Abstract
Glycogen of normal human blood leukocytes was studied in cell suspensions containing chiefly neutrophiles.
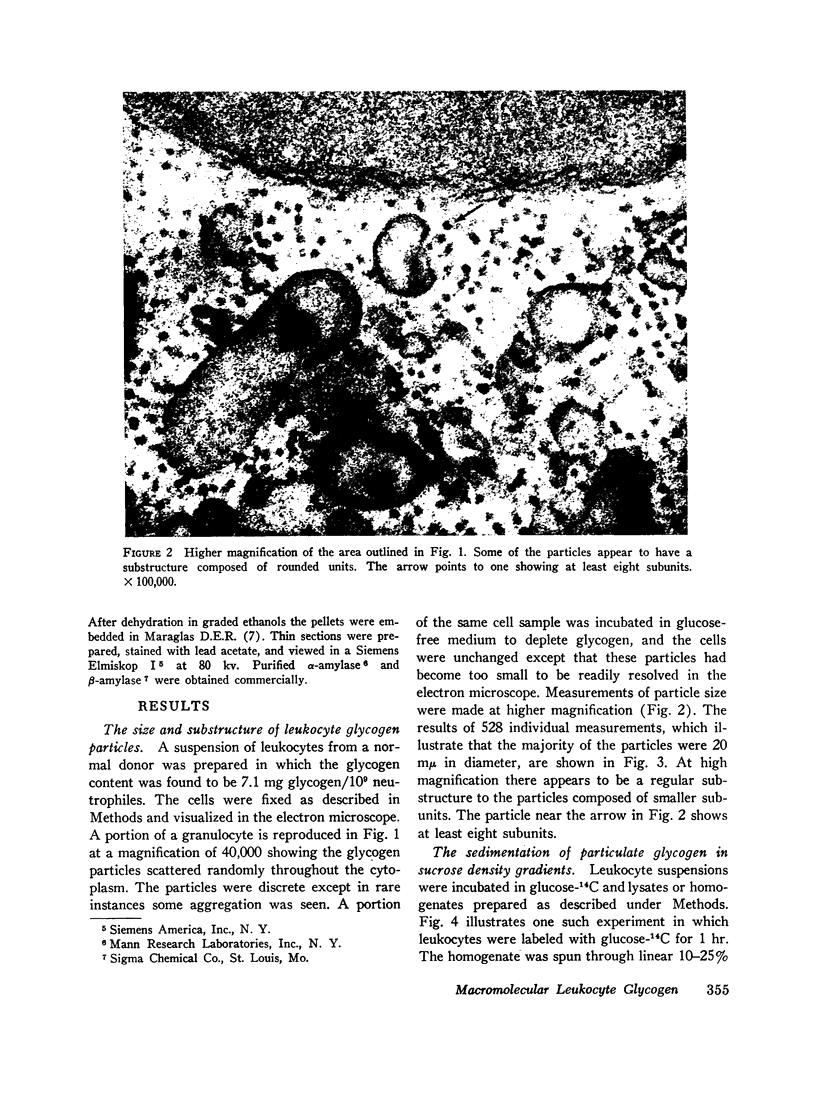
In electron micrographs of neutrophiles stained with lead the glycogen particles appear to be relatively uniform with a diameter of 20 mμ. At high magnification the 20 mμ particle appears to be composed of at least eight subunits.
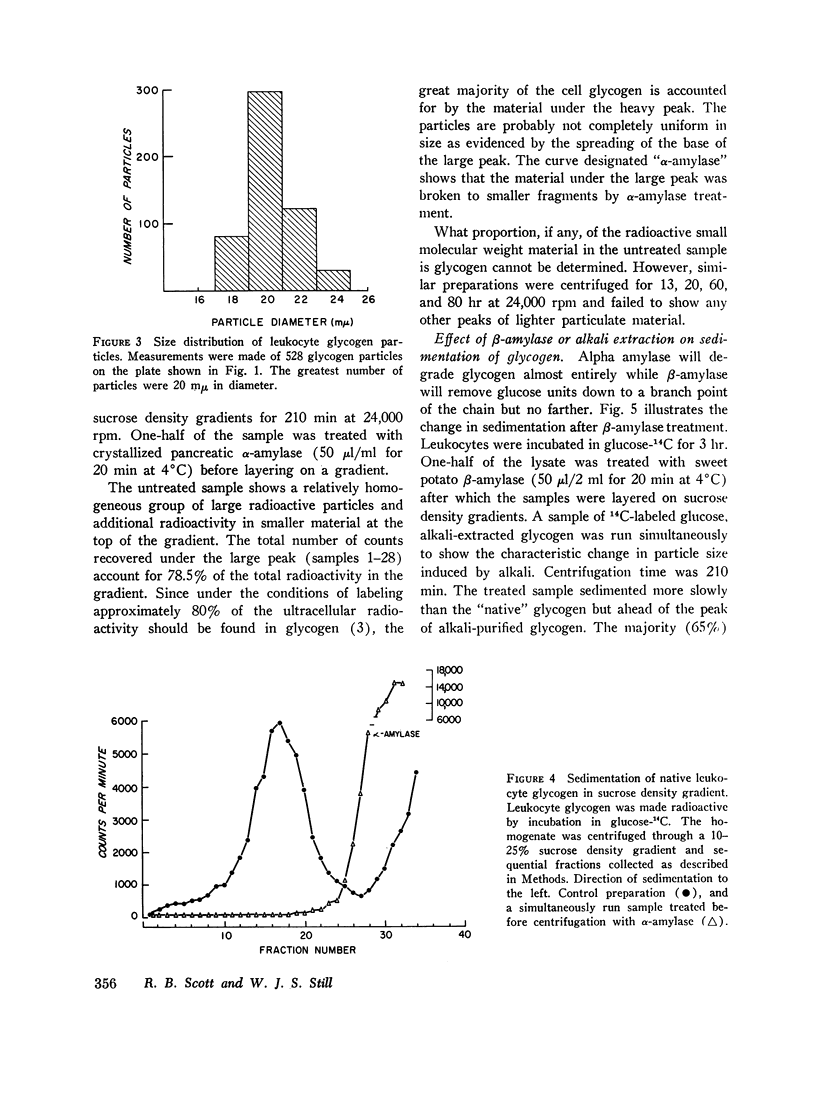
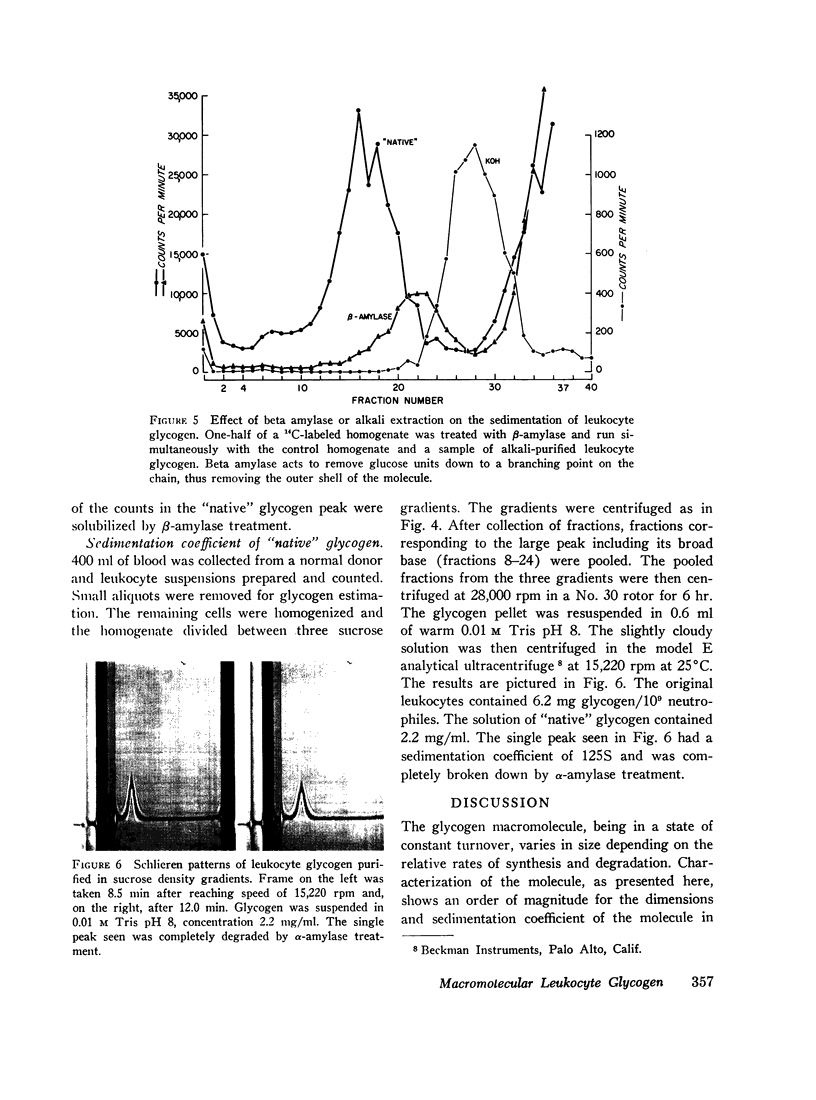
Leukocyte glycogen released by lysis or homogenization sediments as a single peak of high molecular weight material. The great majority of the cell glycogen can be accounted for in the large molecular weight material. The large molecular weight material is degraded to small fragments by α-amylase and partially degraded by β-amylase. Purification of cell glycogen by alkali extraction and ethanol precipitation produces a relatively uniform particle smaller than the original native macromolecule.
Native glycogen was prepared in pure form by a sucrose density gradient technique and its purity demonstrated by its susceptibility to purified α-amylase and by analytical ultracentrifugation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. A., Harris W. W., Anderson N. G. Isolation of native glycogen by combined rate-zonal and isopycnic centrifugation. Natl Cancer Inst Monogr. 1966 Jun;21:285–302. [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROCHMANS P. [Morphology of glycogen. Electron microscopic study of the negative stains of particulate glycogen]. J Ultrastruct Res. 1962 Apr;6:141–163. doi: 10.1016/s0022-5320(62)90050-3. [DOI] [PubMed] [Google Scholar]
- ERLANDSON R. A. A NEW MAGAGLAS, D.E.R.(R) 732, EMBEDMENT FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:704–709. doi: 10.1083/jcb.22.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- STETTEN M. R., STETTEN D., Jr A study of the nature of glycogen regeneration in the intact animal. J Biol Chem. 1954 Mar;207(1):331–340. [PubMed] [Google Scholar]
- SUTHERLAND E. W., WOSILAIT W. D. The relationship of epinephrine and glucagon to liver phosphorylase. I. Liver phosphorylase; preparation and properties. J Biol Chem. 1956 Jan;218(1):459–468. [PubMed] [Google Scholar]
- Scott R. B. Glycogen in human peripheral blood leukocytes. I. Characteristics of the synthesis and turnover of glycogen in vitro. J Clin Invest. 1968 Feb;47(2):344–352. doi: 10.1172/JCI105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE W. N., BECK W. S., FOLLETTE J. H., MILLS H., LAWRENCE J. S. Biochemical studies in chronic myelocytic leukemia, polycythemia vera and other idiopathic myeloproliferative disorders. Blood. 1952 Oct;7(10):959–977. [PubMed] [Google Scholar]