Abstract
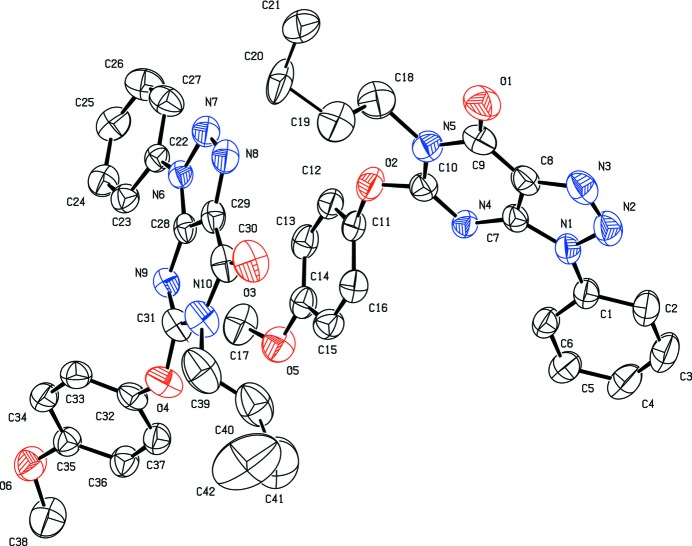
The asymmetric unit of the title compound, C21H21N5O3, consists of two geometrically similar molecules. The fused rings of the triazolo[4,5-d]pyrimidine system are nearly coplanar, making dihedral angels of 1.48 (18) and 1.34 (16)°, and the phenyl rings are twisted by 12.3 (1) and 8.7 (1)° with respect to the triazolopyrimidine plane. The ethyl groups of the n-butyl side chains are disordered over two sites in each of the independent molecules, the ratios of occupancies being 0.60:0.40 and 0.61:0.39.
Related literature
For the biological activity of 8-azaguanine derivatives, see: Roblin et al. (1945 ▶); Ding et al. (2004 ▶); Mitchell et al. (1950 ▶); Levine et al. (1963 ▶); Montgomery et al. (1962 ▶)); Yamamoto et al. (1967 ▶); Bariana (1971 ▶); Holland et al. (1975 ▶). For related structures, see: Wang et al. (2006 ▶); Zeng et al. (2006 ▶, 2009 ▶); Zhao, Hu et al. (2005 ▶); Zhao, Wang & Ding (2005 ▶); Zhao, Xie et al. (2005 ▶).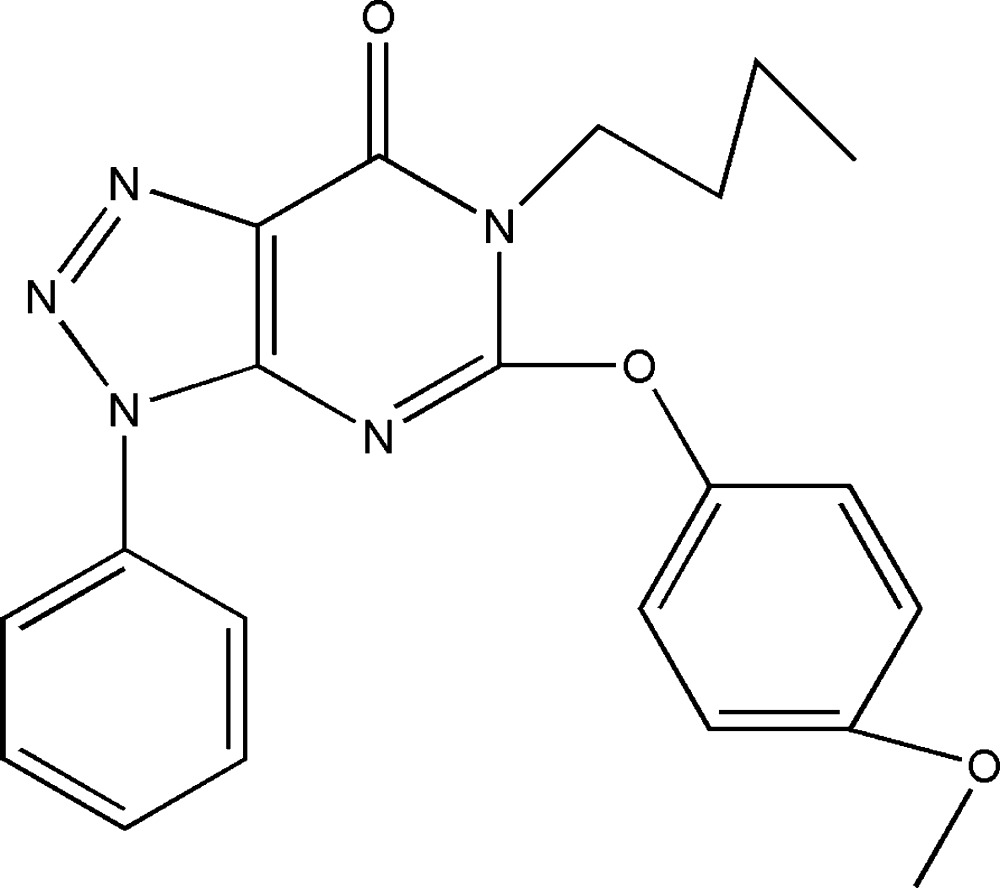
Experimental
Crystal data
C21H21N5O3
M r = 391.43
Triclinic,
a = 11.5167 (13) Å
b = 12.4026 (13) Å
c = 14.9353 (16) Å
α = 78.795 (2)°
β = 76.207 (2)°
γ = 76.360 (2)°
V = 1991.7 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 298 K
0.15 × 0.12 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.987, T max = 0.991
11898 measured reflections
6940 independent reflections
5252 reflections with I > 2σ(I)
R int = 0.061
Refinement
R[F 2 > 2σ(F 2)] = 0.079
wR(F 2) = 0.212
S = 1.07
6940 reflections
567 parameters
12 restraints
H-atom parameters constrained
Δρmax = 0.44 e Å−3
Δρmin = −0.22 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXTL97 (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809046017/ya2108sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809046017/ya2108Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We gratefully acknowledge financial support of this work by the National Basic Research Program of China (2003CB114400), the National Natural Science Foundation of China (20372023, 20102001), the Educational Commission of Hubei Province (grant Nos. B200624004, B20092412), the Shiyan Municipal Science and Technology Bureau (grant No. 20061835) and Yunyang Medical College (grant Nos. 2007QDJ15, 2007ZQB19, 2007ZQB20).
supplementary crystallographic information
Comment
The derivatives of heterocycles containing 8-azaguanine system, which are well known bioisosteres of guanine, are of great importance because of their remarkable biological properties, such as antimicrobial or antifungal activities (Roblin et al., 1945; Ding et al., 2004), encephaloma cell inhibitor (Mitchell et al., 1950; Levine et al., 1963), antileukemia (Montgomery et al., 1962), hypersusceptibility inhibitor and acesodyne activities (Yamamoto et al., 1967; Bariana, 1971; Holland et al., 1975).
In recent years, Zhao's group succeeded in synthesizing the derivatives of 8-azaguanine via aza-Wittig reaction of beta-ethoxycarbonyl iminophosphorane with aromatic isocyanates (Zhao, Xie et al., 2005). As a continuation of the quest for new biologically active derivatives of 8-azaguanine, the title compound was obtained from beta-ethoxycarbonyl iminophosphorane and aliphatic isocyanate and structurally characterized.
The asymmetric unit of the crystal of the title compound, C21H21N5O3, consists of two geometrically similar molecules (Fig. 1), the bond lengths and angles in triazolopyrimidinone moiety are in good agreement with those observed for a closely related structures (Zhao, Hu et al., 2005; Zhao, Wang & Ding, 2005). The fused rings of the triazolo[4,5-d]pyrimidine ring system are as usual coplanar (e.g. see structures by Wang et al., 2006 and Zeng et al., 2009), and the phenyl rings are twisted with respect to the triazolopyrimidine plane by 12.3 (1)° and 8.7 (1)° in each of the two independent molecules.
Experimental
To the solution of carbodiimide, prepared according to Zeng et al. (2006) in a mixed solvent (CH2Cl2/CH3CN, 1:4 v/v, 15 ml), was added 4-chlorophenol (3 mmol) and K2CO3 (6 mmol). After the reaction mixture was stirred for 12 h, the solvent was removed under reduced pressure and the residue was recrystallized from EtOH to give the title compound in 93% yield (m.p. 426 K). Elemental analysis: calculated for C21H21N5O3: C, 64.44; H, 5.41; N, 17.89%. Found: C, 63.62; H, 5.68; N, 17.51%. Crystals suitable for X-ray diffraction study were obtained by recrystallization from hexane and dichloromethane (1:3 v/v) at room temperature.
Refinement
All H atoms were placed in the geometrically calculated positions and treated as riding atoms, with C—H = 0.93–0.97 Å, and Uiso(H) = 1.2Ueq(C) [1.5Ueq(C) for methyl H atoms]. The terminal ethyl groups of the n-butyl substituent are disordered in both molecules; the occupancy refinement yielded ratio 0.60 : 0.40 and 0.61 : 0.39 for each of the two molecules. The bond lengths and the 1,3-distances in these groups were restrained during the refinement to be within the ranges of 1,54 ± 0.01 Å and 2.45 ± 0.01 Å respectively.
Figures
Fig. 1.
The asymmetric unit of the title compound with atomic numbering scheme and displacement ellipsoids drawn at the 30% probability level; only major components of disordered butyl groups are shown. H-Atoms are omitted.
Crystal data
| C21H21N5O3 | Z = 4 |
| Mr = 391.43 | F(000) = 824 |
| Triclinic, P1 | Dx = 1.305 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.5167 (13) Å | Cell parameters from 4234 reflections |
| b = 12.4026 (13) Å | θ = 2.3–25.4° |
| c = 14.9353 (16) Å | µ = 0.09 mm−1 |
| α = 78.795 (2)° | T = 298 K |
| β = 76.207 (2)° | Block, colorless |
| γ = 76.360 (2)° | 0.15 × 0.12 × 0.10 mm |
| V = 1991.7 (4) Å3 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 6940 independent reflections |
| Radiation source: fine-focus sealed tube | 5252 reflections with I > 2σ(I) |
| graphite | Rint = 0.061 |
| φ and ω scans | θmax = 25.0°, θmin = 2.0° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −13→13 |
| Tmin = 0.987, Tmax = 0.991 | k = −13→14 |
| 11898 measured reflections | l = −17→17 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.079 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.212 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0878P)2 + 0.7377P] where P = (Fo2 + 2Fc2)/3 |
| 6940 reflections | (Δ/σ)max < 0.001 |
| 567 parameters | Δρmax = 0.44 e Å−3 |
| 12 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.3813 (3) | 1.1282 (3) | −0.0630 (2) | 0.0704 (8) | |
| C2 | 0.4159 (3) | 1.2053 (3) | −0.1390 (3) | 0.0929 (11) | |
| H2 | 0.4473 | 1.2648 | −0.1315 | 0.112* | |
| C3 | 0.4038 (4) | 1.1937 (4) | −0.2255 (3) | 0.1134 (15) | |
| H3 | 0.4286 | 1.2451 | −0.2767 | 0.136* | |
| C4 | 0.3560 (4) | 1.1082 (4) | −0.2378 (3) | 0.1053 (13) | |
| H4 | 0.3469 | 1.1021 | −0.2968 | 0.126* | |
| C5 | 0.3215 (3) | 1.0312 (3) | −0.1625 (3) | 0.0918 (10) | |
| H5 | 0.2897 | 0.9721 | −0.1703 | 0.110* | |
| C6 | 0.3341 (3) | 1.0418 (3) | −0.0745 (2) | 0.0789 (9) | |
| H6 | 0.3103 | 0.9900 | −0.0233 | 0.095* | |
| C7 | 0.3573 (3) | 1.0900 (2) | 0.1139 (2) | 0.0642 (7) | |
| C8 | 0.4017 (3) | 1.1368 (3) | 0.1700 (3) | 0.0748 (8) | |
| C9 | 0.3731 (3) | 1.1058 (3) | 0.2691 (3) | 0.0856 (10) | |
| C10 | 0.2607 (3) | 0.9846 (3) | 0.2292 (2) | 0.0708 (8) | |
| C11 | 0.1502 (3) | 0.8491 (3) | 0.2166 (2) | 0.0695 (8) | |
| C12 | 0.0289 (3) | 0.8462 (3) | 0.2362 (2) | 0.0759 (9) | |
| H12 | −0.0257 | 0.8930 | 0.2758 | 0.091* | |
| C13 | −0.0129 (3) | 0.7744 (3) | 0.1976 (2) | 0.0775 (9) | |
| H13 | −0.0955 | 0.7721 | 0.2110 | 0.093* | |
| C14 | 0.0688 (3) | 0.7057 (3) | 0.1388 (2) | 0.0732 (8) | |
| C15 | 0.1902 (3) | 0.7121 (3) | 0.1176 (2) | 0.0810 (9) | |
| H15 | 0.2447 | 0.6676 | 0.0761 | 0.097* | |
| C16 | 0.2326 (3) | 0.7834 (3) | 0.1569 (2) | 0.0767 (9) | |
| H16 | 0.3149 | 0.7867 | 0.1432 | 0.092* | |
| C17 | −0.0777 (4) | 0.6012 (4) | 0.1313 (3) | 0.1118 (14) | |
| H17A | −0.0869 | 0.5741 | 0.1969 | 0.168* | |
| H17B | −0.0848 | 0.5438 | 0.0993 | 0.168* | |
| H17C | −0.1401 | 0.6663 | 0.1212 | 0.168* | |
| C18 | 0.2467 (5) | 0.9939 (4) | 0.3997 (3) | 0.1173 (14) | |
| H18A | 0.2351 | 1.0582 | 0.4312 | 0.141* | |
| H18B | 0.1682 | 0.9732 | 0.4084 | 0.141* | |
| C19 | 0.3312 (5) | 0.9008 (5) | 0.4395 (3) | 0.1241 (15) | |
| H19A | 0.4072 | 0.9247 | 0.4342 | 0.149* | 0.60 |
| H19B | 0.3485 | 0.8401 | 0.4030 | 0.149* | 0.60 |
| H19C | 0.4133 | 0.9143 | 0.4292 | 0.149* | 0.40 |
| H19D | 0.3321 | 0.8293 | 0.4218 | 0.149* | 0.40 |
| C20 | 0.2846 (11) | 0.8550 (9) | 0.5437 (5) | 0.121 (4) | 0.60 |
| H20A | 0.2018 | 0.8435 | 0.5531 | 0.145* | 0.60 |
| H20B | 0.3363 | 0.7840 | 0.5619 | 0.145* | 0.60 |
| C21 | 0.2888 (10) | 0.9402 (8) | 0.5999 (5) | 0.132 (3) | 0.60 |
| H21A | 0.3635 | 0.9671 | 0.5768 | 0.198* | 0.60 |
| H21B | 0.2846 | 0.9068 | 0.6639 | 0.198* | 0.60 |
| H21C | 0.2209 | 1.0016 | 0.5952 | 0.198* | 0.60 |
| C22 | −0.1449 (3) | 0.6338 (2) | 0.43768 (18) | 0.0621 (7) | |
| C23 | −0.1744 (3) | 0.5483 (3) | 0.4068 (2) | 0.0720 (8) | |
| H23 | −0.1134 | 0.4941 | 0.3790 | 0.086* | |
| C24 | −0.2963 (3) | 0.5440 (3) | 0.4179 (2) | 0.0820 (9) | |
| H24 | −0.3169 | 0.4866 | 0.3967 | 0.098* | |
| C25 | −0.3869 (3) | 0.6231 (4) | 0.4594 (2) | 0.0891 (10) | |
| H25 | −0.4685 | 0.6199 | 0.4663 | 0.107* | |
| C26 | −0.3555 (4) | 0.7063 (4) | 0.4904 (3) | 0.1013 (12) | |
| H26 | −0.4164 | 0.7599 | 0.5191 | 0.122* | |
| C27 | −0.2362 (4) | 0.7126 (3) | 0.4800 (3) | 0.0895 (10) | |
| H27 | −0.2165 | 0.7701 | 0.5016 | 0.107* | |
| C28 | 0.0845 (3) | 0.5873 (2) | 0.37838 (19) | 0.0593 (7) | |
| C29 | 0.1745 (3) | 0.6332 (3) | 0.3916 (2) | 0.0678 (8) | |
| C30 | 0.2988 (3) | 0.5944 (3) | 0.3509 (3) | 0.0815 (10) | |
| C31 | 0.2108 (3) | 0.4696 (3) | 0.2899 (2) | 0.0733 (8) | |
| C32 | 0.1543 (3) | 0.3377 (3) | 0.2199 (2) | 0.0700 (8) | |
| C33 | 0.0893 (3) | 0.2762 (3) | 0.2924 (2) | 0.0785 (9) | |
| H33 | 0.0932 | 0.2781 | 0.3536 | 0.094* | |
| C34 | 0.0188 (3) | 0.2120 (3) | 0.2740 (2) | 0.0786 (9) | |
| H34 | −0.0263 | 0.1710 | 0.3232 | 0.094* | |
| C35 | 0.0135 (3) | 0.2071 (3) | 0.1841 (2) | 0.0701 (8) | |
| C36 | 0.0764 (3) | 0.2719 (3) | 0.1126 (2) | 0.0783 (9) | |
| H36 | 0.0713 | 0.2714 | 0.0515 | 0.094* | |
| C37 | 0.1466 (3) | 0.3373 (3) | 0.1306 (2) | 0.0781 (9) | |
| H37 | 0.1887 | 0.3812 | 0.0818 | 0.094* | |
| C38 | −0.0400 (5) | 0.1100 (4) | 0.0814 (3) | 0.1248 (17) | |
| H38A | −0.0841 | 0.1719 | 0.0454 | 0.187* | |
| H38B | −0.0717 | 0.0439 | 0.0850 | 0.187* | |
| H38C | 0.0448 | 0.0972 | 0.0521 | 0.187* | |
| C39 | 0.4370 (5) | 0.4412 (4) | 0.2681 (5) | 0.1387 (19) | |
| H39A | 0.4898 | 0.4463 | 0.3083 | 0.166* | |
| H39B | 0.4363 | 0.3626 | 0.2704 | 0.166* | |
| C40 | 0.4816 (4) | 0.4881 (4) | 0.1756 (5) | 0.1389 (19) | |
| H40A | 0.4737 | 0.5671 | 0.1781 | 0.167* | 0.61 |
| H40B | 0.4220 | 0.4841 | 0.1407 | 0.167* | 0.61 |
| H40C | 0.4748 | 0.5685 | 0.1698 | 0.167* | 0.39 |
| H40D | 0.4417 | 0.4716 | 0.1310 | 0.167* | 0.39 |
| C41 | 0.6105 (11) | 0.4531 (16) | 0.1097 (10) | 0.266 (13) | 0.61 |
| H41A | 0.6056 | 0.4016 | 0.0701 | 0.319* | 0.61 |
| H41B | 0.6361 | 0.5188 | 0.0703 | 0.319* | 0.61 |
| C42 | 0.6979 (10) | 0.3988 (19) | 0.1703 (12) | 0.284 (11) | 0.61 |
| H42A | 0.7216 | 0.4550 | 0.1942 | 0.427* | 0.61 |
| H42B | 0.7687 | 0.3562 | 0.1350 | 0.427* | 0.61 |
| H42C | 0.6605 | 0.3497 | 0.2212 | 0.427* | 0.61 |
| C20' | 0.2534 (19) | 0.9186 (14) | 0.5406 (7) | 0.208 (14) | 0.40 |
| H20C | 0.2578 | 0.9900 | 0.5557 | 0.250* | 0.40 |
| H20D | 0.1686 | 0.9167 | 0.5446 | 0.250* | 0.40 |
| C21' | 0.3110 (14) | 0.8215 (16) | 0.6063 (8) | 0.170 (7) | 0.40 |
| H21D | 0.3036 | 0.7518 | 0.5914 | 0.255* | 0.40 |
| H21E | 0.2700 | 0.8287 | 0.6694 | 0.255* | 0.40 |
| H21F | 0.3957 | 0.8229 | 0.5992 | 0.255* | 0.40 |
| C41' | 0.6174 (7) | 0.4221 (14) | 0.1683 (10) | 0.131 (6) | 0.39 |
| H41C | 0.6526 | 0.4362 | 0.2167 | 0.157* | 0.39 |
| H41D | 0.6214 | 0.3421 | 0.1743 | 0.157* | 0.39 |
| C42' | 0.6838 (12) | 0.4652 (15) | 0.0738 (11) | 0.174 (8) | 0.39 |
| H42D | 0.6476 | 0.4508 | 0.0268 | 0.261* | 0.39 |
| H42E | 0.7680 | 0.4279 | 0.0651 | 0.261* | 0.39 |
| H42F | 0.6785 | 0.5445 | 0.0689 | 0.261* | 0.39 |
| N1 | 0.3984 (2) | 1.1397 (2) | 0.0256 (2) | 0.0718 (7) | |
| N2 | 0.4667 (3) | 1.2151 (2) | 0.0314 (3) | 0.0924 (9) | |
| N3 | 0.4686 (3) | 1.2126 (2) | 0.1183 (3) | 0.0922 (9) | |
| N4 | 0.2861 (2) | 1.0120 (2) | 0.13914 (17) | 0.0651 (6) | |
| N5 | 0.2979 (3) | 1.0252 (2) | 0.29391 (19) | 0.0853 (8) | |
| N6 | −0.0203 (2) | 0.64224 (19) | 0.42687 (15) | 0.0627 (6) | |
| N7 | 0.0072 (3) | 0.7202 (2) | 0.46881 (18) | 0.0786 (8) | |
| N8 | 0.1237 (3) | 0.7147 (2) | 0.44767 (18) | 0.0818 (8) | |
| N9 | 0.0976 (2) | 0.50531 (19) | 0.32598 (16) | 0.0607 (6) | |
| N10 | 0.3093 (2) | 0.5042 (2) | 0.3012 (2) | 0.0888 (9) | |
| O1 | 0.4041 (3) | 1.1411 (2) | 0.3284 (2) | 0.1146 (9) | |
| O2 | 0.1887 (2) | 0.9109 (2) | 0.26967 (15) | 0.0907 (7) | |
| O3 | 0.3883 (2) | 0.6278 (2) | 0.3538 (2) | 0.1099 (9) | |
| O4 | 0.2423 (2) | 0.3919 (2) | 0.23281 (19) | 0.0926 (7) | |
| O5 | 0.0382 (2) | 0.6299 (2) | 0.09702 (18) | 0.0975 (8) | |
| O6 | −0.0535 (2) | 0.1350 (2) | 0.17288 (18) | 0.0985 (8) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0525 (16) | 0.0621 (19) | 0.080 (2) | −0.0022 (14) | −0.0041 (14) | 0.0059 (16) |
| C2 | 0.083 (2) | 0.082 (2) | 0.095 (3) | −0.0164 (19) | −0.0054 (19) | 0.016 (2) |
| C3 | 0.103 (3) | 0.118 (4) | 0.089 (3) | −0.017 (3) | −0.004 (2) | 0.031 (3) |
| C4 | 0.088 (3) | 0.134 (4) | 0.071 (2) | −0.006 (3) | −0.0051 (19) | 0.006 (2) |
| C5 | 0.086 (2) | 0.104 (3) | 0.075 (2) | −0.012 (2) | −0.0074 (18) | −0.008 (2) |
| C6 | 0.069 (2) | 0.080 (2) | 0.072 (2) | −0.0116 (17) | −0.0004 (15) | 0.0038 (17) |
| C7 | 0.0537 (16) | 0.0508 (16) | 0.079 (2) | −0.0017 (13) | −0.0093 (14) | −0.0045 (15) |
| C8 | 0.0682 (19) | 0.0572 (18) | 0.096 (2) | −0.0068 (15) | −0.0154 (17) | −0.0129 (17) |
| C9 | 0.092 (2) | 0.073 (2) | 0.098 (3) | −0.0042 (19) | −0.031 (2) | −0.026 (2) |
| C10 | 0.0747 (19) | 0.0667 (19) | 0.070 (2) | −0.0118 (16) | −0.0160 (15) | −0.0097 (16) |
| C11 | 0.085 (2) | 0.0651 (18) | 0.0525 (16) | −0.0167 (17) | −0.0152 (15) | 0.0094 (14) |
| C12 | 0.072 (2) | 0.071 (2) | 0.0683 (19) | −0.0076 (16) | −0.0023 (15) | 0.0062 (16) |
| C13 | 0.0637 (19) | 0.084 (2) | 0.075 (2) | −0.0144 (17) | −0.0173 (16) | 0.0143 (18) |
| C14 | 0.072 (2) | 0.074 (2) | 0.0682 (19) | −0.0107 (17) | −0.0227 (16) | 0.0077 (16) |
| C15 | 0.074 (2) | 0.084 (2) | 0.078 (2) | −0.0077 (18) | −0.0110 (16) | −0.0093 (18) |
| C16 | 0.0640 (19) | 0.080 (2) | 0.082 (2) | −0.0157 (17) | −0.0182 (16) | 0.0023 (18) |
| C17 | 0.113 (3) | 0.139 (4) | 0.100 (3) | −0.057 (3) | −0.036 (2) | 0.002 (3) |
| C18 | 0.135 (4) | 0.123 (4) | 0.108 (3) | −0.033 (3) | −0.042 (3) | −0.021 (3) |
| C19 | 0.124 (4) | 0.139 (4) | 0.119 (4) | −0.041 (3) | −0.033 (3) | −0.011 (3) |
| C20 | 0.171 (8) | 0.147 (10) | 0.065 (5) | −0.097 (7) | −0.039 (5) | 0.034 (5) |
| C21 | 0.197 (9) | 0.137 (8) | 0.071 (5) | −0.052 (7) | −0.016 (5) | −0.029 (5) |
| C22 | 0.082 (2) | 0.0595 (17) | 0.0467 (14) | −0.0145 (15) | −0.0234 (13) | 0.0009 (13) |
| C23 | 0.081 (2) | 0.075 (2) | 0.0632 (18) | −0.0214 (17) | −0.0150 (15) | −0.0092 (15) |
| C24 | 0.089 (2) | 0.094 (2) | 0.073 (2) | −0.032 (2) | −0.0205 (18) | −0.0121 (18) |
| C25 | 0.075 (2) | 0.116 (3) | 0.075 (2) | −0.011 (2) | −0.0279 (18) | −0.006 (2) |
| C26 | 0.088 (3) | 0.105 (3) | 0.112 (3) | 0.010 (2) | −0.038 (2) | −0.033 (2) |
| C27 | 0.104 (3) | 0.079 (2) | 0.096 (3) | −0.007 (2) | −0.040 (2) | −0.027 (2) |
| C28 | 0.0784 (19) | 0.0533 (16) | 0.0523 (15) | −0.0204 (15) | −0.0306 (14) | 0.0081 (13) |
| C29 | 0.090 (2) | 0.0656 (18) | 0.0599 (17) | −0.0326 (17) | −0.0351 (16) | 0.0073 (15) |
| C30 | 0.086 (2) | 0.078 (2) | 0.097 (2) | −0.0317 (19) | −0.049 (2) | 0.0062 (18) |
| C31 | 0.068 (2) | 0.0630 (19) | 0.097 (2) | −0.0120 (15) | −0.0364 (17) | −0.0077 (17) |
| C32 | 0.0551 (17) | 0.0622 (18) | 0.093 (2) | −0.0032 (14) | −0.0152 (15) | −0.0213 (17) |
| C33 | 0.087 (2) | 0.078 (2) | 0.071 (2) | −0.0079 (18) | −0.0181 (17) | −0.0196 (17) |
| C34 | 0.086 (2) | 0.081 (2) | 0.069 (2) | −0.0279 (18) | −0.0039 (16) | −0.0120 (17) |
| C35 | 0.0668 (18) | 0.0706 (19) | 0.073 (2) | −0.0176 (15) | −0.0114 (15) | −0.0085 (16) |
| C36 | 0.089 (2) | 0.083 (2) | 0.0656 (19) | −0.0233 (19) | −0.0201 (16) | −0.0038 (17) |
| C37 | 0.076 (2) | 0.074 (2) | 0.080 (2) | −0.0204 (17) | −0.0094 (16) | −0.0014 (17) |
| C38 | 0.166 (5) | 0.150 (4) | 0.093 (3) | −0.082 (4) | −0.041 (3) | −0.017 (3) |
| C39 | 0.111 (4) | 0.105 (4) | 0.219 (7) | −0.035 (3) | −0.059 (4) | −0.020 (4) |
| C40 | 0.109 (4) | 0.106 (4) | 0.202 (6) | −0.027 (3) | −0.032 (4) | −0.015 (4) |
| C41 | 0.162 (15) | 0.182 (13) | 0.32 (3) | 0.038 (11) | 0.090 (15) | 0.014 (17) |
| C42 | 0.108 (9) | 0.44 (3) | 0.254 (19) | 0.042 (14) | −0.034 (11) | −0.048 (19) |
| C20' | 0.41 (4) | 0.164 (19) | 0.071 (10) | −0.15 (2) | −0.052 (14) | 0.041 (11) |
| C21' | 0.121 (11) | 0.22 (2) | 0.132 (13) | −0.017 (12) | 0.003 (9) | 0.008 (13) |
| C41' | 0.044 (6) | 0.194 (18) | 0.154 (13) | −0.035 (8) | −0.026 (7) | −0.002 (11) |
| C42' | 0.100 (11) | 0.192 (16) | 0.205 (18) | −0.034 (11) | 0.076 (12) | −0.095 (15) |
| N1 | 0.0600 (15) | 0.0578 (15) | 0.0877 (19) | −0.0113 (12) | −0.0045 (13) | −0.0002 (13) |
| N2 | 0.082 (2) | 0.0746 (19) | 0.115 (3) | −0.0258 (16) | −0.0075 (17) | −0.0036 (18) |
| N3 | 0.085 (2) | 0.0727 (19) | 0.121 (3) | −0.0197 (16) | −0.0201 (18) | −0.0163 (18) |
| N4 | 0.0657 (15) | 0.0622 (15) | 0.0607 (15) | −0.0086 (12) | −0.0096 (11) | −0.0021 (11) |
| N5 | 0.102 (2) | 0.0855 (19) | 0.0711 (18) | −0.0220 (17) | −0.0203 (15) | −0.0098 (15) |
| N6 | 0.0871 (18) | 0.0588 (14) | 0.0496 (13) | −0.0233 (13) | −0.0235 (12) | −0.0023 (11) |
| N7 | 0.111 (2) | 0.0760 (17) | 0.0627 (15) | −0.0389 (16) | −0.0237 (15) | −0.0095 (13) |
| N8 | 0.117 (2) | 0.0825 (19) | 0.0650 (16) | −0.0486 (18) | −0.0337 (15) | −0.0017 (14) |
| N9 | 0.0634 (15) | 0.0572 (14) | 0.0666 (14) | −0.0112 (11) | −0.0280 (11) | −0.0040 (11) |
| N10 | 0.0599 (16) | 0.0815 (19) | 0.134 (3) | −0.0122 (14) | −0.0391 (16) | −0.0159 (18) |
| O1 | 0.144 (3) | 0.103 (2) | 0.114 (2) | −0.0306 (18) | −0.0407 (18) | −0.0324 (17) |
| O2 | 0.1180 (19) | 0.0982 (17) | 0.0616 (13) | −0.0480 (16) | −0.0136 (12) | 0.0013 (12) |
| O3 | 0.0943 (18) | 0.114 (2) | 0.147 (2) | −0.0462 (16) | −0.0537 (17) | −0.0124 (18) |
| O4 | 0.0615 (13) | 0.0880 (16) | 0.138 (2) | −0.0121 (12) | −0.0179 (13) | −0.0449 (16) |
| O5 | 0.0979 (18) | 0.1090 (19) | 0.0956 (17) | −0.0366 (15) | −0.0251 (14) | −0.0123 (15) |
| O6 | 0.1103 (19) | 0.1090 (19) | 0.0899 (17) | −0.0546 (16) | −0.0113 (14) | −0.0179 (14) |
Geometric parameters (Å, °)
| C1—C6 | 1.366 (4) | C25—H25 | 0.9300 |
| C1—C2 | 1.379 (5) | C26—C27 | 1.364 (5) |
| C1—N1 | 1.422 (4) | C26—H26 | 0.9300 |
| C2—C3 | 1.370 (6) | C27—H27 | 0.9300 |
| C2—H2 | 0.9300 | C28—N6 | 1.354 (4) |
| C3—C4 | 1.365 (6) | C28—N9 | 1.360 (4) |
| C3—H3 | 0.9300 | C28—C29 | 1.363 (4) |
| C4—C5 | 1.373 (5) | C29—N8 | 1.373 (4) |
| C4—H4 | 0.9300 | C29—C30 | 1.422 (5) |
| C5—C6 | 1.390 (5) | C30—O3 | 1.211 (4) |
| C5—H5 | 0.9300 | C30—N10 | 1.426 (4) |
| C6—H6 | 0.9300 | C31—N9 | 1.293 (4) |
| C7—C8 | 1.354 (4) | C31—O4 | 1.339 (4) |
| C7—N4 | 1.355 (4) | C31—N10 | 1.359 (4) |
| C7—N1 | 1.366 (4) | C32—C37 | 1.359 (5) |
| C8—N3 | 1.360 (4) | C32—C33 | 1.371 (5) |
| C8—C9 | 1.432 (5) | C32—O4 | 1.407 (4) |
| C9—O1 | 1.217 (4) | C33—C34 | 1.365 (4) |
| C9—N5 | 1.413 (5) | C33—H33 | 0.9300 |
| C10—N4 | 1.301 (4) | C34—C35 | 1.373 (4) |
| C10—O2 | 1.339 (4) | C34—H34 | 0.9300 |
| C10—N5 | 1.363 (4) | C35—O6 | 1.367 (4) |
| C11—C12 | 1.365 (4) | C35—C36 | 1.371 (4) |
| C11—C16 | 1.368 (5) | C36—C37 | 1.372 (4) |
| C11—O2 | 1.406 (4) | C36—H36 | 0.9300 |
| C12—C13 | 1.376 (5) | C37—H37 | 0.9300 |
| C12—H12 | 0.9300 | C38—O6 | 1.424 (5) |
| C13—C14 | 1.379 (5) | C38—H38A | 0.9600 |
| C13—H13 | 0.9300 | C38—H38B | 0.9600 |
| C14—O5 | 1.374 (4) | C38—H38C | 0.9600 |
| C14—C15 | 1.376 (4) | C39—C40 | 1.414 (8) |
| C15—C16 | 1.382 (5) | C39—N10 | 1.510 (6) |
| C15—H15 | 0.9300 | C39—H39A | 0.9700 |
| C16—H16 | 0.9300 | C39—H39B | 0.9700 |
| C17—O5 | 1.413 (4) | C40—C41' | 1.574 (9) |
| C17—H17A | 0.9600 | C40—C41 | 1.590 (9) |
| C17—H17B | 0.9600 | C40—H40A | 0.9700 |
| C17—H17C | 0.9600 | C40—H40B | 0.9700 |
| C18—C19 | 1.454 (6) | C40—H40C | 0.9700 |
| C18—N5 | 1.556 (5) | C40—H40D | 0.9700 |
| C18—H18A | 0.9700 | C41—C42 | 1.471 (10) |
| C18—H18B | 0.9700 | C41—H41A | 0.9700 |
| C19—C20 | 1.560 (7) | C41—H41B | 0.9700 |
| C19—C20' | 1.587 (10) | C42—H42A | 0.9600 |
| C19—H19A | 0.9700 | C42—H42B | 0.9600 |
| C19—H19B | 0.9700 | C42—H42C | 0.9600 |
| C19—H19C | 0.9700 | C20'—C21' | 1.525 (10) |
| C19—H19D | 0.9700 | C20'—H20C | 0.9700 |
| C20—C21 | 1.486 (8) | C20'—H20D | 0.9700 |
| C20—H20A | 0.9700 | C21'—H21D | 0.9600 |
| C20—H20B | 0.9700 | C21'—H21E | 0.9600 |
| C21—H21A | 0.9600 | C21'—H21F | 0.9600 |
| C21—H21B | 0.9600 | C41'—C42' | 1.499 (10) |
| C21—H21C | 0.9600 | C41'—H41C | 0.9700 |
| C22—C23 | 1.373 (4) | C41'—H41D | 0.9700 |
| C22—C27 | 1.377 (5) | C42'—H42D | 0.9600 |
| C22—N6 | 1.432 (4) | C42'—H42E | 0.9600 |
| C23—C24 | 1.387 (5) | C42'—H42F | 0.9600 |
| C23—H23 | 0.9300 | N1—N2 | 1.384 (4) |
| C24—C25 | 1.372 (5) | N2—N3 | 1.298 (4) |
| C24—H24 | 0.9300 | N6—N7 | 1.379 (3) |
| C25—C26 | 1.361 (5) | N7—N8 | 1.292 (4) |
| C6—C1—C2 | 119.8 (4) | N8—C29—C30 | 129.9 (3) |
| C6—C1—N1 | 121.5 (3) | O3—C30—C29 | 129.1 (4) |
| C2—C1—N1 | 118.8 (3) | O3—C30—N10 | 120.7 (4) |
| C3—C2—C1 | 119.6 (4) | C29—C30—N10 | 110.2 (3) |
| C3—C2—H2 | 120.2 | N9—C31—O4 | 120.8 (3) |
| C1—C2—H2 | 120.2 | N9—C31—N10 | 127.0 (3) |
| C4—C3—C2 | 121.2 (4) | O4—C31—N10 | 112.2 (3) |
| C4—C3—H3 | 119.4 | C37—C32—C33 | 120.5 (3) |
| C2—C3—H3 | 119.4 | C37—C32—O4 | 117.0 (3) |
| C3—C4—C5 | 119.4 (4) | C33—C32—O4 | 122.0 (3) |
| C3—C4—H4 | 120.3 | C34—C33—C32 | 119.3 (3) |
| C5—C4—H4 | 120.3 | C34—C33—H33 | 120.3 |
| C4—C5—C6 | 119.9 (4) | C32—C33—H33 | 120.3 |
| C4—C5—H5 | 120.1 | C33—C34—C35 | 121.0 (3) |
| C6—C5—H5 | 120.1 | C33—C34—H34 | 119.5 |
| C1—C6—C5 | 120.1 (3) | C35—C34—H34 | 119.5 |
| C1—C6—H6 | 119.9 | O6—C35—C36 | 124.8 (3) |
| C5—C6—H6 | 119.9 | O6—C35—C34 | 116.4 (3) |
| C8—C7—N4 | 127.8 (3) | C36—C35—C34 | 118.8 (3) |
| C8—C7—N1 | 105.1 (3) | C35—C36—C37 | 120.5 (3) |
| N4—C7—N1 | 127.0 (3) | C35—C36—H36 | 119.7 |
| C7—C8—N3 | 110.2 (3) | C37—C36—H36 | 119.7 |
| C7—C8—C9 | 120.2 (3) | C32—C37—C36 | 119.8 (3) |
| N3—C8—C9 | 129.5 (3) | C32—C37—H37 | 120.1 |
| O1—C9—N5 | 120.9 (4) | C36—C37—H37 | 120.1 |
| O1—C9—C8 | 128.0 (4) | O6—C38—H38A | 109.5 |
| N5—C9—C8 | 111.0 (3) | O6—C38—H38B | 109.5 |
| N4—C10—O2 | 121.5 (3) | H38A—C38—H38B | 109.5 |
| N4—C10—N5 | 127.2 (3) | O6—C38—H38C | 109.5 |
| O2—C10—N5 | 111.3 (3) | H38A—C38—H38C | 109.5 |
| C12—C11—C16 | 121.4 (3) | H38B—C38—H38C | 109.5 |
| C12—C11—O2 | 116.8 (3) | C40—C39—N10 | 109.1 (4) |
| C16—C11—O2 | 121.2 (3) | C40—C39—H39A | 109.9 |
| C11—C12—C13 | 120.2 (3) | N10—C39—H39A | 109.9 |
| C11—C12—H12 | 119.9 | C40—C39—H39B | 109.9 |
| C13—C12—H12 | 119.9 | N10—C39—H39B | 109.9 |
| C12—C13—C14 | 119.3 (3) | H39A—C39—H39B | 108.3 |
| C12—C13—H13 | 120.3 | C39—C40—C41' | 97.0 (6) |
| C14—C13—H13 | 120.3 | C39—C40—C41 | 129.1 (7) |
| O5—C14—C15 | 115.7 (3) | C41'—C40—C41 | 33.0 (6) |
| O5—C14—C13 | 124.6 (3) | C39—C40—H40A | 105.0 |
| C15—C14—C13 | 119.7 (3) | C41'—C40—H40A | 112.3 |
| C14—C15—C16 | 121.0 (3) | C41—C40—H40A | 105.0 |
| C14—C15—H15 | 119.5 | C39—C40—H40B | 105.0 |
| C16—C15—H15 | 119.5 | C41'—C40—H40B | 128.6 |
| C11—C16—C15 | 118.3 (3) | C41—C40—H40B | 105.0 |
| C11—C16—H16 | 120.9 | H40A—C40—H40B | 105.9 |
| C15—C16—H16 | 120.9 | C39—C40—H40C | 111.7 |
| O5—C17—H17A | 109.5 | C41'—C40—H40C | 112.5 |
| O5—C17—H17B | 109.5 | C41—C40—H40C | 101.3 |
| H17A—C17—H17B | 109.5 | H40A—C40—H40C | 7.0 |
| O5—C17—H17C | 109.5 | H40B—C40—H40C | 101.4 |
| H17A—C17—H17C | 109.5 | C39—C40—H40D | 112.6 |
| H17B—C17—H17C | 109.5 | C41'—C40—H40D | 112.7 |
| C19—C18—N5 | 109.9 (4) | C41—C40—H40D | 89.7 |
| C19—C18—H18A | 109.7 | H40A—C40—H40D | 115.4 |
| N5—C18—H18A | 109.7 | H40B—C40—H40D | 16.0 |
| C19—C18—H18B | 109.7 | H40C—C40—H40D | 109.8 |
| N5—C18—H18B | 109.7 | C42—C41—C40 | 107.2 (9) |
| H18A—C18—H18B | 108.2 | C42—C41—H41A | 110.3 |
| C18—C19—C20 | 114.6 (6) | C40—C41—H41A | 110.3 |
| C18—C19—C20' | 89.4 (7) | C42—C41—H41B | 110.3 |
| C20—C19—C20' | 28.8 (6) | C40—C41—H41B | 110.3 |
| C18—C19—H19A | 108.6 | H41A—C41—H41B | 108.5 |
| C20—C19—H19A | 108.6 | C21'—C20'—C19 | 105.0 (9) |
| C20'—C19—H19A | 106.6 | C21'—C20'—H20C | 110.7 |
| C18—C19—H19B | 108.6 | C19—C20'—H20C | 110.7 |
| C20—C19—H19B | 108.6 | C21'—C20'—H20D | 110.7 |
| C20'—C19—H19B | 133.2 | C19—C20'—H20D | 110.7 |
| H19A—C19—H19B | 107.6 | H20C—C20'—H20D | 108.8 |
| C18—C19—H19C | 113.7 | C20'—C21'—H21D | 109.5 |
| C20—C19—H19C | 111.4 | C20'—C21'—H21E | 109.5 |
| C20'—C19—H19C | 113.5 | H21D—C21'—H21E | 109.5 |
| H19A—C19—H19C | 9.2 | C20'—C21'—H21F | 109.5 |
| H19B—C19—H19C | 98.5 | H21D—C21'—H21F | 109.5 |
| C18—C19—H19D | 113.7 | H21E—C21'—H21F | 109.5 |
| C20—C19—H19D | 90.2 | C42'—C41'—C40 | 105.5 (8) |
| C20'—C19—H19D | 114.1 | C42'—C41'—H41C | 110.6 |
| H19A—C19—H19D | 120.1 | C40—C41'—H41C | 110.6 |
| H19B—C19—H19D | 19.2 | C42'—C41'—H41D | 110.6 |
| H19C—C19—H19D | 111.0 | C40—C41'—H41D | 110.6 |
| C21—C20—C19 | 107.0 (6) | H41C—C41'—H41D | 108.8 |
| C21—C20—H20A | 110.3 | C41'—C42'—H42D | 109.5 |
| C19—C20—H20A | 110.3 | C41'—C42'—H42E | 109.5 |
| C21—C20—H20B | 110.3 | H42D—C42'—H42E | 109.5 |
| C19—C20—H20B | 110.3 | C41'—C42'—H42F | 109.5 |
| H20A—C20—H20B | 108.6 | H42D—C42'—H42F | 109.5 |
| C23—C22—C27 | 119.8 (3) | H42E—C42'—H42F | 109.5 |
| C23—C22—N6 | 120.9 (3) | C7—N1—N2 | 108.1 (3) |
| C27—C22—N6 | 119.3 (3) | C7—N1—C1 | 132.5 (3) |
| C22—C23—C24 | 119.0 (3) | N2—N1—C1 | 119.4 (3) |
| C22—C23—H23 | 120.5 | N3—N2—N1 | 108.7 (3) |
| C24—C23—H23 | 120.5 | N2—N3—C8 | 107.8 (3) |
| C25—C24—C23 | 121.0 (3) | C10—N4—C7 | 111.3 (3) |
| C25—C24—H24 | 119.5 | C10—N5—C9 | 122.4 (3) |
| C23—C24—H24 | 119.5 | C10—N5—C18 | 120.5 (3) |
| C26—C25—C24 | 118.9 (3) | C9—N5—C18 | 116.7 (3) |
| C26—C25—H25 | 120.5 | C28—N6—N7 | 108.7 (2) |
| C24—C25—H25 | 120.5 | C28—N6—C22 | 131.7 (2) |
| C25—C26—C27 | 121.2 (4) | N7—N6—C22 | 119.5 (3) |
| C25—C26—H26 | 119.4 | N8—N7—N6 | 108.7 (3) |
| C27—C26—H26 | 119.4 | N7—N8—C29 | 108.0 (2) |
| C26—C27—C22 | 120.1 (3) | C31—N9—C28 | 111.8 (2) |
| C26—C27—H27 | 119.9 | C31—N10—C30 | 122.8 (3) |
| C22—C27—H27 | 119.9 | C31—N10—C39 | 120.7 (3) |
| N6—C28—N9 | 127.6 (2) | C30—N10—C39 | 116.3 (3) |
| N6—C28—C29 | 105.3 (3) | C10—O2—C11 | 121.3 (2) |
| N9—C28—C29 | 127.0 (3) | C31—O4—C32 | 120.4 (2) |
| C28—C29—N8 | 109.2 (3) | C14—O5—C17 | 118.2 (3) |
| C28—C29—C30 | 121.0 (3) | C35—O6—C38 | 117.6 (3) |
| C6—C1—C2—C3 | −0.7 (5) | N4—C7—N1—N2 | 179.2 (3) |
| N1—C1—C2—C3 | 178.0 (3) | C8—C7—N1—C1 | −178.7 (3) |
| C1—C2—C3—C4 | 1.1 (6) | N4—C7—N1—C1 | 0.4 (5) |
| C2—C3—C4—C5 | −1.2 (6) | C6—C1—N1—C7 | −13.3 (5) |
| C3—C4—C5—C6 | 0.8 (6) | C2—C1—N1—C7 | 168.0 (3) |
| C2—C1—C6—C5 | 0.3 (5) | C6—C1—N1—N2 | 168.0 (3) |
| N1—C1—C6—C5 | −178.3 (3) | C2—C1—N1—N2 | −10.6 (4) |
| C4—C5—C6—C1 | −0.4 (5) | C7—N1—N2—N3 | 0.2 (3) |
| N4—C7—C8—N3 | −179.4 (3) | C1—N1—N2—N3 | 179.1 (3) |
| N1—C7—C8—N3 | −0.3 (3) | N1—N2—N3—C8 | −0.4 (4) |
| N4—C7—C8—C9 | −0.8 (5) | C7—C8—N3—N2 | 0.4 (4) |
| N1—C7—C8—C9 | 178.3 (3) | C9—C8—N3—N2 | −178.0 (3) |
| C7—C8—C9—O1 | −178.8 (3) | O2—C10—N4—C7 | 178.2 (3) |
| N3—C8—C9—O1 | −0.5 (6) | N5—C10—N4—C7 | −0.2 (4) |
| C7—C8—C9—N5 | 0.2 (4) | C8—C7—N4—C10 | 0.8 (4) |
| N3—C8—C9—N5 | 178.5 (3) | N1—C7—N4—C10 | −178.1 (3) |
| C16—C11—C12—C13 | 1.6 (5) | N4—C10—N5—C9 | −0.4 (5) |
| O2—C11—C12—C13 | −170.2 (3) | O2—C10—N5—C9 | −178.9 (3) |
| C11—C12—C13—C14 | −0.2 (4) | N4—C10—N5—C18 | 172.7 (3) |
| C12—C13—C14—O5 | 179.2 (3) | O2—C10—N5—C18 | −5.9 (5) |
| C12—C13—C14—C15 | −1.7 (5) | O1—C9—N5—C10 | 179.4 (3) |
| O5—C14—C15—C16 | −178.5 (3) | C8—C9—N5—C10 | 0.3 (5) |
| C13—C14—C15—C16 | 2.3 (5) | O1—C9—N5—C18 | 6.1 (5) |
| C12—C11—C16—C15 | −1.0 (5) | C8—C9—N5—C18 | −173.0 (3) |
| O2—C11—C16—C15 | 170.4 (3) | C19—C18—N5—C10 | 96.9 (4) |
| C14—C15—C16—C11 | −1.0 (5) | C19—C18—N5—C9 | −89.7 (4) |
| N5—C18—C19—C20 | −175.0 (4) | N9—C28—N6—N7 | −178.9 (2) |
| N5—C18—C19—C20' | 170.7 (10) | C29—C28—N6—N7 | −0.1 (3) |
| C18—C19—C20—C21 | −72.3 (10) | N9—C28—N6—C22 | −0.8 (4) |
| C20'—C19—C20—C21 | −41 (2) | C29—C28—N6—C22 | 178.0 (2) |
| C27—C22—C23—C24 | 0.9 (4) | C23—C22—N6—C28 | 10.2 (4) |
| N6—C22—C23—C24 | −179.4 (3) | C27—C22—N6—C28 | −170.0 (3) |
| C22—C23—C24—C25 | −0.4 (5) | C23—C22—N6—N7 | −171.8 (2) |
| C23—C24—C25—C26 | −0.3 (5) | C27—C22—N6—N7 | 7.9 (4) |
| C24—C25—C26—C27 | 0.5 (6) | C28—N6—N7—N8 | 0.1 (3) |
| C25—C26—C27—C22 | −0.1 (6) | C22—N6—N7—N8 | −178.3 (2) |
| C23—C22—C27—C26 | −0.7 (5) | N6—N7—N8—C29 | 0.0 (3) |
| N6—C22—C27—C26 | 179.6 (3) | C28—C29—N8—N7 | −0.1 (3) |
| N6—C28—C29—N8 | 0.1 (3) | C30—C29—N8—N7 | −179.2 (3) |
| N9—C28—C29—N8 | 178.9 (2) | O4—C31—N9—C28 | −177.3 (3) |
| N6—C28—C29—C30 | 179.3 (3) | N10—C31—N9—C28 | 1.1 (4) |
| N9—C28—C29—C30 | −1.9 (4) | N6—C28—N9—C31 | −179.1 (3) |
| C28—C29—C30—O3 | 177.9 (3) | C29—C28—N9—C31 | 2.4 (4) |
| N8—C29—C30—O3 | −3.1 (6) | N9—C31—N10—C30 | −5.1 (5) |
| C28—C29—C30—N10 | −1.8 (4) | O4—C31—N10—C30 | 173.4 (3) |
| N8—C29—C30—N10 | 177.2 (3) | N9—C31—N10—C39 | 169.6 (4) |
| C37—C32—C33—C34 | 1.7 (5) | O4—C31—N10—C39 | −11.9 (5) |
| O4—C32—C33—C34 | −170.3 (3) | O3—C30—N10—C31 | −174.8 (3) |
| C32—C33—C34—C35 | 0.9 (5) | C29—C30—N10—C31 | 5.0 (4) |
| C33—C34—C35—O6 | 176.0 (3) | O3—C30—N10—C39 | 10.3 (5) |
| C33—C34—C35—C36 | −2.8 (5) | C29—C30—N10—C39 | −169.9 (4) |
| O6—C35—C36—C37 | −176.5 (3) | C40—C39—N10—C31 | 91.3 (5) |
| C34—C35—C36—C37 | 2.3 (5) | C40—C39—N10—C30 | −93.7 (5) |
| C33—C32—C37—C36 | −2.2 (5) | N4—C10—O2—C11 | 8.4 (5) |
| O4—C32—C37—C36 | 170.1 (3) | N5—C10—O2—C11 | −173.0 (3) |
| C35—C36—C37—C32 | 0.2 (5) | C12—C11—O2—C10 | −132.0 (3) |
| N10—C39—C40—C41' | 170.7 (7) | C16—C11—O2—C10 | 56.2 (4) |
| N10—C39—C40—C41 | 179.6 (11) | N9—C31—O4—C32 | −7.8 (5) |
| C39—C40—C41—C42 | −25 (2) | N10—C31—O4—C32 | 173.6 (3) |
| C41'—C40—C41—C42 | −9.0 (16) | C37—C32—O4—C31 | 128.2 (3) |
| C18—C19—C20'—C21' | 174.3 (17) | C33—C32—O4—C31 | −59.6 (4) |
| C20—C19—C20'—C21' | 22.1 (13) | C15—C14—O5—C17 | 166.1 (3) |
| C39—C40—C41'—C42' | −177.3 (11) | C13—C14—O5—C17 | −14.8 (5) |
| C41—C40—C41'—C42' | 15.4 (17) | C36—C35—O6—C38 | 11.9 (5) |
| C8—C7—N1—N2 | 0.1 (3) | C34—C35—O6—C38 | −166.9 (4) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: YA2108).
References
- Bariana, D. S. (1971). J. Med. Chem. 14, 535–543. [DOI] [PubMed]
- Bruker (2001). SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Ding, M. W., Xu, S. Z. & Zhao, J. F. (2004). J. Org. Chem. 69, 8366–8371. [DOI] [PubMed]
- Holland, A., Jackson, D., Chaplen, P., Lunt, E., Marshall, S., Pain, C. L. & Wooldridge, K. R. H. (1975). Eur. J. Med. Chem. 10, 447–449.
- Levine, R. J., Hall, T. C. & Harris, C. A. (1963). Cancer (N. Y.), 16, 269–272. [DOI] [PubMed]
- Mitchell, J. H., Skipper, H. E. & Bennett, L. L. (1950). Cancer Res. 10, 647–649. [PubMed]
- Montgomery, J. A., Schabel, F. M. & Skipper, H. E. (1962). Cancer Res. 22, 504–509. [PubMed]
- Roblin, R. O., Lampen, J. O., English, J. P., Cole, Q. P. & Vaughan, J. R. (1945). J. Am. Chem. Soc. 67, 290–294.
- Sheldrick, G. M. (1996). SADABS. University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wang, H.-M., Zeng, X.-H., Hu, Z.-Q., Li, G.-H. & Tian, J.-H. (2006). Acta Cryst. E62, o5038–o5040.
- Yamamoto, I., Inoki, R., Tamari, Y. & Iwatsubo, K. (1967). Jpn J. Pharmacol. 17, 140–142. [DOI] [PubMed]
- Zeng, X.-H., Liu, X.-L., Deng, S.-H., Chen, P. & Wang, H.-M. (2009). Acta Cryst. E65, o2583–o2584. [DOI] [PMC free article] [PubMed]
- Zeng, X.-H., Wang, H.-M., Ding, M.-W. & He, H.-W. (2006). Acta Cryst. E62, o1888–o1890.
- Zhao, J.-F., Hu, Y.-G., Ding, M.-W. & He, H.-W. (2005). Acta Cryst. E61, o2791–o2792.
- Zhao, J. F., Wang, C. G. & Ding, M. W. (2005). Chin. J. Struct. Chem. 24, 439–444.
- Zhao, J. F., Xie, C., Ding, M. W. & He, H. W. (2005). Chem. Lett. 34, 1020–1022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809046017/ya2108sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809046017/ya2108Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report