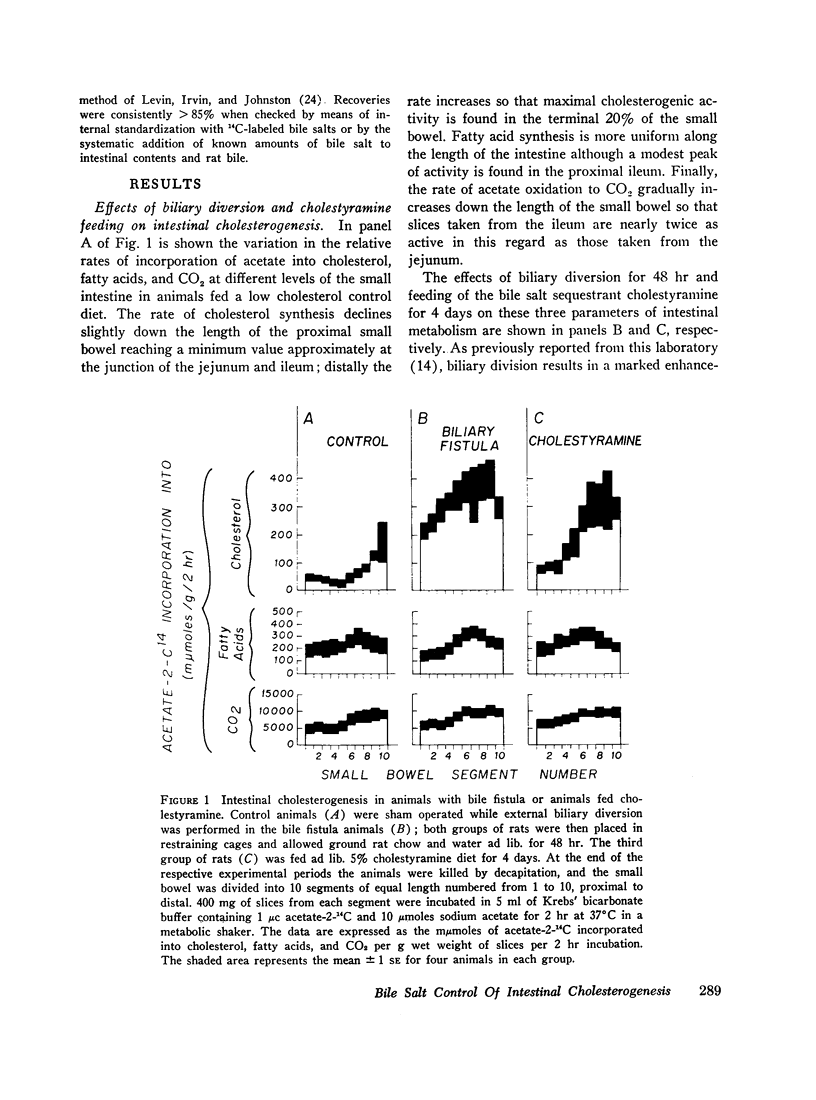
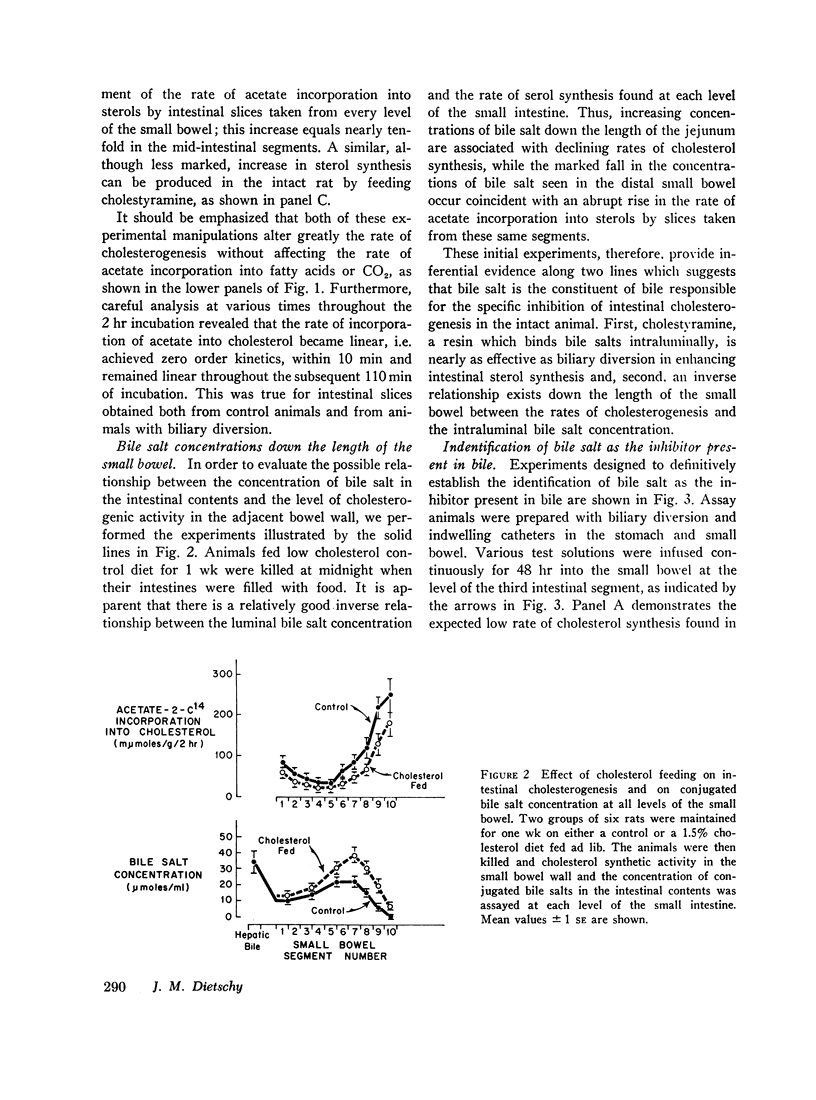
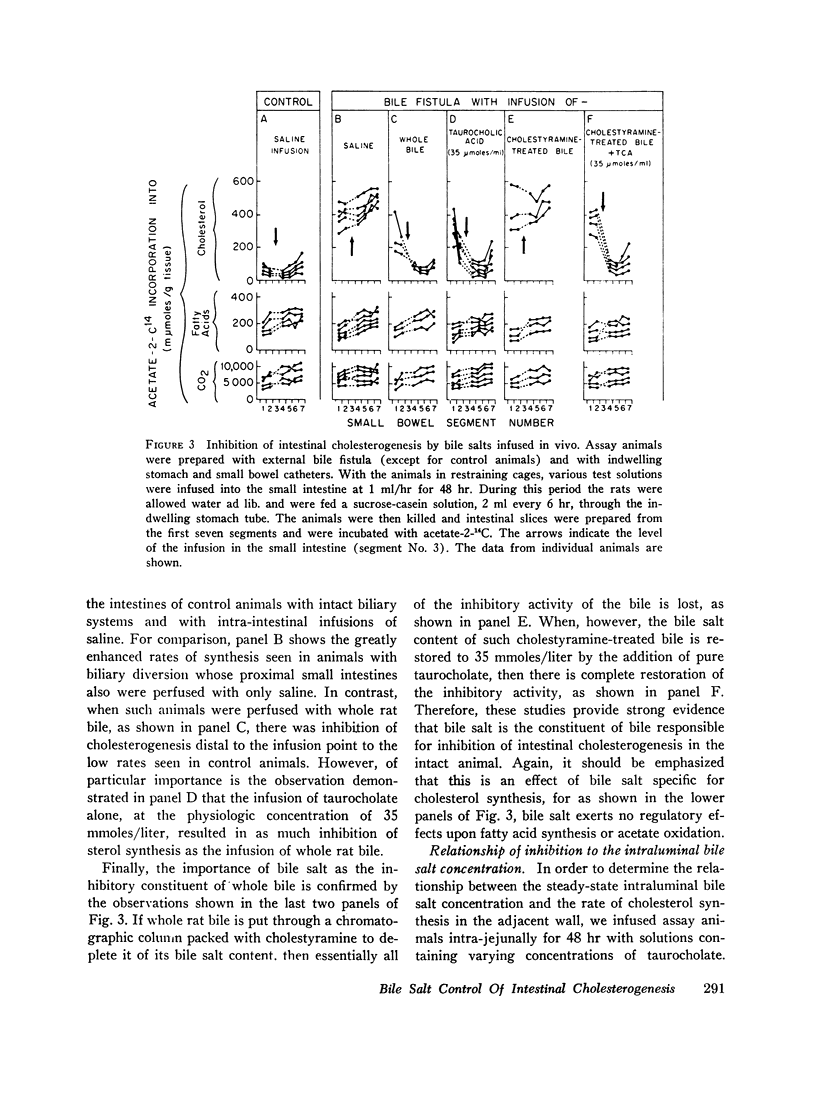
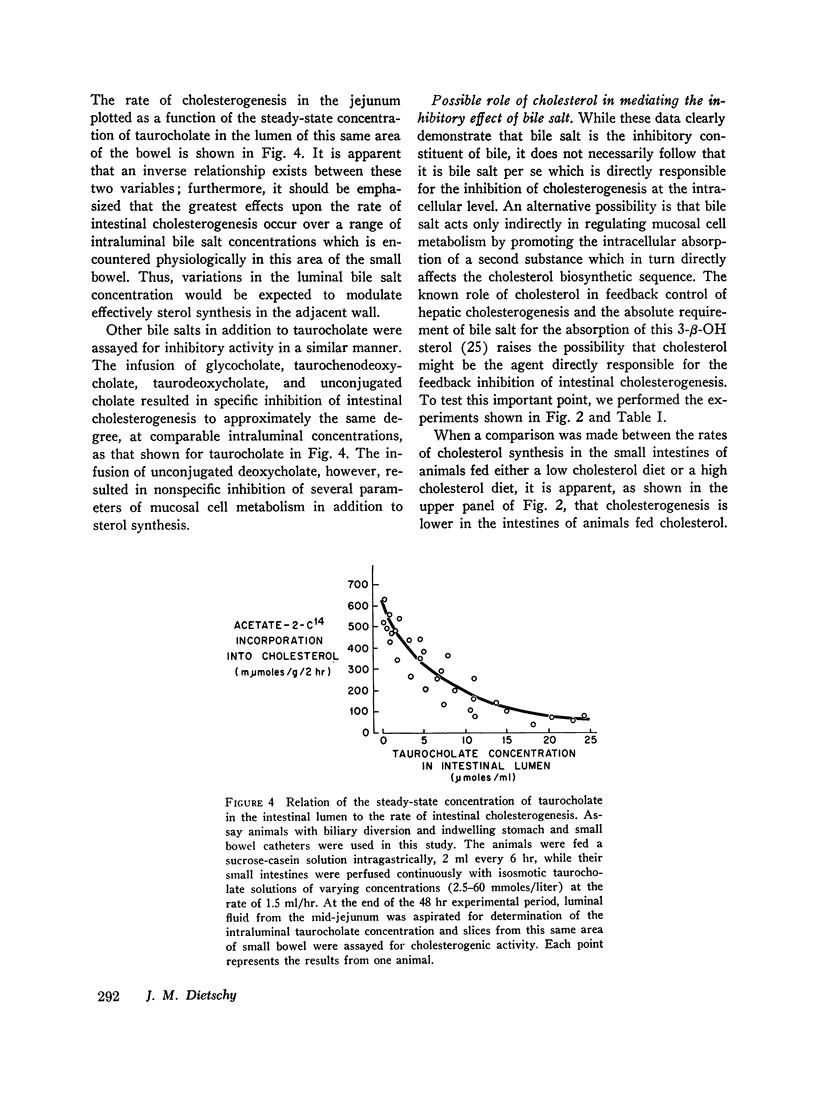
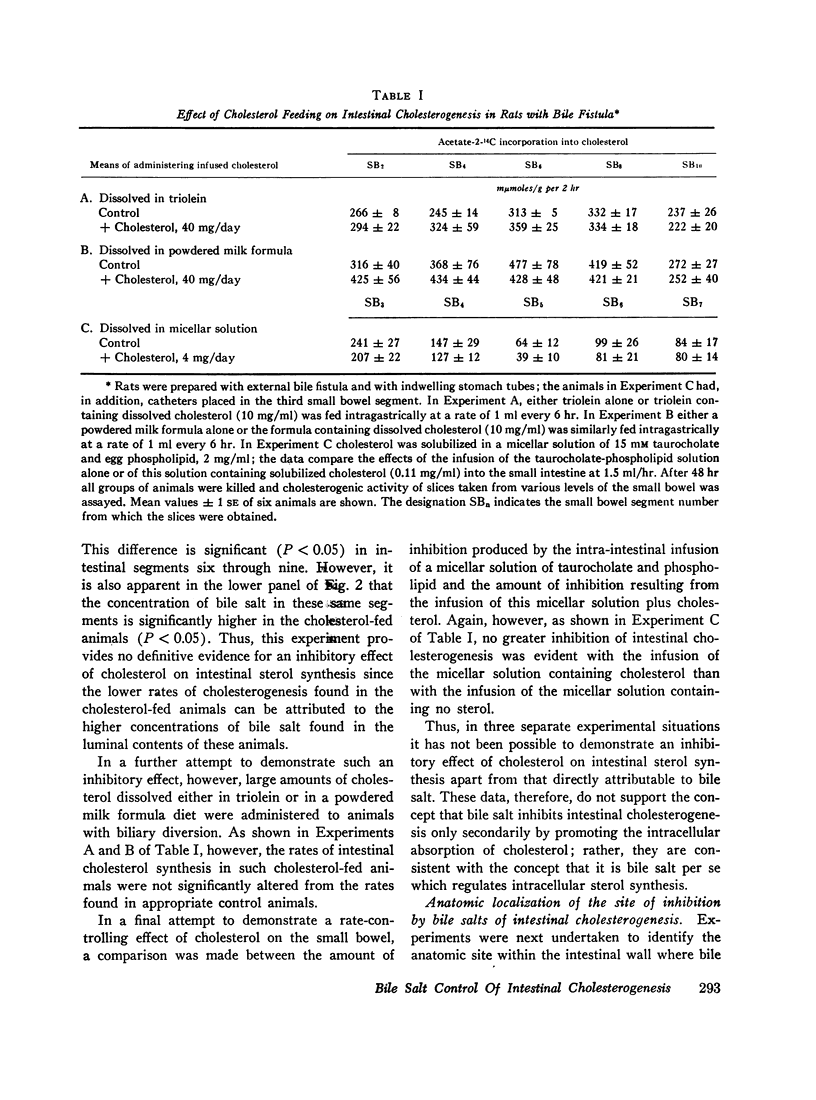
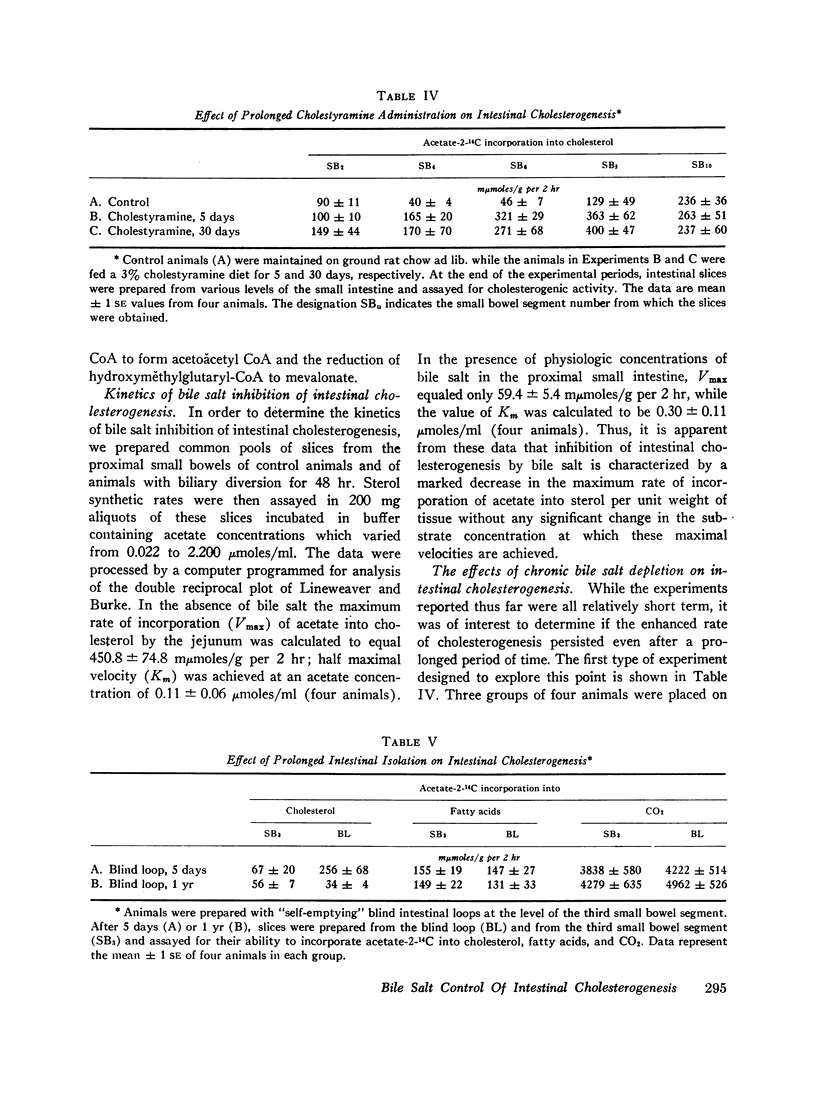
After either biliary diversion or cholestyramine feeding, there is a marked enhancement of cholesterogenesis at every level of the small intestine; this effect is specific for sterol synthesis since acetate incorporation into fatty acids and CO2 is unaffected by these experimental manipulations. In the present investigation bile salt has been shown to be the constituent of whole bile responsible for the inhibited rate of sterol synthesis found in the intact animal, and in addition, an inverse relationship has been shown to exist between the steady-state intraluminal bile salt concentration and the rate of cholesterogenesis in the adjacent bowel wall.
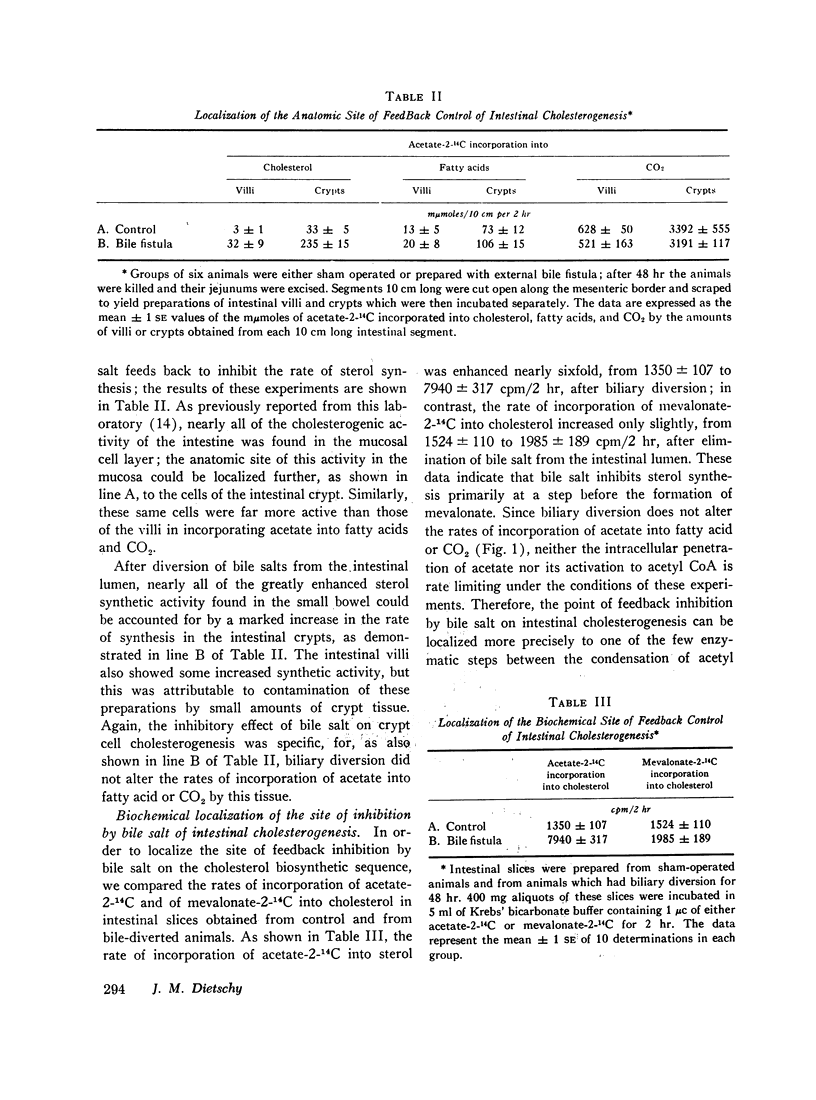
The inhibitory effect of bile salt is directed at the cells of the intestinal crypt, the major anatomic site for sterol synthesis in the small bowel. This feedback inhibition has been localized in the biosynthetic sequence to a step between acetyl CoA and mevalonic acid and, presumably, is at the enzymatic step mediated by hydroxymethylglutaryl reductase.
These studies emphasize the close interrelationship which exists between the mechanisms of control of cholesterogenesis in the liver and small intestine. Sterol synthesis in the liver is regulated by exogenous cholesterol intake, whereas the rate of intestinal sterol synthesis is controlled by bile salt, the major end product of the hepatic catabolism of cholesterol.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEHER W. T., BAKER G. D. Build-up and regression of inhibitory effects of cholic acid on in vivo liver cholesterol synthesis. Proc Soc Exp Biol Med. 1959 Jun;101(2):214–217. doi: 10.3181/00379727-101-24887. [DOI] [PubMed] [Google Scholar]
- BUCHER N. L., McGARRAHAN K., GOULD E., LOUD A. V. Cholesterol biosynthesis in preparations of liver from normal, fasting, x-irradiated, cholesterol-fed, triton, or delta 4-cholesten-3-one-treated rats. J Biol Chem. 1959 Feb;234(2):262–267. [PubMed] [Google Scholar]
- BUCHER N. L., OVERATH P., LYNEN F. beta-Hydroxy-beta-methyl-glutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim Biophys Acta. 1960 Jun 3;40:491–501. doi: 10.1016/0006-3002(60)91390-1. [DOI] [PubMed] [Google Scholar]
- CAMERON D. G., WATSON G. M., WITTS L. J. The alimentary tract of rats with intestinal culs-de-sac. Br J Exp Pathol. 1950 Jun;31(3):349–354. [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENEROTH P. THIN-LAYER CHROMATOGRAPHY OF BILE ACIDS. J Lipid Res. 1963 Jan;4:11–16. [PubMed] [Google Scholar]
- FRIEDMAN M., BYERS S. O., MICHAELIS F. Production and excretion of cholesterol in mammals. Iv. Role of liver in restoration of plasma cholesterol after experimentally induced hypocholesteremia. Am J Physiol. 1951 Mar;164(3):789–791. doi: 10.1152/ajplegacy.1951.164.3.789. [DOI] [PubMed] [Google Scholar]
- GOULD R. G. Lipid metabolism and atherosclerosis. Am J Med. 1951 Aug;11(2):209–227. doi: 10.1016/0002-9343(51)90107-6. [DOI] [PubMed] [Google Scholar]
- GOULD R. G., TAYLOR C. B., HAGERMAN J. S., WARNER I., CAMPBELL D. J. Cholesterol metabolism. I. Effect of dietary cholesterol on the synthesis of cholesterol in dog tissue in vitro. J Biol Chem. 1953 Apr;201(2):519–528. [PubMed] [Google Scholar]
- Gregg J. A. New solvent systems for thin-layer chromatography of bile acids. J Lipid Res. 1966 Jul;7(4):579–581. [PubMed] [Google Scholar]
- HARPER P. V., Jr, NEAL W. B., Jr, HLAVACEK G. R. Lipid synthesis and transport in the dog. Metabolism. 1953 Jan;2(1):69–80. [PubMed] [Google Scholar]
- HOTTA S., CHAIKOFF I. L. The role of the liver in the turnover of plasma cholesterol. Arch Biochem Biophys. 1955 May;56(1):28–37. doi: 10.1016/0003-9861(55)90330-1. [DOI] [PubMed] [Google Scholar]
- LANGDON R. G., BLOCH K. The effect of some dietary additions on the synthesis of cholesterol from acetate in vitro. J Biol Chem. 1953 May;202(1):77–81. [PubMed] [Google Scholar]
- LINDSEY C. A., Jr, WILSON J. D. EVIDENCE FOR A CONTRIBUTION BY THE INTESTINAL WALL TO THE SERUM CHOLESTEROL OF THE RAT. J Lipid Res. 1965 Apr;6:173–181. [PubMed] [Google Scholar]
- PARKINSON T. M., OLSON J. A. Inhibitory effects of bile acids on the uptake, metabolism, and transport of water-soluble substances in the small intestine of the rat. Life Sci. 1963 Jun;6:393–398. doi: 10.1016/0024-3205(63)90123-1. [DOI] [PubMed] [Google Scholar]
- Pope J. L. Crystallization of sodium taurocholate. J Lipid Res. 1967 Mar;8(2):146–147. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Feedback control of mevalonate synthesis by dietary cholesterol. J Biol Chem. 1966 Feb 10;241(3):602–609. [PubMed] [Google Scholar]
- TOMKINS G. M., CHAIKOFF I. L. Cholesterol synthesis by liver. I. Influence of fasting and of diet. J Biol Chem. 1952 May;196(2):569–573. [PubMed] [Google Scholar]
- TOMKINS G. M., SHEPPARD H., CHAIKOFF I. L. Cholesterol synthesis by liver. III. Its regulation by ingested cholesterol. J Biol Chem. 1953 Mar;201(1):137–141. [PubMed] [Google Scholar]
- Wilson J. D. Biosynthetic origin of serum cholesterol in the squirrel monkey: evidence for a contribution by the intestinal wall. J Clin Invest. 1968 Jan;47(1):175–187. doi: 10.1172/JCI105707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. The quantification of cholesterol excretion and degradation in the isotopic steady state in the rat: the influence of dietary cholesterol. J Lipid Res. 1964 Jul;5(3):409–417. [PubMed] [Google Scholar]



