Abstract
The crystal structure of β-cyclodextrin, C42H70O35·10.41H2O, consists of truncated cone-shaped β-cyclodextrin molecules that are herringbone packed. The primary hydroxy groups form an intramolecular hydrogen-bonded array. The semipolar cavity of the cyclodextrin host is filled with water molecules, which show partial occupancy and disorder.
Related literature
For an overview of cyclodextrin chemistry, see: Atwood et al. (1996 ▶), Szejtli (1998 ▶). For applications of cyclodextrins, see: Del Valle (2004 ▶). For previous X-ray crystal structure determinations of various β-cyclodextrin hydrates, see: Hamilton et al. (1968 ▶); Szejtli & Budai (1977 ▶); Lindner & Saenger (1978 ▶, 1982 ▶); Stezowski & Maclennan (1980 ▶); Fujiwara et al. (1983 ▶); Betzel et al. (1984 ▶); Steiner & Koellner (1994 ▶); Damodharan et al. (2004 ▶); Kurokawa, et al. (2004 ▶). For a low temperature single-crystal neutron diffraction study of deutero-β-CD·11D2O, see Zabel et al. (1986 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶).
Experimental
Crystal data
C42H70O35·10.41H2O
M r = 1322.53
Monoclinic,
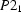
a = 20.8353 (4) Å
b = 9.9397 (1) Å
c = 15.2043 (3) Å
β = 110.630 (2)°
V = 2946.84 (9) Å3
Z = 2
Mo Kα radiation
μ = 0.14 mm−1
T = 110 K
0.37 × 0.33 × 0.28 mm
Data collection
Oxford Diffraction XcaliburTM2 diffractometer
Absorption correction: multi-scan (ABSPACK in CrysAlis Pro; Oxford Diffraction, 2009 ▶) T min = 0.951, T max = 0.963
40031 measured reflections
7019 independent reflections
6090 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.081
S = 0.99
7019 reflections
868 parameters
36 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.39 e Å−3
Δρmin = −0.24 e Å−3
Data collection: CrysAlis Pro (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis Pro; data reduction: CrysAlis Pro; method used to solve structure: initial coordinates of the β-cyclodextrin scaffold taken from Lindner & Saenger (1982 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2009 ▶); software used to prepare material for publication: enCIFer (Allen et al., 2004 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680904865X/tk2569sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680904865X/tk2569Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯O7 | 0.84 | 2.36 | 3.065 (3) | 142 |
| O2—H2A⋯O45i | 0.84 | 2.00 | 2.809 (3) | 161 |
| O2—H2A⋯O45′i | 0.84 | 1.95 | 2.633 (11) | 138 |
| O5—H5A⋯O47 | 0.84 | 1.76 | 2.578 (15) | 163 |
| O5—H5A⋯O45ii | 0.84 | 2.46 | 3.024 (4) | 125 |
| O5′—H5′⋯O45ii | 0.84 | 1.66 | 2.47 (3) | 163 |
| O5′—H5′⋯O45′ii | 0.84 | 2.40 | 3.20 (3) | 159 |
| O6—H6C⋯O12 | 0.84 | 2.04 | 2.845 (2) | 161 |
| O6—H6C⋯O13 | 0.84 | 2.31 | 2.742 (2) | 112 |
| O7—H7A⋯O26i | 0.84 | 2.14 | 2.956 (3) | 165 |
| O11—H11A⋯O40 | 0.84 | 1.78 | 2.595 (2) | 163 |
| O12—H12C⋯O31iii | 0.84 | 1.85 | 2.676 (2) | 167 |
| O15—H15A⋯O41iv | 0.84 | 2.04 | 2.870 (3) | 172 |
| O16—H16A⋯O38v | 0.84 | 1.90 | 2.733 (3) | 174 |
| O17—H17A⋯O11 | 0.84 | 2.06 | 2.889 (2) | 171 |
| O20—H20A⋯O43 | 0.84 | 1.93 | 2.725 (3) | 157 |
| O20—H20A⋯O50 | 0.84 | 2.12 | 2.80 (3) | 138 |
| O21—H21A⋯O27 | 0.84 | 1.99 | 2.810 (2) | 167 |
| O21—H21A⋯O28 | 0.84 | 2.36 | 2.794 (2) | 112 |
| O22—H22A⋯O16 | 0.84 | 1.97 | 2.776 (2) | 160 |
| O22—H22A⋯O23 | 0.84 | 2.41 | 2.821 (2) | 111 |
| O25—H25A⋯O39vi | 0.84 | 1.90 | 2.743 (3) | 178 |
| O26—H26A⋯O37iii | 0.84 | 1.83 | 2.668 (3) | 176 |
| O27—H27A⋯O5v | 0.84 | 2.09 | 2.821 (3) | 145 |
| O30—H30C⋯O15vii | 0.84 | 2.06 | 2.858 (3) | 158 |
| O30—H30C⋯O14vii | 0.84 | 2.37 | 2.945 (2) | 126 |
| O31—H31A⋯O2 | 0.84 | 2.01 | 2.844 (2) | 172 |
| O32—H32A⋯O26 | 0.84 | 2.04 | 2.871 (2) | 170 |
| O35—H35A⋯O37i | 0.84 | 2.08 | 2.890 (2) | 161 |
| O36—H36⋯O11ii | 0.84 | 2.02 | 2.854 (2) | 173 |
| O37—H37A⋯O6 | 0.824 (17) | 2.05 (2) | 2.837 (2) | 160 (3) |
| O37—H37B⋯O32viii | 0.849 (17) | 1.910 (18) | 2.753 (2) | 171 (3) |
| O38—H38A⋯O20 | 0.828 (17) | 2.11 (2) | 2.865 (3) | 152 (3) |
| O38—H38B⋯O30ix | 0.817 (17) | 1.857 (18) | 2.672 (3) | 176 (3) |
| O39—H39A⋯O22ii | 0.856 (18) | 1.898 (18) | 2.749 (3) | 173 (3) |
| O39—H39B⋯O38 | 0.861 (18) | 2.18 (2) | 2.959 (3) | 151 (3) |
| O40—H40A⋯O41x | 0.879 (18) | 2.21 (3) | 2.787 (3) | 123 (3) |
| O40—H40B⋯O35xi | 0.869 (18) | 1.91 (2) | 2.770 (3) | 168 (3) |
| O41—H41A⋯O20 | 0.844 (17) | 2.134 (18) | 2.976 (3) | 175 (3) |
| O41—H41A⋯O19 | 0.844 (17) | 2.63 (3) | 3.048 (2) | 112 (3) |
| O41—H41B⋯O25 | 0.840 (18) | 1.95 (2) | 2.769 (3) | 166 (3) |
| O42—H42C⋯O21ii | 0.852 (18) | 2.09 (2) | 2.932 (3) | 173 (3) |
| O42—H42D⋯O48 | 0.805 (18) | 1.90 (4) | 2.53 (3) | 135 (3) |
| O42—H42D⋯O39 | 0.805 (18) | 2.34 (3) | 3.038 (3) | 146 (3) |
Symmetry codes: (i) 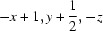 ; (ii)
; (ii) 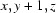 ; (iii)
; (iii) 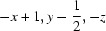 ; (iv)
; (iv) 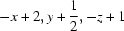 ; (v)
; (v) 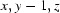 ; (vi)
; (vi) 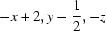 ; (vii)
; (vii) 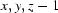 ; (viii)
; (viii) 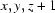 ; (ix)
; (ix) 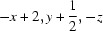 ; (x)
; (x) 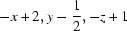 ; (xi)
; (xi) 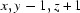 .
.
Acknowledgments
Professor William S. Sheldrick is gratefully acknowledged for generous support. BBK would like to thank the Alexander von Humboldt Foundation for a fellowship.
supplementary crystallographic information
Comment
Cyclodextrins are common, widely studied and cheaply available supramolecular hosts (Atwood et al., 1996; Szejtli, 1998) with a variety of applications in the food, cosmetics and pharmaceutical industries (Del Valle, 2004).
β-Cyclodextrin (β-CD) is a cyclic oligosaccharide comprising seven D-glucopyranoside units, linked through 1,4-glycosidic bonds. The first room temperature crystal structure determination of β-CD dodecahydrate was reported about 40 years ago (Hamilton et al., 1968). A number of room temperature determinations have been reported since (Szejtli & Budai, 1977; Lindner & Saenger, 1978; Lindner & Saenger, 1982; Fujiwara et al., 1983; Betzel et al., 1984; Steiner & Koellner, 1994; Damodharan et al., 2004). A search of the Cambridge Structural Database (CSD; Version 5.3 with September 2009 Updates) (Allen et al., 2002) revealed no low temperature X-ray structure determination of a β-CD hydrate with the exception of those reported by Stezowski & Maclennan (1980) and Kurokawa et al. (2004). Those were apparently reported without atomic coordinates and with an R factor of 13.0% and without refinement details, respectively. Additionally, a neutron diffraction study of deutero-β-CD 11 D2O at 120 K was reported by Zabel et al. (1986); the refinement of which converged at R = 0.049. Herein, we report an X-ray structural study of β-CD 10.41 hydrate at 110 K with R = 0.032 in order to provide an improved model of the β-CD host, (I).
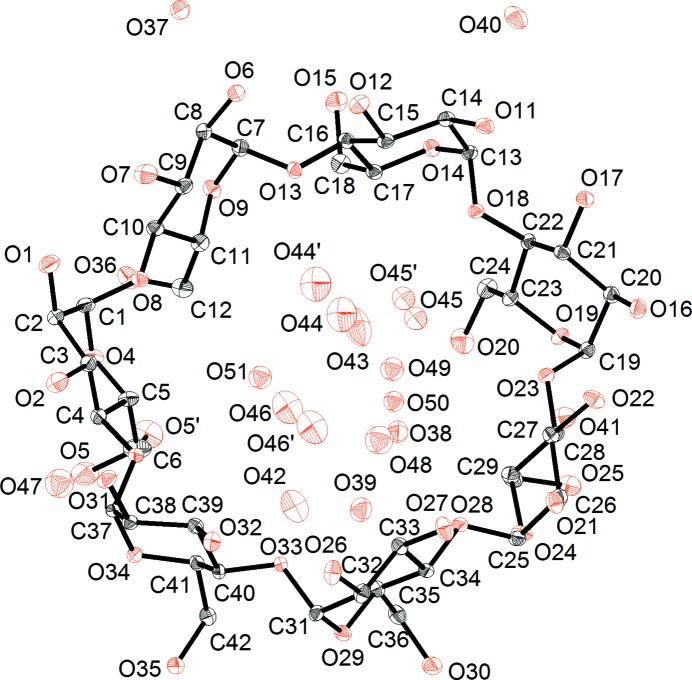
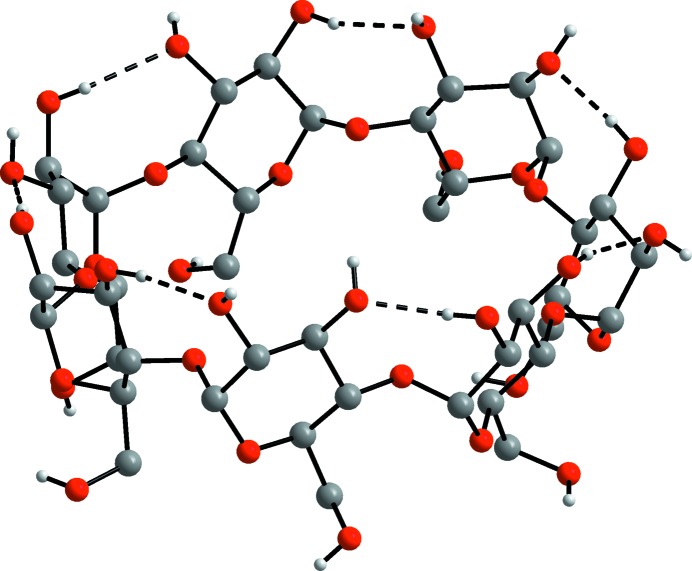
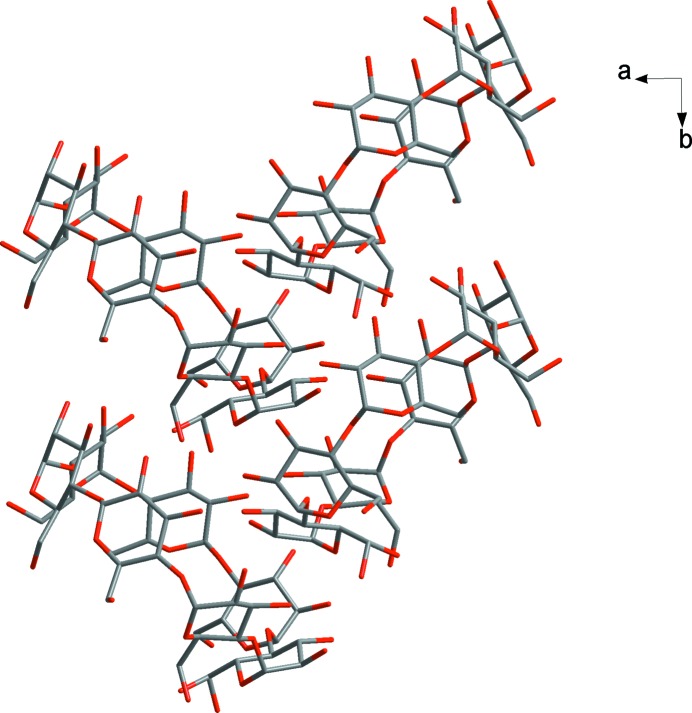
Figs 1 and 2 show a displacement ellipsoid plot and an illustration of the molecular structure of the β-CD host, respectively. The shape of the molecule is a truncated cone. The geometric parameters lie within expected ranges. All D-glucopyranoside units exhibit the C1 chair conformation. The primary hydroxy groups at the wider end of the torus form an array of intramolecular H bonds with O—H···O contacts in the range of 2.776 (2)–3.065 (3) Å. The estimated volume of the semi-polar cavity of a β-CD host is 262 Å3 (Szejtli, 1998). In (I), the cavity was found to be statistically occupied by approximately 5.41 water molecules. Three of the water positions are each disordered over two positions, while five are considered to be partially occupied (see Refinement). In the vast number of structural studies dealing with β-CD hydrates, different solvent water contents were encountered. The solvent water content depends on the crystallization conditions and the humidity (Steiner & Koellner, 1994). In the crystal structure of (I), the β-CD molecules are arranged in herringbone-packed layers that are stacked along the c axis direction (Fig. 3).
Experimental
Crystals of (I) were obtained unintentionally from an aqueous solution of β-cyclodextrin during an attempt to prepare an inclusion compound with an organic dye. A heterogeneous solid of (I) and the dye was obtained instead of the desired inclusion compound when the solvent was allowed to evaporate slowly. The solid was filtered off and dried over P4O10. A colourless crystal of (I) could be separated for the X-ray analysis.
Refinement
In the absence of significant anomalous scattering effects, 5934 Friedel pairs were merged. Anisotropic displacement parameters were introduced for all non-hydrogen atoms with the exception of O47, O48, O49, O50 and O51, the positions of which are not fully occupied. One of the secondary hydroxy groups of the β-cyclodextrin host was found to be disordered over two positions. The ratio of occupancies of O5 and O5' was refined by means of a free variable and converged at 0.917 (5):0.083 (5). Standard similarity restraints on geometry and displacement parameters as well as rigid bond restraints were applied to the disordered group. The O44, O45 and O46 atoms of the solvent water molecules were each found to be disordered over two positions. The refinement of the occupancies by means of a free variable in each case yielded: 0.903 (5):0.097 (5), 0.803 (6):0.197 (6) and 0.884 (9):0.116 (9) for O44, O45 and O46, respectively. The parts of disordered water oxygen atoms were each refined with equivalent anisotropic displacement parameters. The site occupancy factors of O47, O48, O49, O50 and O51 were allowed to refine freely to yield 0.167 (9), 0.081 (8), 0.061 (8), 0.060 (8) and 0.039 (8), respectively. Four of the calculated intermolecular O···O distances (O43···O50 ca 2.01 Å, O44···O49 ca 1.73 Å, O45···O47 ca 2.29 Å and O46···O51 ca 2.42) indicate that the two positions cannot be occupied simultaneously in each case. The C-bound H atoms were placed at geometrically calculated positions (C—H = 0.99-1.00 Å) and refined with a riding model and with Uiso(H) = 1.2 Ueq(C). The hydroxy- and water-H atoms were localized in difference Fourier syntheses. The hydroxy-H atoms were subsequently refined with O—H = 0.84 Å and constrained tetrahedral C—O—H angles. The O—H bond lengths of the water molecules were restrained to a target value of 0.84 (2) Å. The 1,3-H,H distances of the water molecules were restrained to be similar with an effective standard deviation of 0.04 Å. The hydroxy- and water-H atoms were refined with Uiso(H) = 1.2 Ueq(O). The positions for some of the H atoms in some of the water molecules could not be determined reliably and were therefore excluded from the refinement.
Figures
Fig. 1.
The asymmetric unit of (I), with displacement ellipsoids drawn at the 50% probability level. Positions of disordered O atoms with minor occupancy and those not fully occupied are drawn as empty ellipsoids. Hydrogen atoms are omitted for clarity.
Fig. 2.
Molecular Structure of the β-cyclodextrin host in (I). H bonds are represented by dashed lines. H atoms attached to carbon are omitted for clarity.
Fig. 3.
View of the crystal structure of (I) viewed down the c axis, showing the herringbone packing of the β-cyclodextrin molecules. H atoms and water molecules are omitted for clarity.
Crystal data
| C42H70O35·10.41H2O | F(000) = 1412 |
| Mr = 1322.53 | Dx = 1.490 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 20832 reflections |
| a = 20.8353 (4) Å | θ = 2.7–28.1° |
| b = 9.9397 (1) Å | µ = 0.14 mm−1 |
| c = 15.2043 (3) Å | T = 110 K |
| β = 110.630 (2)° | Prism, colourless |
| V = 2946.84 (9) Å3 | 0.37 × 0.33 × 0.28 mm |
| Z = 2 |
Data collection
| Oxford Diffraction Xcalibur diffractometer | 7019 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 6090 reflections with I > 2σ(I) |
| graphite | Rint = 0.026 |
| Detector resolution: 8.4171 pixels mm-1 | θmax = 27.5°, θmin = 2.9° |
| ω scans | h = −26→26 |
| Absorption correction: multi-scan (ABSPACK in CrysAlis PRO; Oxford Diffraction, 2009) | k = −12→12 |
| Tmin = 0.951, Tmax = 0.963 | l = −19→19 |
| 40031 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: the initial coordinates of the β-cyclodextrin scaffold were taken from Lindner & Saenger (1982) |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.081 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.99 | w = 1/[σ2(Fo2) + (0.0578P)2] where P = (Fo2 + 2Fc2)/3 |
| 7019 reflections | (Δ/σ)max = 0.001 |
| 868 parameters | Δρmax = 0.39 e Å−3 |
| 36 restraints | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.43109 (9) | 0.4795 (2) | 0.06964 (12) | 0.0237 (4) | |
| H1A | 0.4451 | 0.4043 | 0.0941 | 0.028* | |
| O2 | 0.41763 (9) | 0.4374 (2) | −0.12954 (12) | 0.0238 (4) | |
| H2A | 0.3885 | 0.3942 | −0.1142 | 0.029* | |
| O3 | 0.54177 (8) | 0.39543 (17) | −0.15508 (11) | 0.0154 (3) | |
| O4 | 0.59435 (9) | 0.59993 (18) | 0.05896 (11) | 0.0207 (4) | |
| O5 | 0.64406 (13) | 0.6978 (2) | −0.07616 (17) | 0.0368 (7) | 0.917 (5) |
| H5A | 0.6758 | 0.7505 | −0.0465 | 0.044* | 0.917 (5) |
| C6 | 0.65945 (15) | 0.5684 (3) | −0.03927 (19) | 0.0294 (6) | 0.917 (5) |
| H6A | 0.7026 | 0.5711 | 0.0158 | 0.035* | 0.917 (5) |
| H6B | 0.6669 | 0.5091 | −0.0871 | 0.035* | 0.917 (5) |
| O5' | 0.7150 (11) | 0.620 (3) | 0.0298 (15) | 0.040 (5) | 0.083 (5) |
| H5' | 0.7025 | 0.6822 | 0.0573 | 0.059* | 0.083 (5) |
| C6' | 0.65945 (15) | 0.5684 (3) | −0.03927 (19) | 0.0294 (6) | 0.083 (5) |
| H6A' | 0.6756 | 0.4974 | −0.0722 | 0.035* | 0.083 (5) |
| H6B' | 0.6391 | 0.6408 | −0.0854 | 0.035* | 0.083 (5) |
| O6 | 0.58391 (8) | 0.16416 (19) | 0.41646 (12) | 0.0189 (4) | |
| H6C | 0.6091 | 0.1016 | 0.4102 | 0.023* | |
| O7 | 0.49901 (9) | 0.3003 (2) | 0.24149 (13) | 0.0260 (4) | |
| H7A | 0.4845 | 0.3179 | 0.2853 | 0.031* | |
| O8 | 0.57078 (8) | 0.42611 (17) | 0.14463 (11) | 0.0157 (3) | |
| O9 | 0.70256 (8) | 0.41357 (17) | 0.38138 (11) | 0.0166 (4) | |
| O11 | 0.77159 (9) | −0.27659 (17) | 0.45930 (12) | 0.0177 (4) | |
| H11A | 0.7667 | −0.3267 | 0.5010 | 0.021* | |
| O12 | 0.66776 (8) | −0.06812 (18) | 0.43729 (12) | 0.0178 (4) | |
| H12C | 0.6448 | −0.1177 | 0.3926 | 0.021* | |
| O13 | 0.70736 (8) | 0.18126 (17) | 0.39046 (11) | 0.0146 (3) | |
| O14 | 0.87332 (8) | 0.03010 (17) | 0.53239 (11) | 0.0152 (3) | |
| O15 | 0.84497 (9) | 0.26449 (19) | 0.60728 (12) | 0.0232 (4) | |
| H15A | 0.8597 | 0.3377 | 0.6346 | 0.028* | |
| O16 | 0.92260 (9) | −0.49406 (17) | 0.26437 (12) | 0.0186 (4) | |
| H16A | 0.9550 | −0.5406 | 0.2600 | 0.022* | |
| O17 | 0.87877 (8) | −0.40676 (18) | 0.41115 (11) | 0.0179 (4) | |
| H17A | 0.8453 | −0.3770 | 0.4238 | 0.022* | |
| O18 | 0.86032 (8) | −0.11803 (17) | 0.40904 (11) | 0.0139 (3) | |
| O19 | 0.99620 (8) | −0.15637 (17) | 0.31140 (11) | 0.0154 (3) | |
| O20 | 0.99809 (10) | 0.12933 (19) | 0.33502 (15) | 0.0297 (4) | |
| H20A | 0.9622 | 0.1707 | 0.3035 | 0.045* | |
| O21 | 0.79734 (9) | −0.43799 (18) | −0.12551 (11) | 0.0195 (4) | |
| H21A | 0.7657 | −0.3839 | −0.1532 | 0.023* | |
| O22 | 0.85197 (9) | −0.47175 (18) | 0.07245 (12) | 0.0198 (4) | |
| H22A | 0.8666 | −0.4645 | 0.1313 | 0.024* | |
| O23 | 0.91264 (8) | −0.24262 (17) | 0.17883 (11) | 0.0150 (3) | |
| O24 | 0.91996 (8) | −0.15919 (18) | −0.05281 (11) | 0.0175 (4) | |
| O25 | 1.04195 (9) | −0.1142 (2) | 0.11668 (12) | 0.0235 (4) | |
| H25A | 1.0516 | −0.1317 | 0.0687 | 0.028* | |
| O26 | 0.57670 (8) | −0.12304 (18) | −0.36578 (12) | 0.0212 (4) | |
| H26A | 0.5657 | −0.1960 | −0.3951 | 0.025* | |
| O27 | 0.68202 (9) | −0.29122 (18) | −0.23675 (13) | 0.0230 (4) | |
| H27A | 0.6554 | −0.3069 | −0.2073 | 0.035* | |
| O28 | 0.80158 (8) | −0.15711 (17) | −0.12746 (11) | 0.0147 (3) | |
| O29 | 0.73057 (8) | 0.07842 (18) | −0.32427 (11) | 0.0159 (3) | |
| O30 | 0.87316 (9) | 0.0327 (2) | −0.27399 (13) | 0.0238 (4) | |
| H30C | 0.8677 | 0.0859 | −0.3190 | 0.036 (9)* | |
| O31 | 0.42147 (8) | 0.30039 (18) | −0.29183 (12) | 0.0194 (4) | |
| H31A | 0.4242 | 0.3384 | −0.2413 | 0.023* | |
| O32 | 0.48828 (8) | 0.09599 (17) | −0.36044 (11) | 0.0174 (3) | |
| H32A | 0.5137 | 0.0286 | −0.3554 | 0.021* | |
| O33 | 0.63304 (8) | 0.11283 (16) | −0.28602 (11) | 0.0137 (3) | |
| O34 | 0.58413 (8) | 0.46343 (16) | −0.27119 (11) | 0.0153 (3) | |
| O35 | 0.65236 (8) | 0.44776 (18) | −0.39480 (11) | 0.0178 (4) | |
| H35A | 0.6216 | 0.5041 | −0.3976 | 0.021* | |
| O36 | 0.70072 (9) | 0.65475 (19) | 0.26713 (13) | 0.0251 (4) | |
| H36 | 0.7188 | 0.6715 | 0.3248 | 0.030* | |
| C1 | 0.54900 (12) | 0.5510 (3) | 0.10087 (16) | 0.0164 (5) | |
| H1 | 0.5462 | 0.6171 | 0.1489 | 0.020* | |
| C2 | 0.47733 (12) | 0.5308 (3) | 0.02720 (16) | 0.0170 (5) | |
| H2 | 0.4599 | 0.6208 | −0.0002 | 0.020* | |
| C3 | 0.48101 (11) | 0.4415 (3) | −0.05196 (15) | 0.0163 (5) | |
| H3 | 0.4935 | 0.3481 | −0.0274 | 0.020* | |
| C4 | 0.53467 (12) | 0.4933 (2) | −0.08968 (16) | 0.0162 (5) | |
| H4 | 0.5197 | 0.5815 | −0.1221 | 0.019* | |
| C5 | 0.60351 (12) | 0.5095 (3) | −0.00976 (16) | 0.0181 (5) | |
| H5 | 0.6187 | 0.4199 | 0.0202 | 0.022* | |
| C7 | 0.68164 (12) | 0.2994 (2) | 0.41849 (16) | 0.0161 (5) | |
| H7 | 0.6998 | 0.3052 | 0.4886 | 0.019* | |
| C8 | 0.60300 (12) | 0.2877 (3) | 0.38360 (16) | 0.0159 (5) | |
| H8 | 0.5850 | 0.3637 | 0.4113 | 0.019* | |
| C9 | 0.57234 (12) | 0.2998 (3) | 0.27719 (16) | 0.0160 (5) | |
| H9 | 0.5878 | 0.2200 | 0.2499 | 0.019* | |
| C10 | 0.60044 (12) | 0.4254 (3) | 0.24549 (15) | 0.0157 (5) | |
| H10 | 0.5866 | 0.5080 | 0.2719 | 0.019* | |
| C11 | 0.67885 (12) | 0.4133 (3) | 0.28019 (16) | 0.0164 (5) | |
| H11 | 0.6908 | 0.3246 | 0.2590 | 0.020* | |
| C12 | 0.71698 (13) | 0.5221 (3) | 0.24961 (18) | 0.0209 (5) | |
| H12A | 0.7668 | 0.5079 | 0.2822 | 0.025* | |
| H12B | 0.7073 | 0.5122 | 0.1813 | 0.025* | |
| C13 | 0.85802 (12) | −0.1031 (2) | 0.49974 (16) | 0.0142 (5) | |
| H13 | 0.8922 | −0.1654 | 0.5436 | 0.017* | |
| C14 | 0.78611 (12) | −0.1431 (2) | 0.49470 (16) | 0.0151 (5) | |
| H14 | 0.7839 | −0.1397 | 0.5593 | 0.018* | |
| C15 | 0.73386 (11) | −0.0454 (2) | 0.43193 (16) | 0.0142 (5) | |
| H15 | 0.7313 | −0.0574 | 0.3655 | 0.017* | |
| C16 | 0.75377 (11) | 0.0987 (2) | 0.46217 (16) | 0.0140 (5) | |
| H16 | 0.7475 | 0.1162 | 0.5234 | 0.017* | |
| C17 | 0.82787 (12) | 0.1293 (2) | 0.47187 (16) | 0.0142 (5) | |
| H17 | 0.8322 | 0.1264 | 0.4084 | 0.017* | |
| C18 | 0.85261 (13) | 0.2635 (3) | 0.51694 (17) | 0.0188 (5) | |
| H18A | 0.9013 | 0.2770 | 0.5244 | 0.023* | |
| H18B | 0.8253 | 0.3369 | 0.4772 | 0.023* | |
| C19 | 0.97083 (12) | −0.2731 (2) | 0.25883 (16) | 0.0146 (5) | |
| H19 | 1.0073 | −0.3129 | 0.2382 | 0.018* | |
| C20 | 0.94960 (12) | −0.3762 (2) | 0.31866 (16) | 0.0152 (5) | |
| H20 | 0.9903 | −0.4010 | 0.3749 | 0.018* | |
| C21 | 0.89510 (11) | −0.3156 (2) | 0.35013 (15) | 0.0138 (5) | |
| H21 | 0.8530 | −0.3000 | 0.2936 | 0.017* | |
| C22 | 0.91912 (11) | −0.1811 (2) | 0.39884 (15) | 0.0129 (5) | |
| H22 | 0.9551 | −0.1961 | 0.4620 | 0.016* | |
| C23 | 0.94635 (11) | −0.0876 (2) | 0.34095 (16) | 0.0139 (5) | |
| H23 | 0.9074 | −0.0573 | 0.2842 | 0.017* | |
| C24 | 0.98264 (13) | 0.0341 (3) | 0.39539 (18) | 0.0198 (5) | |
| H24A | 0.9532 | 0.0773 | 0.4261 | 0.024* | |
| H24B | 1.0257 | 0.0054 | 0.4451 | 0.024* | |
| C25 | 0.86255 (12) | −0.2313 (2) | −0.11112 (16) | 0.0152 (5) | |
| H25 | 0.8683 | −0.2489 | −0.1727 | 0.018* | |
| C26 | 0.85543 (12) | −0.3653 (2) | −0.06681 (16) | 0.0162 (5) | |
| H26 | 0.8971 | −0.4200 | −0.0604 | 0.019* | |
| C27 | 0.85369 (12) | −0.3433 (2) | 0.03141 (16) | 0.0150 (5) | |
| H27 | 0.8115 | −0.2916 | 0.0270 | 0.018* | |
| C28 | 0.91664 (12) | −0.2633 (2) | 0.08818 (16) | 0.0143 (5) | |
| H28 | 0.9589 | −0.3157 | 0.0942 | 0.017* | |
| C29 | 0.91743 (12) | −0.1305 (3) | 0.03929 (16) | 0.0166 (5) | |
| H29 | 0.8745 | −0.0795 | 0.0321 | 0.020* | |
| C30 | 0.97906 (12) | −0.0434 (3) | 0.08994 (18) | 0.0206 (5) | |
| H30A | 0.9807 | 0.0323 | 0.0485 | 0.025* | |
| H30B | 0.9734 | −0.0050 | 0.1468 | 0.025* | |
| C31 | 0.66024 (11) | 0.0500 (2) | −0.34856 (16) | 0.0147 (5) | |
| H31 | 0.6349 | 0.0818 | −0.4141 | 0.018* | |
| C32 | 0.64881 (12) | −0.1005 (3) | −0.34219 (16) | 0.0163 (5) | |
| H32 | 0.6648 | −0.1489 | −0.3883 | 0.020* | |
| C33 | 0.68954 (12) | −0.1496 (2) | −0.24365 (16) | 0.0156 (5) | |
| H33 | 0.6725 | −0.1033 | −0.1977 | 0.019* | |
| C34 | 0.76454 (12) | −0.1134 (2) | −0.22174 (15) | 0.0135 (5) | |
| H34 | 0.7824 | −0.1603 | −0.2666 | 0.016* | |
| C35 | 0.77121 (11) | 0.0386 (2) | −0.22966 (16) | 0.0148 (5) | |
| H35 | 0.7525 | 0.0837 | −0.1850 | 0.018* | |
| C36 | 0.84314 (12) | 0.0888 (3) | −0.21093 (17) | 0.0185 (5) | |
| H36A | 0.8422 | 0.1881 | −0.2169 | 0.022* | |
| H36B | 0.8721 | 0.0659 | −0.1456 | 0.022* | |
| C37 | 0.52490 (12) | 0.4371 (2) | −0.24926 (15) | 0.0144 (5) | |
| H37 | 0.4964 | 0.5206 | −0.2598 | 0.017* | |
| C38 | 0.48255 (11) | 0.3247 (2) | −0.31085 (16) | 0.0145 (5) | |
| H38 | 0.4692 | 0.3534 | −0.3780 | 0.017* | |
| C39 | 0.52559 (12) | 0.1989 (2) | −0.29771 (16) | 0.0139 (5) | |
| H39 | 0.5379 | 0.1666 | −0.2315 | 0.017* | |
| C40 | 0.59077 (11) | 0.2293 (2) | −0.31679 (16) | 0.0125 (4) | |
| H40 | 0.5799 | 0.2433 | −0.3856 | 0.015* | |
| C41 | 0.62916 (12) | 0.3508 (2) | −0.26224 (16) | 0.0138 (5) | |
| H41 | 0.6515 | 0.3266 | −0.1944 | 0.017* | |
| C42 | 0.68285 (12) | 0.3999 (2) | −0.30030 (16) | 0.0157 (5) | |
| H42A | 0.7096 | 0.4734 | −0.2599 | 0.019* | |
| H42B | 0.7149 | 0.3255 | −0.2986 | 0.019* | |
| O37 | 0.45907 (9) | 0.13938 (19) | 0.45062 (12) | 0.0208 (4) | |
| H37A | 0.4952 (11) | 0.126 (3) | 0.4413 (19) | 0.025* | |
| H37B | 0.4700 (14) | 0.134 (3) | 0.5099 (13) | 0.025* | |
| O38 | 1.02376 (10) | 0.3565 (2) | 0.23620 (13) | 0.0248 (4) | |
| H38A | 1.0311 (15) | 0.290 (2) | 0.271 (2) | 0.030* | |
| H38B | 1.0561 (13) | 0.409 (3) | 0.250 (2) | 0.030* | |
| O39 | 0.92858 (10) | 0.3215 (2) | 0.04040 (14) | 0.0299 (4) | |
| H39A | 0.9075 (15) | 0.387 (3) | 0.055 (2) | 0.036* | |
| H39B | 0.9646 (12) | 0.311 (3) | 0.0898 (17) | 0.036* | |
| O40 | 0.75974 (10) | −0.3861 (2) | 0.60776 (14) | 0.0293 (4) | |
| H40A | 0.7851 (14) | −0.456 (3) | 0.607 (2) | 0.035* | |
| H40B | 0.7227 (11) | −0.433 (3) | 0.599 (2) | 0.035* | |
| O41 | 1.11226 (10) | 0.0083 (2) | 0.28708 (14) | 0.0282 (4) | |
| H41A | 1.0788 (13) | 0.038 (3) | 0.300 (2) | 0.034* | |
| H41B | 1.0977 (15) | −0.029 (3) | 0.2341 (16) | 0.034* | |
| O42 | 0.77658 (12) | 0.2871 (2) | −0.07080 (17) | 0.0400 (5) | |
| H42C | 0.7787 (17) | 0.367 (2) | −0.090 (2) | 0.048* | |
| H42D | 0.8137 (12) | 0.263 (4) | −0.036 (2) | 0.048* | |
| O43 | 0.87096 (13) | 0.2433 (3) | 0.2749 (2) | 0.0585 (7) | |
| O44 | 0.74718 (14) | 0.1038 (3) | 0.1869 (2) | 0.0523 (8) | 0.903 (5) |
| O44' | 0.7000 (14) | 0.109 (3) | 0.1997 (19) | 0.0523 (8) | 0.097 (5) |
| O45 | 0.70193 (13) | −0.1792 (3) | 0.1178 (2) | 0.0287 (7) | 0.803 (6) |
| O45' | 0.7106 (6) | −0.1310 (13) | 0.1604 (9) | 0.0287 (7) | 0.197 (6) |
| O46 | 0.68237 (15) | 0.1660 (4) | 0.0002 (3) | 0.0502 (11) | 0.884 (9) |
| O46' | 0.6883 (13) | 0.103 (3) | −0.033 (2) | 0.0502 (11) | 0.116 (9) |
| O47 | 0.7268 (7) | 0.8957 (15) | −0.0091 (10) | 0.044 (5)* | 0.167 (9) |
| O48 | 0.8452 (15) | 0.143 (3) | 0.068 (2) | 0.043 (11)* | 0.081 (8) |
| O49 | 0.7843 (16) | 0.013 (3) | 0.120 (2) | 0.030 (12)* | 0.062 (8) |
| O50 | 0.8898 (15) | 0.167 (3) | 0.164 (2) | 0.022 (11)* | 0.060 (8) |
| O51 | 0.744 (3) | 0.337 (5) | 0.111 (3) | 0.029 (18)* | 0.039 (8) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0206 (9) | 0.0320 (11) | 0.0213 (9) | 0.0035 (8) | 0.0110 (8) | −0.0001 (8) |
| O2 | 0.0146 (8) | 0.0375 (11) | 0.0167 (9) | 0.0022 (8) | 0.0023 (7) | −0.0030 (8) |
| O3 | 0.0204 (8) | 0.0150 (8) | 0.0118 (7) | 0.0038 (7) | 0.0069 (6) | 0.0017 (6) |
| O4 | 0.0256 (9) | 0.0228 (9) | 0.0144 (8) | −0.0058 (8) | 0.0081 (7) | −0.0017 (7) |
| O5 | 0.0467 (15) | 0.0275 (13) | 0.0468 (15) | −0.0080 (11) | 0.0296 (12) | 0.0026 (11) |
| C6 | 0.0291 (15) | 0.0368 (16) | 0.0245 (14) | −0.0102 (13) | 0.0123 (12) | −0.0028 (12) |
| O5' | 0.027 (8) | 0.047 (9) | 0.041 (9) | −0.010 (8) | 0.007 (7) | 0.001 (8) |
| C6' | 0.0291 (15) | 0.0368 (16) | 0.0245 (14) | −0.0102 (13) | 0.0123 (12) | −0.0028 (12) |
| O6 | 0.0178 (8) | 0.0206 (9) | 0.0211 (9) | 0.0032 (7) | 0.0105 (7) | 0.0046 (7) |
| O7 | 0.0140 (8) | 0.0407 (11) | 0.0233 (9) | 0.0001 (8) | 0.0064 (7) | 0.0040 (8) |
| O8 | 0.0183 (8) | 0.0170 (8) | 0.0108 (8) | 0.0007 (7) | 0.0040 (6) | −0.0001 (7) |
| O9 | 0.0181 (8) | 0.0151 (8) | 0.0139 (8) | −0.0009 (7) | 0.0022 (7) | −0.0002 (7) |
| O11 | 0.0233 (9) | 0.0139 (8) | 0.0186 (9) | −0.0024 (7) | 0.0108 (7) | 0.0014 (7) |
| O12 | 0.0117 (8) | 0.0208 (9) | 0.0222 (9) | −0.0050 (7) | 0.0076 (7) | −0.0038 (7) |
| O13 | 0.0135 (8) | 0.0162 (8) | 0.0116 (7) | 0.0030 (7) | 0.0014 (6) | 0.0004 (6) |
| O14 | 0.0124 (8) | 0.0178 (8) | 0.0135 (8) | 0.0002 (7) | 0.0022 (6) | 0.0000 (7) |
| O15 | 0.0275 (10) | 0.0203 (9) | 0.0218 (9) | −0.0042 (8) | 0.0087 (8) | −0.0072 (7) |
| O16 | 0.0202 (9) | 0.0157 (8) | 0.0190 (8) | 0.0029 (7) | 0.0059 (7) | −0.0017 (7) |
| O17 | 0.0179 (8) | 0.0180 (9) | 0.0205 (8) | 0.0006 (7) | 0.0099 (7) | 0.0040 (7) |
| O18 | 0.0115 (8) | 0.0187 (9) | 0.0121 (7) | 0.0017 (7) | 0.0048 (6) | 0.0017 (6) |
| O19 | 0.0112 (8) | 0.0188 (8) | 0.0176 (8) | −0.0009 (7) | 0.0068 (6) | −0.0027 (7) |
| O20 | 0.0291 (10) | 0.0207 (10) | 0.0408 (11) | −0.0065 (8) | 0.0140 (9) | 0.0032 (8) |
| O21 | 0.0190 (9) | 0.0173 (9) | 0.0159 (8) | 0.0014 (7) | −0.0016 (7) | −0.0001 (7) |
| O22 | 0.0260 (9) | 0.0171 (9) | 0.0135 (8) | −0.0046 (8) | 0.0034 (7) | 0.0003 (7) |
| O23 | 0.0124 (8) | 0.0206 (9) | 0.0113 (8) | 0.0028 (7) | 0.0032 (6) | −0.0001 (7) |
| O24 | 0.0137 (8) | 0.0253 (9) | 0.0125 (8) | −0.0029 (7) | 0.0032 (6) | 0.0035 (7) |
| O25 | 0.0164 (8) | 0.0309 (11) | 0.0211 (9) | −0.0048 (8) | 0.0040 (7) | 0.0014 (8) |
| O26 | 0.0126 (8) | 0.0187 (9) | 0.0267 (9) | −0.0005 (7) | 0.0002 (7) | −0.0049 (7) |
| O27 | 0.0184 (9) | 0.0159 (9) | 0.0314 (10) | −0.0015 (7) | 0.0047 (8) | 0.0052 (8) |
| O28 | 0.0140 (8) | 0.0190 (8) | 0.0108 (7) | 0.0036 (7) | 0.0043 (6) | 0.0025 (6) |
| O29 | 0.0122 (8) | 0.0206 (9) | 0.0151 (8) | 0.0015 (7) | 0.0048 (6) | 0.0050 (7) |
| O30 | 0.0195 (9) | 0.0308 (11) | 0.0241 (9) | 0.0047 (8) | 0.0115 (7) | 0.0090 (8) |
| O31 | 0.0135 (8) | 0.0268 (10) | 0.0194 (8) | 0.0015 (7) | 0.0075 (7) | −0.0013 (7) |
| O32 | 0.0146 (8) | 0.0156 (8) | 0.0216 (8) | −0.0011 (7) | 0.0058 (7) | −0.0012 (7) |
| O33 | 0.0156 (8) | 0.0132 (8) | 0.0130 (8) | 0.0046 (7) | 0.0059 (6) | 0.0029 (6) |
| O34 | 0.0174 (8) | 0.0130 (8) | 0.0177 (8) | 0.0011 (7) | 0.0091 (7) | 0.0014 (7) |
| O35 | 0.0177 (8) | 0.0200 (9) | 0.0171 (8) | 0.0030 (7) | 0.0077 (7) | 0.0030 (7) |
| O36 | 0.0263 (10) | 0.0214 (10) | 0.0235 (9) | −0.0020 (8) | 0.0039 (8) | 0.0031 (8) |
| C1 | 0.0205 (12) | 0.0173 (12) | 0.0136 (11) | −0.0005 (10) | 0.0086 (9) | −0.0013 (9) |
| C2 | 0.0204 (12) | 0.0170 (12) | 0.0155 (11) | 0.0029 (10) | 0.0088 (10) | −0.0004 (9) |
| C3 | 0.0151 (11) | 0.0195 (12) | 0.0124 (11) | 0.0032 (10) | 0.0023 (9) | −0.0003 (9) |
| C4 | 0.0202 (12) | 0.0149 (12) | 0.0140 (11) | 0.0035 (10) | 0.0065 (9) | 0.0003 (9) |
| C5 | 0.0198 (12) | 0.0225 (13) | 0.0134 (11) | −0.0017 (10) | 0.0076 (10) | −0.0016 (10) |
| C7 | 0.0167 (11) | 0.0175 (12) | 0.0125 (11) | 0.0009 (10) | 0.0031 (9) | −0.0011 (9) |
| C8 | 0.0149 (11) | 0.0175 (12) | 0.0153 (11) | 0.0032 (10) | 0.0054 (9) | 0.0018 (9) |
| C9 | 0.0123 (11) | 0.0217 (12) | 0.0142 (11) | 0.0007 (10) | 0.0049 (9) | −0.0013 (10) |
| C10 | 0.0154 (11) | 0.0199 (12) | 0.0108 (11) | 0.0011 (10) | 0.0033 (9) | −0.0008 (9) |
| C11 | 0.0148 (11) | 0.0184 (12) | 0.0147 (11) | −0.0011 (10) | 0.0035 (9) | −0.0022 (9) |
| C12 | 0.0160 (12) | 0.0268 (14) | 0.0175 (12) | −0.0034 (11) | 0.0032 (10) | 0.0008 (10) |
| C13 | 0.0139 (11) | 0.0178 (12) | 0.0110 (10) | 0.0023 (9) | 0.0045 (9) | 0.0003 (9) |
| C14 | 0.0166 (11) | 0.0160 (11) | 0.0144 (11) | −0.0012 (9) | 0.0075 (9) | 0.0000 (9) |
| C15 | 0.0104 (10) | 0.0190 (12) | 0.0139 (11) | −0.0009 (9) | 0.0053 (9) | 0.0007 (9) |
| C16 | 0.0119 (11) | 0.0163 (12) | 0.0130 (10) | 0.0001 (9) | 0.0036 (8) | 0.0017 (9) |
| C17 | 0.0130 (11) | 0.0169 (12) | 0.0120 (10) | 0.0005 (9) | 0.0037 (9) | 0.0012 (9) |
| C18 | 0.0182 (12) | 0.0176 (12) | 0.0194 (12) | −0.0037 (10) | 0.0051 (10) | −0.0018 (10) |
| C19 | 0.0115 (11) | 0.0177 (12) | 0.0135 (11) | 0.0015 (9) | 0.0030 (9) | −0.0014 (9) |
| C20 | 0.0150 (11) | 0.0159 (12) | 0.0130 (11) | 0.0016 (9) | 0.0029 (9) | 0.0000 (9) |
| C21 | 0.0121 (10) | 0.0164 (12) | 0.0123 (10) | −0.0004 (9) | 0.0036 (9) | 0.0019 (9) |
| C22 | 0.0093 (10) | 0.0162 (12) | 0.0127 (10) | 0.0020 (9) | 0.0030 (8) | 0.0001 (9) |
| C23 | 0.0114 (10) | 0.0157 (11) | 0.0155 (11) | −0.0005 (9) | 0.0058 (9) | −0.0011 (9) |
| C24 | 0.0168 (12) | 0.0188 (12) | 0.0252 (13) | −0.0043 (10) | 0.0093 (10) | −0.0028 (10) |
| C25 | 0.0127 (11) | 0.0206 (13) | 0.0125 (11) | 0.0006 (9) | 0.0045 (9) | 0.0015 (9) |
| C26 | 0.0160 (11) | 0.0164 (12) | 0.0144 (11) | 0.0030 (9) | 0.0031 (9) | −0.0005 (9) |
| C27 | 0.0157 (11) | 0.0140 (11) | 0.0148 (11) | −0.0001 (9) | 0.0049 (9) | 0.0007 (9) |
| C28 | 0.0137 (11) | 0.0166 (11) | 0.0129 (11) | 0.0001 (9) | 0.0049 (9) | −0.0015 (9) |
| C29 | 0.0157 (12) | 0.0186 (12) | 0.0144 (11) | −0.0010 (10) | 0.0040 (9) | 0.0000 (9) |
| C30 | 0.0198 (12) | 0.0188 (13) | 0.0212 (12) | −0.0037 (10) | 0.0046 (10) | 0.0033 (10) |
| C31 | 0.0124 (11) | 0.0181 (12) | 0.0135 (11) | 0.0039 (9) | 0.0045 (9) | 0.0000 (9) |
| C32 | 0.0118 (11) | 0.0179 (12) | 0.0171 (11) | 0.0010 (9) | 0.0023 (9) | −0.0025 (10) |
| C33 | 0.0173 (12) | 0.0120 (11) | 0.0177 (11) | −0.0014 (9) | 0.0065 (9) | 0.0006 (9) |
| C34 | 0.0143 (11) | 0.0163 (12) | 0.0099 (10) | 0.0020 (9) | 0.0041 (8) | 0.0015 (9) |
| C35 | 0.0139 (11) | 0.0160 (12) | 0.0137 (11) | 0.0028 (9) | 0.0039 (9) | 0.0018 (9) |
| C36 | 0.0173 (12) | 0.0161 (12) | 0.0207 (12) | −0.0007 (10) | 0.0051 (9) | 0.0033 (10) |
| C37 | 0.0154 (11) | 0.0177 (12) | 0.0123 (10) | 0.0048 (9) | 0.0076 (9) | 0.0036 (9) |
| C38 | 0.0107 (11) | 0.0201 (12) | 0.0135 (11) | 0.0022 (9) | 0.0053 (9) | 0.0031 (9) |
| C39 | 0.0138 (11) | 0.0143 (11) | 0.0124 (10) | −0.0004 (9) | 0.0033 (9) | 0.0006 (9) |
| C40 | 0.0120 (11) | 0.0121 (11) | 0.0141 (11) | 0.0028 (9) | 0.0053 (9) | 0.0025 (9) |
| C41 | 0.0153 (11) | 0.0132 (11) | 0.0118 (10) | 0.0019 (9) | 0.0035 (9) | 0.0011 (9) |
| C42 | 0.0157 (11) | 0.0137 (11) | 0.0163 (11) | −0.0007 (9) | 0.0039 (9) | −0.0001 (9) |
| O37 | 0.0183 (9) | 0.0247 (10) | 0.0182 (9) | 0.0013 (8) | 0.0051 (7) | −0.0002 (7) |
| O38 | 0.0228 (10) | 0.0224 (10) | 0.0311 (11) | −0.0006 (8) | 0.0118 (8) | 0.0028 (8) |
| O39 | 0.0258 (10) | 0.0335 (12) | 0.0287 (10) | 0.0057 (9) | 0.0074 (8) | −0.0002 (9) |
| O40 | 0.0235 (10) | 0.0296 (11) | 0.0358 (11) | −0.0020 (9) | 0.0115 (9) | 0.0132 (9) |
| O41 | 0.0213 (10) | 0.0391 (12) | 0.0237 (10) | −0.0036 (9) | 0.0074 (8) | −0.0022 (9) |
| O42 | 0.0337 (12) | 0.0314 (12) | 0.0432 (13) | −0.0093 (10) | −0.0011 (10) | 0.0006 (11) |
| O43 | 0.0398 (14) | 0.0378 (14) | 0.094 (2) | −0.0030 (11) | 0.0194 (14) | 0.0242 (14) |
| O44 | 0.0400 (16) | 0.0510 (17) | 0.0615 (18) | −0.0047 (14) | 0.0123 (13) | −0.0022 (14) |
| O44' | 0.0400 (16) | 0.0510 (17) | 0.0615 (18) | −0.0047 (14) | 0.0123 (13) | −0.0022 (14) |
| O45 | 0.0278 (12) | 0.0282 (16) | 0.0290 (16) | −0.0001 (11) | 0.0088 (12) | 0.0024 (12) |
| O45' | 0.0278 (12) | 0.0282 (16) | 0.0290 (16) | −0.0001 (11) | 0.0088 (12) | 0.0024 (12) |
| O46 | 0.0424 (15) | 0.046 (2) | 0.055 (2) | −0.0070 (15) | 0.0087 (14) | 0.0050 (17) |
| O46' | 0.0424 (15) | 0.046 (2) | 0.055 (2) | −0.0070 (15) | 0.0087 (14) | 0.0050 (17) |
Geometric parameters (Å, °)
| O1—C2 | 1.429 (3) | C8—H8 | 1.0000 |
| O1—H1A | 0.8400 | C9—C10 | 1.527 (3) |
| O2—C3 | 1.428 (3) | C9—H9 | 1.0000 |
| O2—H2A | 0.8400 | C10—C11 | 1.534 (3) |
| O3—C37 | 1.411 (3) | C10—H10 | 1.0000 |
| O3—C4 | 1.436 (3) | C11—C12 | 1.509 (3) |
| O4—C1 | 1.400 (3) | C11—H11 | 1.0000 |
| O4—C5 | 1.441 (3) | C12—H12A | 0.9900 |
| O5—C6 | 1.395 (4) | C12—H12B | 0.9900 |
| O5—H5A | 0.8400 | C13—C14 | 1.526 (3) |
| C6—C5 | 1.507 (4) | C13—H13 | 1.0000 |
| C6—H6A | 0.9900 | C14—C15 | 1.519 (3) |
| C6—H6B | 0.9900 | C14—H14 | 1.0000 |
| O5'—H5' | 0.8400 | C15—C16 | 1.517 (3) |
| O6—C8 | 1.433 (3) | C15—H15 | 1.0000 |
| O6—H6C | 0.8400 | C16—C17 | 1.529 (3) |
| O7—C9 | 1.430 (3) | C16—H16 | 1.0000 |
| O7—H7A | 0.8400 | C17—C18 | 1.506 (3) |
| O8—C1 | 1.406 (3) | C17—H17 | 1.0000 |
| O8—C10 | 1.437 (3) | C18—H18A | 0.9900 |
| O9—C7 | 1.403 (3) | C18—H18B | 0.9900 |
| O9—C11 | 1.441 (3) | C19—C20 | 1.536 (3) |
| O11—C14 | 1.424 (3) | C19—H19 | 1.0000 |
| O11—H11A | 0.8400 | C20—C21 | 1.505 (3) |
| O12—C15 | 1.426 (3) | C20—H20 | 1.0000 |
| O12—H12C | 0.8400 | C21—C22 | 1.525 (3) |
| O13—C7 | 1.417 (3) | C21—H21 | 1.0000 |
| O13—C16 | 1.433 (3) | C22—C23 | 1.520 (3) |
| O14—C13 | 1.410 (3) | C22—H22 | 1.0000 |
| O14—C17 | 1.449 (3) | C23—C24 | 1.510 (3) |
| O15—C18 | 1.437 (3) | C23—H23 | 1.0000 |
| O15—H15A | 0.8400 | C24—H24A | 0.9900 |
| O16—C20 | 1.429 (3) | C24—H24B | 0.9900 |
| O16—H16A | 0.8400 | C25—C26 | 1.523 (3) |
| O17—C21 | 1.421 (3) | C25—H25 | 1.0000 |
| O17—H17A | 0.8400 | C26—C27 | 1.522 (3) |
| O18—C13 | 1.405 (3) | C26—H26 | 1.0000 |
| O18—C22 | 1.432 (3) | C27—C28 | 1.517 (3) |
| O19—C19 | 1.402 (3) | C27—H27 | 1.0000 |
| O19—C23 | 1.441 (3) | C28—C29 | 1.518 (3) |
| O20—C24 | 1.432 (3) | C28—H28 | 1.0000 |
| O20—H20A | 0.8400 | C29—C30 | 1.515 (3) |
| O21—C26 | 1.422 (3) | C29—H29 | 1.0000 |
| O21—H21A | 0.8400 | C30—H30A | 0.9900 |
| O22—C27 | 1.427 (3) | C30—H30B | 0.9900 |
| O22—H22A | 0.8400 | C31—C32 | 1.524 (4) |
| O23—C19 | 1.415 (3) | C31—H31 | 1.0000 |
| O23—C28 | 1.425 (3) | C32—C33 | 1.519 (3) |
| O24—C25 | 1.408 (3) | C32—H32 | 1.0000 |
| O24—C29 | 1.448 (3) | C33—C34 | 1.522 (3) |
| O25—C30 | 1.415 (3) | C33—H33 | 1.0000 |
| O25—H25A | 0.8400 | C34—C35 | 1.526 (3) |
| O26—C32 | 1.434 (3) | C34—H34 | 1.0000 |
| O26—H26A | 0.8400 | C35—C36 | 1.508 (3) |
| O27—C33 | 1.424 (3) | C35—H35 | 1.0000 |
| O27—H27A | 0.8400 | C36—H36A | 0.9900 |
| O28—C25 | 1.413 (3) | C36—H36B | 0.9900 |
| O28—C34 | 1.435 (3) | C37—C38 | 1.524 (3) |
| O29—C31 | 1.408 (3) | C37—H37 | 1.0000 |
| O29—C35 | 1.444 (3) | C38—C39 | 1.510 (3) |
| O30—C36 | 1.430 (3) | C38—H38 | 1.0000 |
| O30—H30C | 0.8400 | C39—C40 | 1.516 (3) |
| O31—C38 | 1.422 (3) | C39—H39 | 1.0000 |
| O31—H31A | 0.8400 | C40—C41 | 1.523 (3) |
| O32—C39 | 1.428 (3) | C40—H40 | 1.0000 |
| O32—H32A | 0.8400 | C41—C42 | 1.510 (3) |
| O33—C31 | 1.412 (3) | C41—H41 | 1.0000 |
| O33—C40 | 1.430 (3) | C42—H42A | 0.9900 |
| O34—C37 | 1.411 (3) | C42—H42B | 0.9900 |
| O34—C41 | 1.436 (3) | O37—H37A | 0.824 (17) |
| O35—C42 | 1.432 (3) | O37—H37B | 0.849 (17) |
| O35—H35A | 0.8400 | O38—H38A | 0.828 (17) |
| O36—C12 | 1.410 (3) | O38—H38B | 0.817 (17) |
| O36—H36 | 0.8400 | O39—H39A | 0.856 (18) |
| C1—C2 | 1.532 (3) | O39—H39B | 0.861 (18) |
| C1—H1 | 1.0000 | O40—H40A | 0.879 (18) |
| C2—C3 | 1.519 (3) | O40—H40B | 0.869 (18) |
| C2—H2 | 1.0000 | O41—H41A | 0.844 (17) |
| C3—C4 | 1.515 (3) | O41—H41B | 0.840 (18) |
| C3—H3 | 1.0000 | O42—H42C | 0.852 (18) |
| C4—C5 | 1.527 (3) | O42—H42D | 0.805 (18) |
| C4—H4 | 1.0000 | O44—O44' | 1.07 (3) |
| C5—H5 | 1.0000 | O44—O49 | 1.73 (3) |
| C7—C8 | 1.539 (3) | O45—O45' | 0.774 (13) |
| C7—H7 | 1.0000 | O46—O46' | 0.84 (4) |
| C8—C9 | 1.521 (3) | O48—O50 | 1.46 (4) |
| C2—O1—H1A | 109.5 | O23—C19—H19 | 109.3 |
| C3—O2—H2A | 109.5 | C20—C19—H19 | 109.3 |
| C37—O3—C4 | 117.01 (18) | O16—C20—C21 | 108.93 (18) |
| C1—O4—C5 | 113.34 (18) | O16—C20—C19 | 109.99 (18) |
| C6—O5—H5A | 109.5 | C21—C20—C19 | 109.15 (19) |
| O5—C6—C5 | 112.7 (2) | O16—C20—H20 | 109.6 |
| O5—C6—H6A | 109.1 | C21—C20—H20 | 109.6 |
| C5—C6—H6A | 109.1 | C19—C20—H20 | 109.6 |
| O5—C6—H6B | 109.1 | O17—C21—C20 | 109.46 (19) |
| C5—C6—H6B | 109.1 | O17—C21—C22 | 110.80 (18) |
| H6A—C6—H6B | 107.8 | C20—C21—C22 | 110.51 (18) |
| C8—O6—H6C | 109.5 | O17—C21—H21 | 108.7 |
| C9—O7—H7A | 109.5 | C20—C21—H21 | 108.7 |
| C1—O8—C10 | 117.08 (18) | C22—C21—H21 | 108.7 |
| C7—O9—C11 | 112.94 (18) | O18—C22—C23 | 107.67 (18) |
| C14—O11—H11A | 109.5 | O18—C22—C21 | 106.81 (17) |
| C15—O12—H12C | 109.5 | C23—C22—C21 | 112.33 (18) |
| C7—O13—C16 | 118.09 (17) | O18—C22—H22 | 110.0 |
| C13—O14—C17 | 113.50 (17) | C23—C22—H22 | 110.0 |
| C18—O15—H15A | 109.5 | C21—C22—H22 | 110.0 |
| C20—O16—H16A | 109.5 | O19—C23—C24 | 105.91 (18) |
| C21—O17—H17A | 109.5 | O19—C23—C22 | 110.36 (19) |
| C13—O18—C22 | 118.62 (16) | C24—C23—C22 | 112.89 (19) |
| C19—O19—C23 | 113.68 (17) | O19—C23—H23 | 109.2 |
| C24—O20—H20A | 109.5 | C24—C23—H23 | 109.2 |
| C26—O21—H21A | 109.5 | C22—C23—H23 | 109.2 |
| C27—O22—H22A | 109.5 | O20—C24—C23 | 111.1 (2) |
| C19—O23—C28 | 118.45 (17) | O20—C24—H24A | 109.4 |
| C25—O24—C29 | 113.37 (17) | C23—C24—H24A | 109.4 |
| C30—O25—H25A | 109.5 | O20—C24—H24B | 109.4 |
| C32—O26—H26A | 109.5 | C23—C24—H24B | 109.4 |
| C33—O27—H27A | 109.5 | H24A—C24—H24B | 108.0 |
| C25—O28—C34 | 117.86 (17) | O24—C25—O28 | 110.90 (19) |
| C31—O29—C35 | 113.41 (17) | O24—C25—C26 | 111.10 (18) |
| C36—O30—H30C | 109.5 | O28—C25—C26 | 108.21 (18) |
| C38—O31—H31A | 109.5 | O24—C25—H25 | 108.9 |
| C39—O32—H32A | 109.5 | O28—C25—H25 | 108.9 |
| C31—O33—C40 | 118.99 (16) | C26—C25—H25 | 108.9 |
| C37—O34—C41 | 115.44 (17) | O21—C26—C27 | 112.28 (19) |
| C42—O35—H35A | 109.5 | O21—C26—C25 | 111.14 (18) |
| C12—O36—H36 | 109.5 | C27—C26—C25 | 110.38 (19) |
| O4—C1—O8 | 111.42 (19) | O21—C26—H26 | 107.6 |
| O4—C1—C2 | 110.68 (18) | C27—C26—H26 | 107.6 |
| O8—C1—C2 | 107.42 (19) | C25—C26—H26 | 107.6 |
| O4—C1—H1 | 109.1 | O22—C27—C28 | 111.73 (19) |
| O8—C1—H1 | 109.1 | O22—C27—C26 | 108.29 (19) |
| C2—C1—H1 | 109.1 | C28—C27—C26 | 108.59 (19) |
| O1—C2—C3 | 112.3 (2) | O22—C27—H27 | 109.4 |
| O1—C2—C1 | 110.80 (18) | C28—C27—H27 | 109.4 |
| C3—C2—C1 | 110.34 (19) | C26—C27—H27 | 109.4 |
| O1—C2—H2 | 107.7 | O23—C28—C27 | 107.03 (18) |
| C3—C2—H2 | 107.7 | O23—C28—C29 | 111.26 (19) |
| C1—C2—H2 | 107.7 | C27—C28—C29 | 109.50 (19) |
| O2—C3—C4 | 106.73 (18) | O23—C28—H28 | 109.7 |
| O2—C3—C2 | 112.39 (19) | C27—C28—H28 | 109.7 |
| C4—C3—C2 | 110.6 (2) | C29—C28—H28 | 109.7 |
| O2—C3—H3 | 109.0 | O24—C29—C30 | 106.93 (19) |
| C4—C3—H3 | 109.0 | O24—C29—C28 | 108.22 (19) |
| C2—C3—H3 | 109.0 | C30—C29—C28 | 113.7 (2) |
| O3—C4—C3 | 107.56 (19) | O24—C29—H29 | 109.3 |
| O3—C4—C5 | 109.00 (19) | C30—C29—H29 | 109.3 |
| C3—C4—C5 | 110.25 (18) | C28—C29—H29 | 109.3 |
| O3—C4—H4 | 110.0 | O25—C30—C29 | 113.3 (2) |
| C3—C4—H4 | 110.0 | O25—C30—H30A | 108.9 |
| C5—C4—H4 | 110.0 | C29—C30—H30A | 108.9 |
| O4—C5—C6 | 106.7 (2) | O25—C30—H30B | 108.9 |
| O4—C5—C4 | 108.40 (19) | C29—C30—H30B | 108.9 |
| C6—C5—C4 | 114.3 (2) | H30A—C30—H30B | 107.7 |
| O4—C5—H5 | 109.1 | O29—C31—O33 | 111.25 (18) |
| C6—C5—H5 | 109.1 | O29—C31—C32 | 110.58 (19) |
| C4—C5—H5 | 109.1 | O33—C31—C32 | 106.41 (19) |
| O9—C7—O13 | 110.19 (18) | O29—C31—H31 | 109.5 |
| O9—C7—C8 | 111.52 (19) | O33—C31—H31 | 109.5 |
| O13—C7—C8 | 107.64 (19) | C32—C31—H31 | 109.5 |
| O9—C7—H7 | 109.2 | O26—C32—C33 | 111.62 (19) |
| O13—C7—H7 | 109.2 | O26—C32—C31 | 108.11 (19) |
| C8—C7—H7 | 109.2 | C33—C32—C31 | 109.44 (19) |
| O6—C8—C9 | 112.0 (2) | O26—C32—H32 | 109.2 |
| O6—C8—C7 | 109.72 (19) | C33—C32—H32 | 109.2 |
| C9—C8—C7 | 110.97 (18) | C31—C32—H32 | 109.2 |
| O6—C8—H8 | 108.0 | O27—C33—C32 | 110.6 (2) |
| C9—C8—H8 | 108.0 | O27—C33—C34 | 110.42 (19) |
| C7—C8—H8 | 108.0 | C32—C33—C34 | 108.28 (19) |
| O7—C9—C8 | 113.35 (18) | O27—C33—H33 | 109.2 |
| O7—C9—C10 | 110.8 (2) | C32—C33—H33 | 109.2 |
| C8—C9—C10 | 109.68 (19) | C34—C33—H33 | 109.2 |
| O7—C9—H9 | 107.6 | O28—C34—C33 | 107.25 (18) |
| C8—C9—H9 | 107.6 | O28—C34—C35 | 110.28 (19) |
| C10—C9—H9 | 107.6 | C33—C34—C35 | 109.39 (19) |
| O8—C10—C9 | 105.82 (18) | O28—C34—H34 | 110.0 |
| O8—C10—C11 | 111.88 (18) | C33—C34—H34 | 110.0 |
| C9—C10—C11 | 107.94 (19) | C35—C34—H34 | 110.0 |
| O8—C10—H10 | 110.4 | O29—C35—C36 | 107.00 (18) |
| C9—C10—H10 | 110.4 | O29—C35—C34 | 108.20 (19) |
| C11—C10—H10 | 110.4 | C36—C35—C34 | 115.1 (2) |
| O9—C11—C12 | 107.95 (19) | O29—C35—H35 | 108.8 |
| O9—C11—C10 | 106.86 (18) | C36—C35—H35 | 108.8 |
| C12—C11—C10 | 116.8 (2) | C34—C35—H35 | 108.8 |
| O9—C11—H11 | 108.3 | O30—C36—C35 | 112.6 (2) |
| C12—C11—H11 | 108.3 | O30—C36—H36A | 109.1 |
| C10—C11—H11 | 108.3 | C35—C36—H36A | 109.1 |
| O36—C12—C11 | 115.1 (2) | O30—C36—H36B | 109.1 |
| O36—C12—H12A | 108.5 | C35—C36—H36B | 109.1 |
| C11—C12—H12A | 108.5 | H36A—C36—H36B | 107.8 |
| O36—C12—H12B | 108.5 | O3—C37—O34 | 111.60 (18) |
| C11—C12—H12B | 108.5 | O3—C37—C38 | 106.94 (18) |
| H12A—C12—H12B | 107.5 | O34—C37—C38 | 110.84 (18) |
| O18—C13—O14 | 111.31 (19) | O3—C37—H37 | 109.1 |
| O18—C13—C14 | 107.20 (18) | O34—C37—H37 | 109.1 |
| O14—C13—C14 | 111.02 (19) | C38—C37—H37 | 109.1 |
| O18—C13—H13 | 109.1 | O31—C38—C39 | 111.40 (19) |
| O14—C13—H13 | 109.1 | O31—C38—C37 | 111.44 (18) |
| C14—C13—H13 | 109.1 | C39—C38—C37 | 109.72 (18) |
| O11—C14—C15 | 110.39 (19) | O31—C38—H38 | 108.0 |
| O11—C14—C13 | 109.65 (19) | C39—C38—H38 | 108.0 |
| C15—C14—C13 | 109.79 (19) | C37—C38—H38 | 108.0 |
| O11—C14—H14 | 109.0 | O32—C39—C38 | 110.27 (18) |
| C15—C14—H14 | 109.0 | O32—C39—C40 | 109.19 (18) |
| C13—C14—H14 | 109.0 | C38—C39—C40 | 109.88 (19) |
| O12—C15—C16 | 107.38 (18) | O32—C39—H39 | 109.2 |
| O12—C15—C14 | 110.84 (18) | C38—C39—H39 | 109.2 |
| C16—C15—C14 | 110.75 (18) | C40—C39—H39 | 109.2 |
| O12—C15—H15 | 109.3 | O33—C40—C39 | 105.44 (17) |
| C16—C15—H15 | 109.3 | O33—C40—C41 | 108.61 (17) |
| C14—C15—H15 | 109.3 | C39—C40—C41 | 112.83 (18) |
| O13—C16—C15 | 105.71 (17) | O33—C40—H40 | 110.0 |
| O13—C16—C17 | 110.33 (18) | C39—C40—H40 | 110.0 |
| C15—C16—C17 | 112.08 (19) | C41—C40—H40 | 110.0 |
| O13—C16—H16 | 109.5 | O34—C41—C42 | 105.01 (18) |
| C15—C16—H16 | 109.5 | O34—C41—C40 | 111.49 (18) |
| C17—C16—H16 | 109.5 | C42—C41—C40 | 111.44 (19) |
| O14—C17—C18 | 105.75 (18) | O34—C41—H41 | 109.6 |
| O14—C17—C16 | 109.75 (18) | C42—C41—H41 | 109.6 |
| C18—C17—C16 | 113.1 (2) | C40—C41—H41 | 109.6 |
| O14—C17—H17 | 109.4 | O35—C42—C41 | 111.42 (18) |
| C18—C17—H17 | 109.4 | O35—C42—H42A | 109.3 |
| C16—C17—H17 | 109.4 | C41—C42—H42A | 109.3 |
| O15—C18—C17 | 107.79 (19) | O35—C42—H42B | 109.3 |
| O15—C18—H18A | 110.1 | C41—C42—H42B | 109.3 |
| C17—C18—H18A | 110.1 | H42A—C42—H42B | 108.0 |
| O15—C18—H18B | 110.1 | H37A—O37—H37B | 105 (2) |
| C17—C18—H18B | 110.1 | H38A—O38—H38B | 113 (3) |
| H18A—C18—H18B | 108.5 | H39A—O39—H39B | 103 (3) |
| O19—C19—O23 | 110.45 (19) | H40A—O40—H40B | 95 (2) |
| O19—C19—C20 | 110.13 (18) | H41A—O41—H41B | 109 (3) |
| O23—C19—C20 | 108.29 (18) | H42C—O42—H42D | 111 (3) |
| O19—C19—H19 | 109.3 | O44'—O44—O49 | 139.5 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···O7 | 0.84 | 2.36 | 3.065 (3) | 142 |
| O2—H2A···O45i | 0.84 | 2.00 | 2.809 (3) | 161 |
| O2—H2A···O45'i | 0.84 | 1.95 | 2.633 (11) | 138 |
| O5—H5A···O47 | 0.84 | 1.76 | 2.578 (15) | 163 |
| O5—H5A···O45ii | 0.84 | 2.46 | 3.024 (4) | 125 |
| O5'—H5'···O45ii | 0.84 | 1.66 | 2.47 (3) | 163 |
| O5'—H5'···O45'ii | 0.84 | 2.40 | 3.20 (3) | 159 |
| O6—H6C···O12 | 0.84 | 2.04 | 2.845 (2) | 161 |
| O6—H6C···O13 | 0.84 | 2.31 | 2.742 (2) | 112 |
| O7—H7A···O26i | 0.84 | 2.14 | 2.956 (3) | 165 |
| O11—H11A···O40 | 0.84 | 1.78 | 2.595 (2) | 163 |
| O12—H12C···O31iii | 0.84 | 1.85 | 2.676 (2) | 167 |
| O15—H15A···O41iv | 0.84 | 2.04 | 2.870 (3) | 172 |
| O16—H16A···O38v | 0.84 | 1.90 | 2.733 (3) | 174 |
| O17—H17A···O11 | 0.84 | 2.06 | 2.889 (2) | 171 |
| O20—H20A···O43 | 0.84 | 1.93 | 2.725 (3) | 157 |
| O20—H20A···O50 | 0.84 | 2.12 | 2.80 (3) | 138 |
| O21—H21A···O27 | 0.84 | 1.99 | 2.810 (2) | 167 |
| O21—H21A···O28 | 0.84 | 2.36 | 2.794 (2) | 112 |
| O22—H22A···O16 | 0.84 | 1.97 | 2.776 (2) | 160 |
| O22—H22A···O23 | 0.84 | 2.41 | 2.821 (2) | 111 |
| O25—H25A···O39vi | 0.84 | 1.90 | 2.743 (3) | 178 |
| O26—H26A···O37iii | 0.84 | 1.83 | 2.668 (3) | 176 |
| O27—H27A···O5v | 0.84 | 2.09 | 2.821 (3) | 145 |
| O30—H30C···O15vii | 0.84 | 2.06 | 2.858 (3) | 158 |
| O30—H30C···O14vii | 0.84 | 2.37 | 2.945 (2) | 126 |
| O31—H31A···O2 | 0.84 | 2.01 | 2.844 (2) | 172 |
| O32—H32A···O26 | 0.84 | 2.04 | 2.871 (2) | 170 |
| O35—H35A···O37i | 0.84 | 2.08 | 2.890 (2) | 161 |
| O36—H36···O11ii | 0.84 | 2.02 | 2.854 (2) | 173 |
| O37—H37A···O6 | 0.82 (2) | 2.05 (2) | 2.837 (2) | 160 (3) |
| O37—H37B···O32viii | 0.85 (2) | 1.91 (2) | 2.753 (2) | 171 (3) |
| O38—H38A···O20 | 0.83 (2) | 2.11 (2) | 2.865 (3) | 152 (3) |
| O38—H38B···O30ix | 0.82 (2) | 1.86 (2) | 2.672 (3) | 176 (3) |
| O39—H39A···O22ii | 0.86 (2) | 1.90 (2) | 2.749 (3) | 173 (3) |
| O39—H39B···O38 | 0.86 (2) | 2.18 (2) | 2.959 (3) | 151 (3) |
| O40—H40A···O41x | 0.88 (2) | 2.21 (3) | 2.787 (3) | 123 (3) |
| O40—H40B···O35xi | 0.87 (2) | 1.91 (2) | 2.770 (3) | 168 (3) |
| O41—H41A···O20 | 0.84 (2) | 2.13 (2) | 2.976 (3) | 175 (3) |
| O41—H41A···O19 | 0.84 (2) | 2.63 (3) | 3.048 (2) | 112 (3) |
| O41—H41B···O25 | 0.84 (2) | 1.95 (2) | 2.769 (3) | 166 (3) |
| O42—H42C···O21ii | 0.85 (2) | 2.09 (2) | 2.932 (3) | 173 (3) |
| O42—H42D···O48 | 0.81 (2) | 1.90 (4) | 2.53 (3) | 135 (3) |
| O42—H42D···O39 | 0.81 (2) | 2.34 (3) | 3.038 (3) | 146 (3) |
Symmetry codes: (i) −x+1, y+1/2, −z; (ii) x, y+1, z; (iii) −x+1, y−1/2, −z; (iv) −x+2, y+1/2, −z+1; (v) x, y−1, z; (vi) −x+2, y−1/2, −z; (vii) x, y, z−1; (viii) x, y, z+1; (ix) −x+2, y+1/2, −z; (x) −x+2, y−1/2, −z+1; (xi) x, y−1, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2569).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Allen, F. H., Johnson, O., Shields, G. P., Smith, B. R. & Towler, M. (2004). J. Appl. Cryst. 37, 335–338.
- Atwood, J. L., Davies, J. E. D., MacNicol, D. D. & Vögtle, F. (1996). Comprehensive Supramolecular Chemistry, Vol. 3, 1st ed. Oxford: Pergamon.
- Betzel, C., Saenger, W., Hingerty, B. E. & Brown, G. M. (1984). J. Am. Chem. Soc. 106, 7545–7557.
- Brandenburg, K. (2009). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Damodharan, L., Pattabhi, V. & Nagarajan, K. (2004). Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A, 423, 17–35.
- Del Valle, E. M. M. (2004). Process Biochem. 39, 1033–1046.
- Fujiwara, T., Yamazaki, M., Tomizu, Y., Tokuoka, R., Tomita, K.-I., Matsuo, T., Suga, H. & Saenger, W. (1983). Nippon Kagaku Kaishi (J. Chem. Soc. Jpn), pp. 181–187.
- Hamilton, J. A., Steinrauf, L. K. & Van Etten, R. L. (1968). Acta Cryst. B24, 1560–1562. [DOI] [PubMed]
- Kurokawa, C., Sekii, M., Ishida, T. & Nogami, T. (2004). Supramol. Chem. 16, 381–384.
- Lindner, K. & Saenger, W. (1978). Angew. Chem. Int. Ed. 17, 694–695.
- Lindner, K. & Saenger, W. (1982). Carbohydr. Res. 99, 7–16.
- Oxford Diffraction (2009). CrysAlis Pro. Oxford Diffraction Ltd., Yarnton, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Steiner, T. & Koellner, G. (1994). J. Am. Chem. Soc. 116, 5122–5128.
- Stezowski, J. J. & Maclennan, J. M. (1980). ACA Ser. 2, 7, 24.
- Szejtli, J. (1998). Chem. Rev. 98, 1743–1753. [DOI] [PubMed]
- Szejtli, J. & Budai, Z. (1977). Acta Chim. Acad. Sci. Hung. 94, 383–390.
- Zabel, V., Saenger, W. & Mason, S. A. (1986). J. Am. Chem. Soc. 108, 3664–3673.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680904865X/tk2569sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680904865X/tk2569Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report