Abstract
In the present paper a model system is described utilizing suspensions of peripheral blood leukocytes in which glycogen synthesis and degradation can be studied.
Leukocyte suspensions containing 72-94% granulocytes were prepared essentially free of platelets and erythrocytes and consisted almost entirely of neutrophile granulocytes. Initial glycogen content averaged 7.36 ± 2.05 mg/109 neutrophiles. In a glucose-free medium glycogenolysis took place with glycogen losses averaging 38% in 2 hr. When adequate glucose was added to the medium, glycogen was resynthesized to the original level.
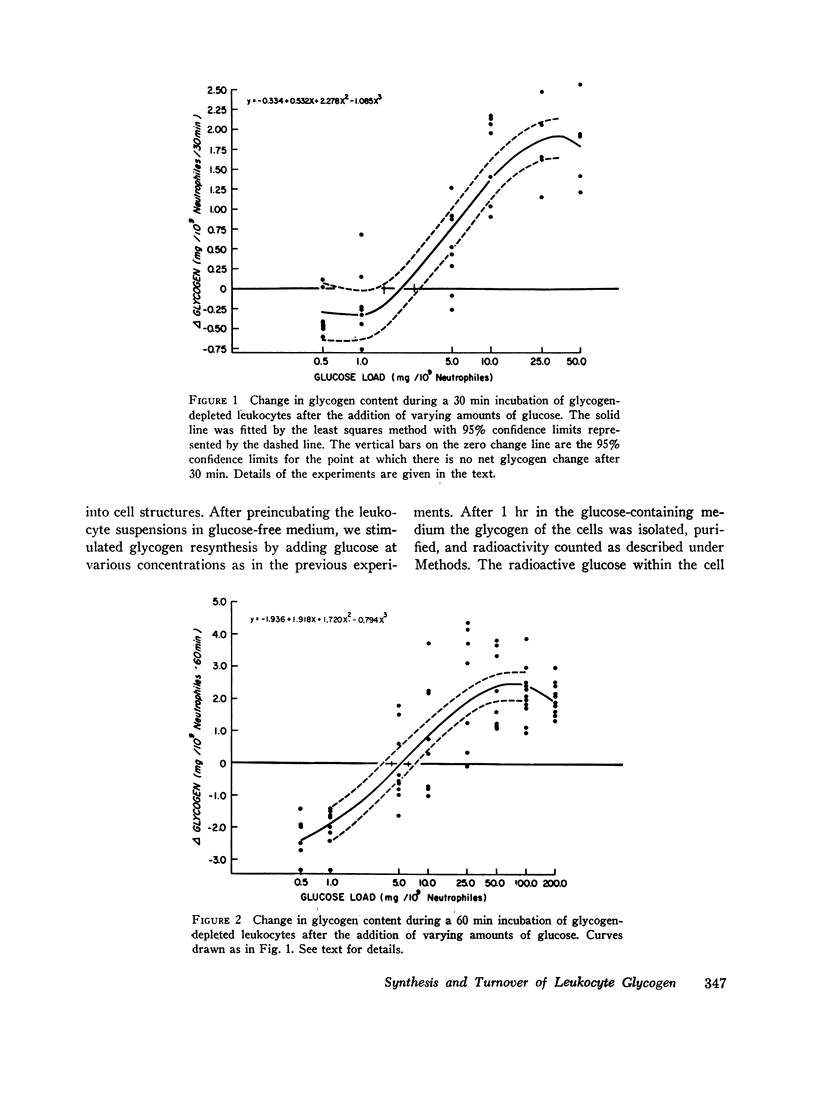
Glycogen resynthesis was studied with varying glucose “loads” to determine (a) the glucose level which was adequate for cell maintenance without utilization of glycogen stores, and (b) the glucose level which provided maximal glycogen resynthesis. With cell densities of 20-50 × 106/ml the minimum glucose load which allowed maintenance of glycogen stores was 2 mg and 5.3 mg/109 neutrophiles for 30 and 60 min, respectively.
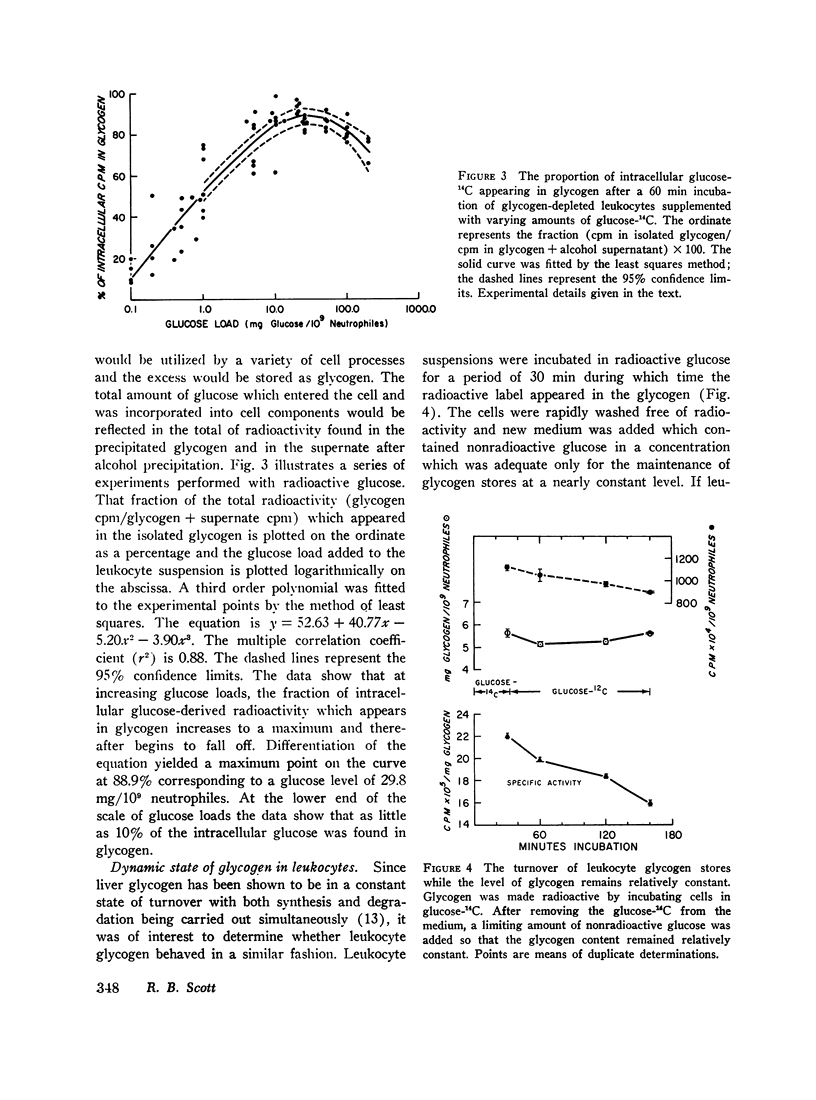
During resynthesis with glucose-14C, as much as 88.9% of the intracellular radioactivity could be found in glycogen. Leukocyte glycogen was made radioactive by a “pulse” of glucose-14C followed by a “chase” with nonradioactive glucose. Specific activity changes in glycogen isolated during the “chase” showed that glycogen was in constant turnover.
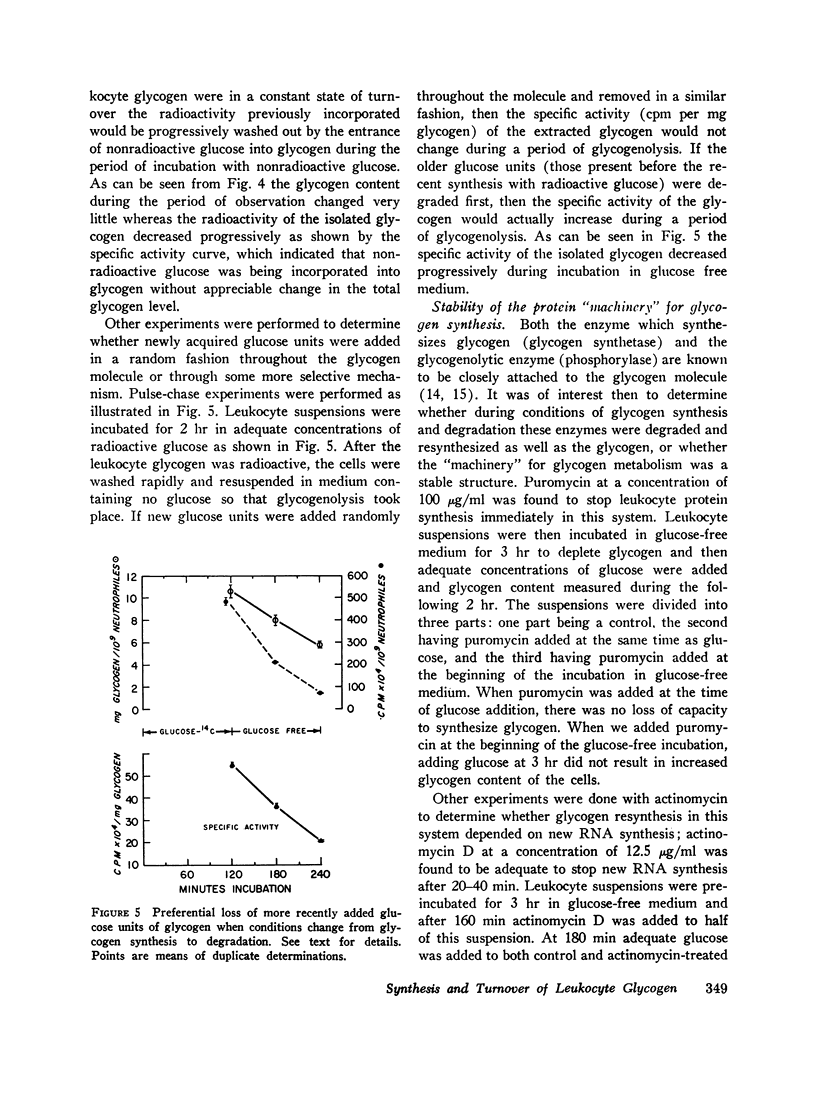
When glycogen was made radioactive by a “pulse” of glucose-14C and the cells placed in glucose-free medium, the specific activity of isolated glycogen fell rapidly. Thus, the most recently added glucose units of the molecule were also the first to be removed when conditions favoring synthesis were changed to conditions favoring degradation.
Even though glycogen is constantly turning over, the enzymatic “machinery” for its synthesis is relatively stable and not dependent on continuous protein or RNA synthesis, as shown by experiments with puromycin and actinomycin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- SOVIK O. EFFECT OF INSULIN ON THE ISOLATED RAT DIAPHRAGM IN THE PRESENCE AND IN THE ABSENCE OF PUROMYCIN AND ACTINOMYCIN D. Acta Physiol Scand. 1965 Mar;63:325–335. doi: 10.1111/j.1748-1716.1965.tb04071.x. [DOI] [PubMed] [Google Scholar]
- STETTEN M. R., STETTEN D., Jr A study of the nature of glycogen regeneration in the intact animal. J Biol Chem. 1954 Mar;207(1):331–340. [PubMed] [Google Scholar]
- SUTHERLAND E. W., WOSILAIT W. D. The relationship of epinephrine and glucagon to liver phosphorylase. I. Liver phosphorylase; preparation and properties. J Biol Chem. 1956 Jan;218(1):459–468. [PubMed] [Google Scholar]
- Scott R. B. Activation of glycogen phosphorylase in blood platelets. Blood. 1967 Sep;30(3):321–330. [PubMed] [Google Scholar]
- Scott R. B., Still W. J. Glycogen in human peripheral blood leukocytes. II. The macromolecular state of leukocyte glycogen. J Clin Invest. 1968 Feb;47(2):353–359. doi: 10.1172/JCI105731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz I. F. Biochemistry of normal and leukemic leucocytes, thrombocytes, and bone marrow cells. Adv Cancer Res. 1965;9:303–410. doi: 10.1016/s0065-230x(08)60449-2. [DOI] [PubMed] [Google Scholar]
- Sovik O. Effect of puromycin and puromycin analogues on glycogen synthesis in the isolated rat diaphragm. Acta Physiol Scand. 1966 Mar;66(3):307–315. doi: 10.1111/j.1748-1716.1966.tb03204.x. [DOI] [PubMed] [Google Scholar]
- VALENTINE W. N., BECK W. S., FOLLETTE J. H., MILLS H., LAWRENCE J. S. Biochemical studies in chronic myelocytic leukemia, polycythemia vera and other idiopathic myeloproliferative disorders. Blood. 1952 Oct;7(10):959–977. [PubMed] [Google Scholar]


