Abstract
Crystals of the title compound, NaScP2O7, were grown by a flux method. The crystal structure is isotypic with those of α-NaTiP2O7, NaYbP2O7 and NaLuP2O7, and is closely related to that of NaYP2O7. The structural set-up consists of a three-dimensional framework of P2O7 units that are corner-shared by ScO6 octahedra, forming tunnels running parallel to [010]. The Na atoms are situated in the tunnels and are surrounded by nine O atoms in a distorted environment.
Related literature
Previous X-ray powder data of NaScP2O7 were reported by Vitins et al. (2000 ▶). NaScP2O7 is isotypic with α-NaTiP2O7 (Leclaire et al., 1988 ▶), NaYbP2O7 (Férid et al., 2004 ▶) and NaLuP2O7 (Yuan et al., 2007 ▶) and shows similar structural features as NaYP2O7 (Hamady & Jouini, 1996 ▶). Both structure types are topologically related to β-cristobalite (Leclaire et al., 1988 ▶). For a detailed review on the structures of A I M IIIP2O7-type diphosphates, see: Li et al. (2005 ▶); Schwendtner & Kolitsch (2004 ▶). For possible applications as scintillators or phosphor materials based on A I M IIIP2O7-type diphosphates, see: Hizhnyi et al. (2007 ▶, 2008 ▶). For background to structural parameters, see: Brese & O’Keeffe (1991 ▶); Robinson et al. (1971 ▶).
Experimental
Crystal data
NaScP2O7
M r = 241.89
Monoclinic,
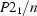
a = 8.9044 (18) Å
b = 5.3300 (11) Å
c = 12.516 (3) Å
β = 104.11 (3)°
V = 576.1 (2) Å3
Z = 4
Mo Kα radiation
μ = 1.89 mm−1
T = 293 K
0.40 × 0.15 × 0.05 mm
Data collection
Kuma KM-4-CCD diffractometer
Absorption correction: multi-scan (CrysAlis CCD; Oxford Diffraction, 2003 ▶) T min = 0.067, T max = 0.093
5082 measured reflections
1018 independent reflections
932 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.025
wR(F 2) = 0.082
S = 1.18
1018 reflections
101 parameters
Δρmax = 0.46 e Å−3
Δρmin = −0.46 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2003 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis RED (Oxford Diffraction, 2003 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ATOMS (Dowty, 2003 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809046224/wm2274sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809046224/wm2274Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Sc—O3 | 2.0217 (19) |
| Sc—O6i | 2.0770 (17) |
| Sc—O7ii | 2.1112 (17) |
| Sc—O1 | 2.1220 (16) |
| Sc—O2 | 2.1220 (16) |
| Sc—O4 | 2.1506 (18) |
| P1—O6 | 1.5088 (17) |
| P1—O7 | 1.5254 (17) |
| P1—O4 | 1.5313 (18) |
| P1—O5iii | 1.6114 (17) |
| P2—O3 | 1.5013 (19) |
| P2—O1iv | 1.5278 (16) |
| P2—O2v | 1.5332 (16) |
| P2—O5 | 1.6151 (17) |
| P1vi—O5—P2 | 125.47 (10) |
Symmetry codes: (i) 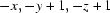 ; (ii)
; (ii) 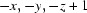 ; (iii)
; (iii) 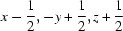 ; (iv)
; (iv) 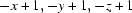 ; (v)
; (v) 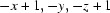 ; (vi)
; (vi) 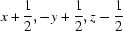 .
.
Acknowledgments
The work was supported by grant MK00009486201.
supplementary crystallographic information
Comment
AIMIIITV2O7-type compounds recently have received an increased attention, partly due to their possible applications as scintillators or phosphor materials (Hizhnyi et al., 2007; Hizhnyi et al., 2008). So far, the AIMIIIP2O7-type diphosphates are known to adopt eight different structure types which depends on the ratio of ionic radii of the alkali metal and the rare earth element or the three-valent metal MIII. Among the eight different structure types, the KAlP2O7-type structures are most common. For a detailed review including also diarsenates, see: Schwendtner & Kolitsch (2004); Li et al. (2005). In this article we present the structure of NaScP2O7 determined from single-crystal x-ray diffaction data. Previous X-ray powder data of NaScP2O7 were reported by Vitins et al. (2000). However, authors could not index all reflections at that time, probably because of by-products. The crystal structure of the title compound is isotypic with α-NaTiP2O7 (Leclaire et al., 1988), NaYbP2O7 (Férid et al., 2004) and NaLuP2O7 (Yuan et al., 2007). It is also closely related to that of NaYP2O7 (Hamady and Jouini, 1996) and β-cristobalite (Leclaire et al., 1988).
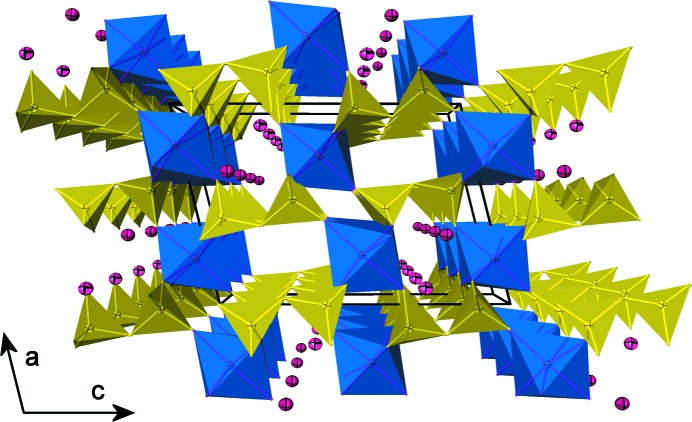
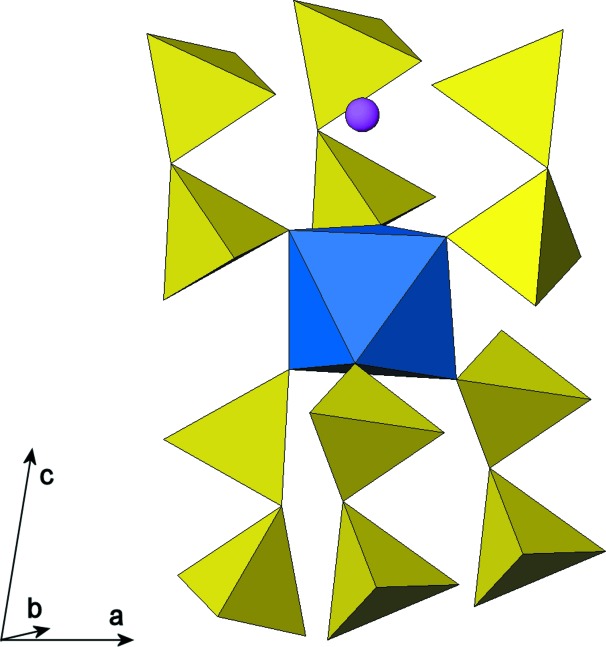
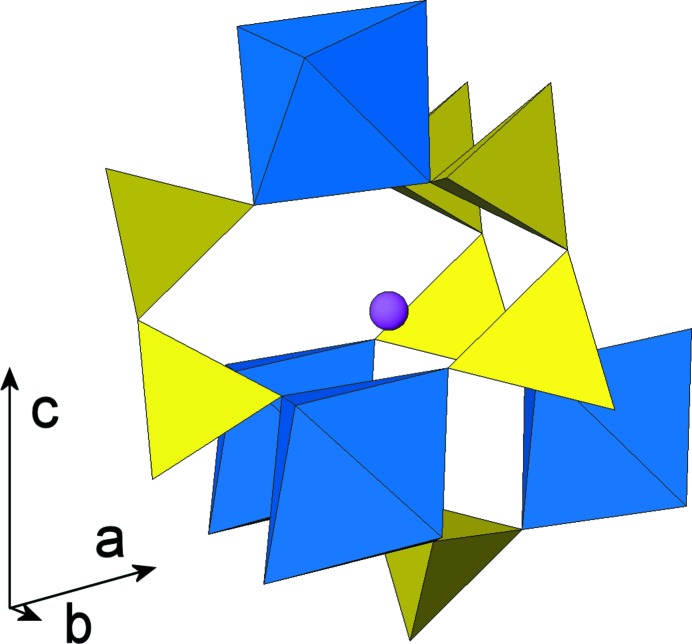
All atoms in the crystal structure occupy general positions. The structure is characterized by a three-dimensional framework of PO4 tetrahedra (forming P2O7 groups via corner-sharing) and ScO6 octahedra leading to narrow tunnels parallel to [010] which are occupied by Na atoms (Fig. 1). One ScO6 octahedron is corner-linked to six tetrahedra of six different diphosphate groups, which are all oriented approximately perpendicular to (001) (Fig. 2). Tunnels are formed by stacking pseudohexagonal rings of [Sc2P4O22] units. A cage enclosing one Na atom is formed by three P2O7 groups, connected to four ScO6 octahedra (Fig. 3).
The P—O bond-lengths range between 1.5088 (17) Å and 1.5332 (16) Å for terminal O of the diphosphate group that are connected to octahedra. The P1—O5bridge—P2 angle is 125.47 (10) °, and corresponding bond lengths to the bridging O atom are 1.6114 (17) Å and 1.6151 (17) Å for <P1—O5> and <P2—O5>, respectively. The average Sc—O bond length is 2.101 Å, corresponding well with the average value for oxide compounds (2.105 Å; Brese & O'Keeffe, 1991). The ScO6 octahedron is significantly less distorted (in terms of quadatic elongation; Robinson et al., 1971) in comparison with the equivalent polyhedra in α-NaTi3+P2O7, NaLuP2O7 and NaYP2O7; the polyhedral distortion is the lowest in NaYbP2O7 structure.
Experimental
NaScP2O7 crystals were grown by the flux-growth technique. The flux, sodium hexametaphosphate (NaPO3)6 (purity 3 N) was mixed together with Sc2O3 (purity 4 N) at a molar ratio of 6:1. The mixture was filled into a platinum crucible, covered by a loose fitting lid, and heated up to 1593 K within 3 h. The temperature was held for 24 h and afterwards slowly cooled down to 1503 K in the course of 72 h. The solidified flux was dissolved in hot water and crystals of NaScP2O7 were mechanically separated. The procedure produced transparent to translucent, colorless skeletal aggregates of tabular to acicular crystals, up to 23 mm in lengths. A fragment of a crystal was used for single-crystal structure determination.
Figures
Fig. 1.
Perspective view of the NaScP2O7 framework structure projected down [010]. Diphosphate groups are corner-linked to the deformed ScO6 octahedra. Tunnels parallel to [010] are occupied by nine-coordinated atoms of Na. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
View on six P2O7 groups corner-linked to the ScO6 polyhedron.
Fig. 3.
Cage formed by three diphosphate groups and four ScO6 polyhedra enclosing the Na cation.
Crystal data
| NaScP2O7 | F(000) = 472 |
| Mr = 241.89 | Dx = 2.789 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 5348 reflections |
| a = 8.9044 (18) Å | θ = 4.2–27.2° |
| b = 5.3300 (11) Å | µ = 1.89 mm−1 |
| c = 12.516 (3) Å | T = 293 K |
| β = 104.11 (3)° | Platy to fibrous fragment, colourless |
| V = 576.1 (2) Å3 | 0.40 × 0.15 × 0.05 mm |
| Z = 4 |
Data collection
| Kuma KM-4-CCD diffractometer | 1018 independent reflections |
| Radiation source: fine-focus sealed tube | 932 reflections with I > 2σ(I) |
| graphite | Rint = 0.028 |
| Detector resolution: 0.06 pixels mm-1 | θmax = 25.0°, θmin = 4.2° |
| ω scans | h = −10→10 |
| Absorption correction: multi-scan (CrysAlis CCD; Oxford Diffraction, 2003) | k = −4→6 |
| Tmin = 0.067, Tmax = 0.093 | l = −14→14 |
| 5082 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.025 | w = 1/[σ2(Fo2) + (0.0535P)2 + 0.089P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.082 | (Δ/σ)max < 0.001 |
| S = 1.18 | Δρmax = 0.46 e Å−3 |
| 1018 reflections | Δρmin = −0.46 e Å−3 |
| 101 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.080 (5) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Sc | 0.26720 (5) | 0.26098 (7) | 0.52783 (4) | 0.0137 (2) | |
| P1 | −0.06473 (7) | 0.22372 (11) | 0.61678 (5) | 0.0140 (2) | |
| P2 | 0.52089 (7) | 0.25413 (10) | 0.35283 (5) | 0.0139 (2) | |
| Na | 0.35939 (11) | 0.23101 (18) | 0.81018 (9) | 0.0278 (3) | |
| O1 | 0.39845 (17) | 0.4953 (3) | 0.65350 (12) | 0.0177 (4) | |
| O2 | 0.36250 (17) | −0.0381 (3) | 0.63494 (13) | 0.0182 (4) | |
| O3 | 0.4256 (2) | 0.2456 (3) | 0.43658 (14) | 0.0210 (4) | |
| O4 | 0.10881 (19) | 0.2727 (3) | 0.63286 (14) | 0.0187 (4) | |
| O5 | 0.39984 (18) | 0.2187 (3) | 0.23463 (13) | 0.0175 (4) | |
| O6 | −0.16444 (17) | 0.4047 (3) | 0.53739 (12) | 0.0220 (4) | |
| O7 | −0.10300 (18) | −0.0507 (3) | 0.58783 (12) | 0.0190 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sc | 0.0124 (3) | 0.0152 (3) | 0.0135 (3) | 0.00023 (15) | 0.0032 (2) | 0.00014 (16) |
| P1 | 0.0126 (4) | 0.0155 (4) | 0.0136 (4) | −0.0001 (2) | 0.0025 (3) | −0.0002 (2) |
| P2 | 0.0127 (4) | 0.0156 (4) | 0.0134 (4) | 0.0001 (2) | 0.0033 (3) | 0.0003 (2) |
| Na | 0.0270 (7) | 0.0245 (7) | 0.0318 (7) | −0.0010 (4) | 0.0069 (5) | 0.0003 (4) |
| O1 | 0.0189 (8) | 0.0161 (9) | 0.0173 (8) | −0.0021 (6) | 0.0028 (6) | 0.0000 (6) |
| O2 | 0.0190 (8) | 0.0172 (9) | 0.0190 (8) | 0.0026 (6) | 0.0055 (6) | 0.0014 (7) |
| O3 | 0.0208 (8) | 0.0240 (11) | 0.0196 (10) | 0.0009 (6) | 0.0077 (7) | 0.0001 (6) |
| O4 | 0.0160 (8) | 0.0238 (10) | 0.0175 (9) | −0.0013 (6) | 0.0062 (7) | −0.0020 (6) |
| O5 | 0.0149 (9) | 0.0220 (10) | 0.0156 (9) | −0.0018 (6) | 0.0037 (7) | 0.0005 (6) |
| O6 | 0.0240 (9) | 0.0188 (10) | 0.0215 (8) | 0.0020 (7) | 0.0022 (7) | 0.0026 (7) |
| O7 | 0.0205 (8) | 0.0168 (9) | 0.0193 (8) | −0.0009 (7) | 0.0041 (6) | −0.0006 (7) |
Geometric parameters (Å, °)
| Sc—O3 | 2.0217 (19) | P2—O2v | 1.5332 (16) |
| Sc—O6i | 2.0770 (17) | P2—O5 | 1.6151 (17) |
| Sc—O7ii | 2.1112 (17) | Na—O1 | 2.5066 (18) |
| Sc—O1 | 2.1220 (16) | Na—O7vi | 2.5176 (19) |
| Sc—O2 | 2.1220 (16) | Na—O4vii | 2.5410 (19) |
| Sc—O4 | 2.1506 (18) | Na—O2vi | 2.5597 (19) |
| P1—O6 | 1.5088 (17) | Na—O2 | 2.6264 (19) |
| P1—O7 | 1.5254 (17) | Na—O4 | 2.746 (2) |
| P1—O4 | 1.5313 (18) | Na—O1vii | 2.7505 (19) |
| P1—O5iii | 1.6114 (17) | Na—O4vi | 2.9710 (19) |
| P2—O3 | 1.5013 (19) | Na—O6viii | 2.992 (2) |
| P2—O1iv | 1.5278 (16) | ||
| O3—Sc—O6i | 96.54 (6) | O4vii—Na—O2vi | 115.31 (6) |
| O3—Sc—O7ii | 93.04 (7) | O1—Na—O2 | 67.77 (6) |
| O6i—Sc—O7ii | 91.19 (6) | O7vi—Na—O2 | 119.51 (6) |
| O3—Sc—O1 | 96.23 (7) | O4vii—Na—O2 | 71.71 (6) |
| O6i—Sc—O1 | 84.07 (7) | O2vi—Na—O2 | 130.74 (5) |
| O7ii—Sc—O1 | 170.01 (6) | O1—Na—O4 | 64.06 (6) |
| O3—Sc—O2 | 95.73 (6) | O7vi—Na—O4 | 143.35 (6) |
| O6i—Sc—O2 | 164.29 (6) | O4vii—Na—O4 | 108.52 (6) |
| O7ii—Sc—O2 | 97.93 (7) | O2vi—Na—O4 | 69.49 (6) |
| O1—Sc—O2 | 84.87 (7) | O2—Na—O4 | 62.69 (6) |
| O3—Sc—O4 | 176.82 (7) | O1—Na—O1vii | 131.81 (5) |
| O6i—Sc—O4 | 85.62 (6) | O7vi—Na—O1vii | 141.08 (7) |
| O7ii—Sc—O4 | 89.25 (6) | O4vii—Na—O1vii | 63.57 (5) |
| O1—Sc—O4 | 81.63 (6) | O2vi—Na—O1vii | 56.31 (6) |
| O2—Sc—O4 | 81.75 (6) | O2—Na—O1vii | 93.88 (6) |
| O6—P1—O7 | 113.28 (9) | O4—Na—O1vii | 67.92 (5) |
| O6—P1—O4 | 112.98 (9) | O1—Na—O4vi | 67.56 (5) |
| O7—P1—O4 | 110.77 (9) | O7vi—Na—O4vi | 53.79 (5) |
| O6—P1—O5iii | 105.42 (9) | O4vii—Na—O4vi | 150.38 (8) |
| O7—P1—O5iii | 108.54 (9) | O2vi—Na—O4vi | 60.19 (5) |
| O4—P1—O5iii | 105.29 (10) | O2—Na—O4vi | 135.34 (6) |
| O3—P2—O1iv | 114.55 (9) | O4—Na—O4vi | 97.26 (6) |
| O3—P2—O2v | 113.07 (9) | O1vii—Na—O4vi | 116.03 (6) |
| O1iv—P2—O2v | 110.27 (9) | O1—Na—O6viii | 159.25 (6) |
| O3—P2—O5 | 105.75 (10) | O7vi—Na—O6viii | 83.21 (6) |
| O1iv—P2—O5 | 105.78 (9) | O4vii—Na—O6viii | 61.94 (5) |
| O2v—P2—O5 | 106.74 (9) | O2vi—Na—O6viii | 67.85 (6) |
| O1—Na—O7vi | 82.54 (6) | O2—Na—O6viii | 132.80 (6) |
| O1—Na—O4vii | 137.09 (7) | O4—Na—O6viii | 123.78 (6) |
| O7vi—Na—O4vii | 106.18 (6) | O1vii—Na—O6viii | 58.46 (5) |
| O1—Na—O2vi | 101.72 (6) | O4vi—Na—O6viii | 91.81 (5) |
| O7vi—Na—O2vi | 105.53 (6) | P1ix—O5—P2 | 125.47 (10) |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x, −y, −z+1; (iii) x−1/2, −y+1/2, z+1/2; (iv) −x+1, −y+1, −z+1; (v) −x+1, −y, −z+1; (vi) −x+1/2, y+1/2, −z+3/2; (vii) −x+1/2, y−1/2, −z+3/2; (viii) x+1/2, −y+1/2, z+1/2; (ix) x+1/2, −y+1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WM2274).
References
- Brese, N. E. & O’Keeffe, M. (1991). Acta Cryst. B47, 192–197.
- Dowty, E. (2003). ATOMS. Shape Software, Kingsport, Tennessee, USA.
- Férid, M., Horchani-Naifer, K. & Trabelsi-Ayedi, M. (2004). Z. Kristallogr. 219, 353–354.
- Hamady, A. & Jouini, T. (1996). Acta Cryst. C52, 2949–2951.
- Hizhnyi, Yu., Gomenyuk, O., Nedilko, S., Oliynyk, A., Okhrimenko, B. & Bojko, V. (2007). Radiat. Meas. 42, 719–722.
- Hizhnyi, Yu., Oliynyk, A., Gomenyuk, O., Nedilko, S., Nagornyi, P., Bojko, R. & Bojko, V. (2008). Opt. Mater. 30, 687–689.
- Leclaire, A., Benmoussa, A., Borel, M. M., Grandin, A. & Raveau, B. (1988). J. Solid State Chem. 77, 299–305.
- Li, M.-R., Liu, W., Chen, H.-H., Yang, X.-X., Wei, Z.-B., Cao, D.-H., Gu, M. & Zhao, J.-T. (2005). Eur. J. Inorg. Chem. pp. 4693–4696.
- Oxford Diffraction (2003). CrysAlis CCD and CrysAlis RED. Oxford Diffraction Ltd, Abingdon, England.
- Robinson, K., Gibbs, G. V. & Ribbe, P. H. (1971). Science, 172, 567–570. [DOI] [PubMed]
- Schwendtner, K. & Kolitsch, U. (2004). Acta Cryst. C60, i79–i83. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Vitins, G., Kanepe, Z., Vitins, A., Ronis, J., Dindune, A. & Lusis, A. (2000). J. Solid State Electrochem. 4, 146–152.
- Yuan, J.-L., Zhang, H., Chen, H.-H., Yang, X.-X., Zhao, J.-T. & Gu, M. (2007). J. Solid State Chem. 180, 3381–3387.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809046224/wm2274sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809046224/wm2274Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report