Abstract
The title compound (systematic name: 5,7-dihydroxy-3,6,8-trimethoxy-4H-chromen-4-one), C18H16O7, is a flavone that was isolated from Ainsliaea henryi. There are two molecules in the asymmetric unit, one of which has a disordered methoxy group [occupancy ratio 0.681 (9):0.319 (9)]. Both molecules have an intramolecular O—H⋯O hydrogen bond. In the crystal, molecules are linked into O—H⋯O hydrogen-bonded chains parallel to [110].
Related literature
For similar compounds and background information, see: Chinese Materia Medica (2007 ▶); Ali et al. (1979 ▶); Cubukcu & Bingol (1984 ▶); Guerreiro et al. (1982 ▶); Horie et al. (1995 ▶); Jakupovic et al. (1989 ▶); Lavault & Richomme (2004 ▶); Mericli et al. (1986 ▶); Torrenegra et al. (1980 ▶); Urzua et al. (1995 ▶); Wollenweber et al. (1993 ▶, 2008 ▶). For the antifungal activity of the title compound, see: Tomas-Lorente et al. (1989 ▶).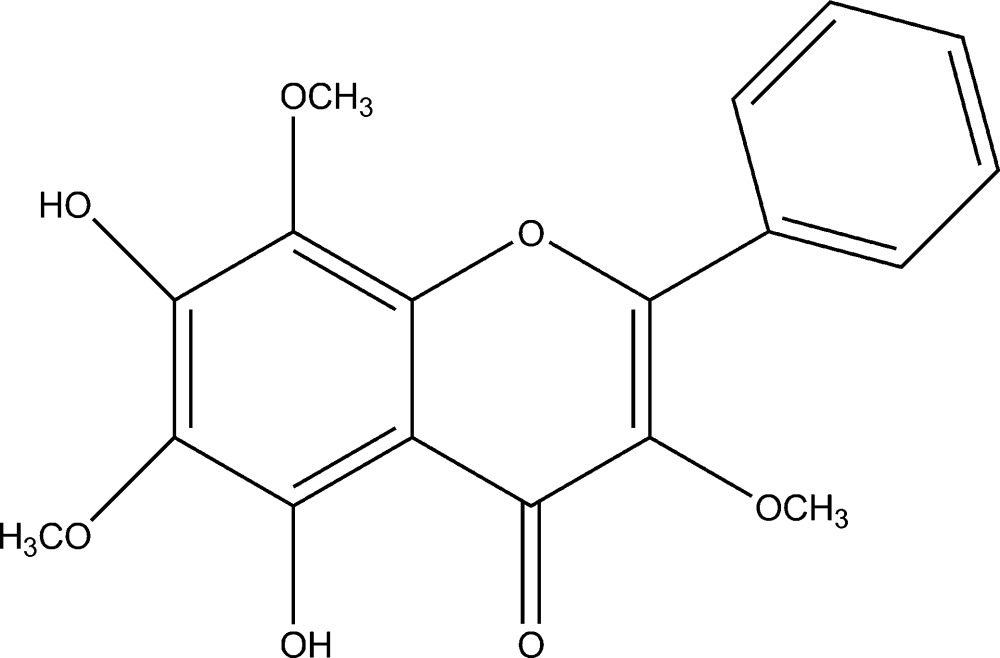
Experimental
Crystal data
C18H16O7
M r = 344.31
Triclinic,
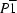
a = 10.147 (4) Å
b = 11.493 (4) Å
c = 14.134 (5) Å
α = 74.233 (5)°
β = 86.461 (5)°
γ = 86.845 (5)°
V = 1582.0 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 293 K
0.30 × 0.20 × 0.20 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.967, T max = 0.978
7698 measured reflections
5590 independent reflections
3283 reflections with I > 2σ(I)
R int = 0.042
Refinement
R[F 2 > 2σ(F 2)] = 0.061
wR(F 2) = 0.171
S = 0.96
5590 reflections
481 parameters
6 restraints
H-atom parameters constrained
Δρmax = 0.25 e Å−3
Δρmin = −0.35 e Å−3
Data collection: SMART (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809050715/pk2205sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809050715/pk2205Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H3⋯O16i | 0.82 | 2.06 | 2.806 (3) | 152 |
| O3—H3⋯O2A | 0.82 | 2.36 | 2.772 (5) | 112 |
| O3—H3⋯O2B | 0.82 | 2.37 | 2.799 (9) | 114 |
| O5—H5⋯O6 | 0.82 | 1.86 | 2.586 (3) | 146 |
| O13—H1⋯O5ii | 0.82 | 2.05 | 2.825 (3) | 158 |
| O13—H1⋯O14 | 0.82 | 2.29 | 2.736 (3) | 115 |
| O15—H2⋯O16 | 0.82 | 1.88 | 2.600 (3) | 147 |
Symmetry codes: (i) 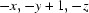 ; (ii)
; (ii) 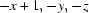 .
.
Acknowledgments
The authors thank Dr Jing-Mei Wang (Center of Analysis and Measurement, Fudan University, Shanghai) for the structure analysis.
supplementary crystallographic information
Comment
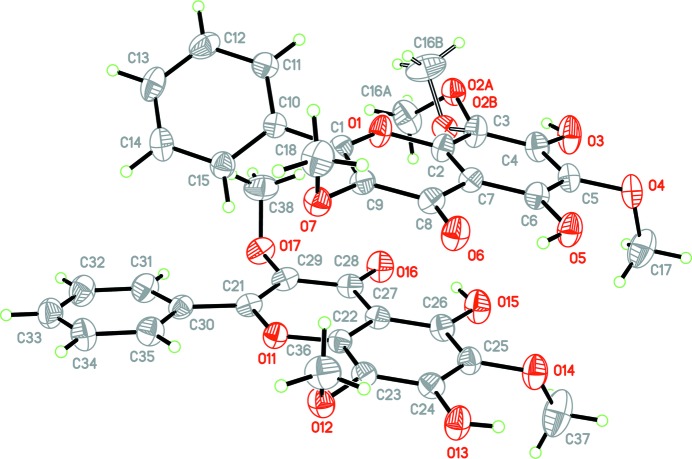
Ainsliaea henryi Diels is mainly distributed in the south-west of China. The whole plant of Ainsliaea henryi has been used in Chinese folk medicine to treat cough, asthma and lumbago (Chinese Materia Medica, 2007). The chemical constituents of this plant have not all been reported previously. Our chemical investigation of this plant for bioactive components resulted in the isolation of the title compound, which was previously obtained from the flowers of Gnaphalium elegans (Torrenegra et al., 1980). The molecular structure is shown in Fig. 1. Bond lengths and angles are within normal ranges.
Experimental
The dry powders (5 kg) of the whole plant of Ainsliaea henryi were refluxed for 1 h with 95% ethanol (50L) three times. After removal of the ethanol under reduced pressure, the extract was suspended in water and then partitioned with petroleum ether, chloroform, ethyl acetate and n-butanol. The chloroform soluble fraction (30 g) was subjected to silica gel column chromatography using gradient elution (petroleum ether/acetone, 15:1 to 2:1, v/v). 5,7-Dihydroxy-3,6,8-trimethoxyflavone was obtained from the fraction eluted by petroleum ether/acetone (2:1). Single crystals suitable for X-ray diffraction analysis were obtained by slow evaporation from acetone after two weeks at room temperature.
Refinement
The hydroxyl H atoms attached to O2 was located in a difference Fourier map and refined isotropically with a constraint distance 0.82 Å to the related oxygen atoms. The remaining H atoms were placed in calculated positions with C—H distances in the range 0.93–0.98 Å. The Uiso values were set equal to 1.5Ueq (C,O) for methyl and hydroxyl H atoms and 1.2Ueq(C) for the remaining H atoms.
Figures
Fig. 1.
The molecular structure showing the atom-labelling scheme with displacement ellipsoids drawn at the 30% probability level. H atoms are shown as small spheres of arbitrary radius.
Crystal data
| C18H16O7 | Z = 4 |
| Mr = 344.31 | F(000) = 720 |
| Triclinic, P1 | Dx = 1.446 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.147 (4) Å | Cell parameters from 688 reflections |
| b = 11.493 (4) Å | θ = 2.7–25.1° |
| c = 14.134 (5) Å | µ = 0.11 mm−1 |
| α = 74.233 (5)° | T = 293 K |
| β = 86.461 (5)° | Block, yellow |
| γ = 86.845 (5)° | 0.30 × 0.20 × 0.20 mm |
| V = 1582.0 (10) Å3 |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 5590 independent reflections |
| Radiation source: fine-focus sealed tube | 3283 reflections with I > 2σ(I) |
| graphite | Rint = 0.042 |
| φ and ω scans | θmax = 25.2°, θmin = 1.5° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −12→12 |
| Tmin = 0.967, Tmax = 0.978 | k = −13→13 |
| 7698 measured reflections | l = −16→9 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.061 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.171 | H-atom parameters constrained |
| S = 0.96 | w = 1/[σ2(Fo2) + (0.0916P)2] where P = (Fo2 + 2Fc2)/3 |
| 5590 reflections | (Δ/σ)max < 0.001 |
| 481 parameters | Δρmax = 0.25 e Å−3 |
| 6 restraints | Δρmin = −0.35 e Å−3 |
| 0 constraints |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O2A | 0.2156 (5) | 0.6030 (5) | 0.0119 (3) | 0.0541 (13) | 0.681 (9) |
| C16A | 0.1025 (6) | 0.5890 (7) | 0.0772 (5) | 0.081 (2) | 0.681 (9) |
| H16A | 0.0614 | 0.5153 | 0.0787 | 0.121* | 0.681 (9) |
| H16B | 0.0412 | 0.6563 | 0.0550 | 0.121* | 0.681 (9) |
| H16C | 0.1282 | 0.5862 | 0.1420 | 0.121* | 0.681 (9) |
| O2B | 0.1539 (9) | 0.5492 (8) | 0.0303 (7) | 0.054 (3) | 0.319 (9) |
| C16B | 0.1933 (16) | 0.6617 (11) | 0.0412 (15) | 0.091 (5) | 0.319 (9) |
| H16D | 0.2672 | 0.6479 | 0.0827 | 0.136* | 0.319 (9) |
| H16E | 0.1211 | 0.6993 | 0.0706 | 0.136* | 0.319 (9) |
| H16F | 0.2181 | 0.7137 | −0.0222 | 0.136* | 0.319 (9) |
| O1 | 0.33194 (18) | 0.44978 (17) | 0.17638 (13) | 0.0552 (5) | |
| O3 | 0.2267 (2) | 0.5264 (2) | −0.15835 (14) | 0.0758 (7) | |
| H3 | 0.1715 | 0.5729 | −0.1418 | 0.114* | |
| O4 | 0.39213 (19) | 0.34365 (19) | −0.18117 (13) | 0.0630 (6) | |
| O5 | 0.52258 (19) | 0.20267 (18) | −0.02531 (14) | 0.0594 (5) | |
| H5 | 0.5487 | 0.1629 | 0.0281 | 0.089* | |
| O6 | 0.57099 (19) | 0.15879 (17) | 0.15881 (14) | 0.0604 (6) | |
| O7 | 0.52849 (17) | 0.21483 (16) | 0.33769 (13) | 0.0507 (5) | |
| C1 | 0.3942 (2) | 0.3804 (2) | 0.25738 (19) | 0.0439 (6) | |
| C2 | 0.3477 (3) | 0.4194 (2) | 0.0884 (2) | 0.0485 (7) | |
| C3 | 0.2788 (3) | 0.4914 (3) | 0.0111 (2) | 0.0583 (8) | |
| C4 | 0.2931 (3) | 0.4636 (3) | −0.0788 (2) | 0.0553 (7) | |
| C5 | 0.3765 (3) | 0.3673 (3) | −0.09125 (19) | 0.0488 (7) | |
| C6 | 0.4439 (2) | 0.2966 (2) | −0.0127 (2) | 0.0463 (7) | |
| C7 | 0.4305 (2) | 0.3227 (2) | 0.07893 (19) | 0.0423 (6) | |
| C8 | 0.4973 (2) | 0.2472 (2) | 0.1637 (2) | 0.0451 (6) | |
| C9 | 0.4720 (2) | 0.2829 (2) | 0.25352 (19) | 0.0430 (6) | |
| C10 | 0.3644 (2) | 0.4280 (2) | 0.34344 (19) | 0.0413 (6) | |
| C11 | 0.3612 (3) | 0.5518 (2) | 0.3333 (2) | 0.0524 (7) | |
| H11 | 0.3771 | 0.6056 | 0.2718 | 0.063* | |
| C12 | 0.3346 (3) | 0.5944 (3) | 0.4150 (3) | 0.0644 (9) | |
| H12 | 0.3342 | 0.6770 | 0.4087 | 0.077* | |
| C13 | 0.3085 (3) | 0.5153 (3) | 0.5058 (3) | 0.0695 (9) | |
| H13 | 0.2911 | 0.5447 | 0.5607 | 0.083* | |
| C14 | 0.3080 (3) | 0.3933 (3) | 0.5155 (2) | 0.0652 (9) | |
| H14 | 0.2881 | 0.3403 | 0.5767 | 0.078* | |
| C15 | 0.3369 (3) | 0.3492 (3) | 0.4351 (2) | 0.0492 (7) | |
| H15 | 0.3380 | 0.2663 | 0.4422 | 0.059* | |
| C17 | 0.2965 (3) | 0.2655 (4) | −0.1966 (3) | 0.0887 (12) | |
| H17A | 0.2953 | 0.1933 | −0.1430 | 0.133* | |
| H17B | 0.3188 | 0.2446 | −0.2571 | 0.133* | |
| H17C | 0.2108 | 0.3058 | −0.2002 | 0.133* | |
| C18 | 0.6633 (3) | 0.2394 (3) | 0.3434 (2) | 0.0677 (9) | |
| H18A | 0.7160 | 0.2122 | 0.2942 | 0.102* | |
| H18B | 0.6930 | 0.1976 | 0.4074 | 0.102* | |
| H18C | 0.6719 | 0.3248 | 0.3326 | 0.102* | |
| O11 | 0.17209 (16) | 0.07702 (15) | 0.40008 (13) | 0.0471 (5) | |
| O12 | 0.34604 (18) | −0.09955 (16) | 0.38200 (13) | 0.0538 (5) | |
| O13 | 0.4014 (2) | −0.13712 (18) | 0.20014 (14) | 0.0615 (6) | |
| H1 | 0.4080 | −0.1414 | 0.1431 | 0.092* | |
| O14 | 0.28707 (19) | 0.00309 (19) | 0.03475 (14) | 0.0652 (6) | |
| O15 | 0.1047 (2) | 0.1880 (2) | 0.05288 (14) | 0.0686 (6) | |
| H2 | 0.0590 | 0.2418 | 0.0681 | 0.103* | |
| O17 | −0.06171 (16) | 0.33282 (17) | 0.35675 (14) | 0.0568 (5) | |
| O16 | −0.01670 (19) | 0.30149 (18) | 0.17101 (14) | 0.0652 (6) | |
| C21 | 0.0844 (2) | 0.1638 (2) | 0.4174 (2) | 0.0449 (7) | |
| C22 | 0.2006 (2) | 0.0650 (2) | 0.30716 (19) | 0.0427 (6) | |
| C23 | 0.2919 (2) | −0.0251 (2) | 0.29875 (19) | 0.0435 (6) | |
| C24 | 0.3162 (2) | −0.0457 (2) | 0.2067 (2) | 0.0478 (7) | |
| C25 | 0.2532 (3) | 0.0272 (3) | 0.1242 (2) | 0.0502 (7) | |
| C26 | 0.1650 (3) | 0.1182 (3) | 0.1336 (2) | 0.0496 (7) | |
| C27 | 0.1367 (2) | 0.1396 (2) | 0.22674 (19) | 0.0435 (6) | |
| C28 | 0.0448 (2) | 0.2327 (2) | 0.2411 (2) | 0.0476 (7) | |
| C29 | 0.0255 (2) | 0.2440 (2) | 0.3408 (2) | 0.0476 (7) | |
| C30 | 0.0705 (2) | 0.1546 (2) | 0.5235 (2) | 0.0470 (7) | |
| C31 | −0.0251 (3) | 0.2212 (3) | 0.5644 (2) | 0.0623 (8) | |
| H31 | −0.0833 | 0.2744 | 0.5237 | 0.075* | |
| C32 | −0.0339 (3) | 0.2089 (3) | 0.6642 (2) | 0.0686 (9) | |
| H32 | −0.0974 | 0.2546 | 0.6903 | 0.082* | |
| C33 | 0.0498 (3) | 0.1302 (3) | 0.7255 (2) | 0.0675 (9) | |
| H33 | 0.0432 | 0.1225 | 0.7928 | 0.081* | |
| C34 | 0.1440 (3) | 0.0619 (3) | 0.6870 (2) | 0.0675 (9) | |
| H34 | 0.2010 | 0.0081 | 0.7284 | 0.081* | |
| C35 | 0.1534 (3) | 0.0740 (3) | 0.5870 (2) | 0.0590 (8) | |
| H35 | 0.2166 | 0.0273 | 0.5617 | 0.071* | |
| C36 | 0.4841 (3) | −0.0855 (3) | 0.3886 (2) | 0.0706 (9) | |
| H36A | 0.5339 | −0.1244 | 0.3451 | 0.106* | |
| H36B | 0.5090 | −0.1215 | 0.4550 | 0.106* | |
| H36C | 0.5022 | −0.0009 | 0.3703 | 0.106* | |
| C37 | 0.1887 (4) | −0.0458 (4) | −0.0052 (3) | 0.1063 (14) | |
| H37A | 0.1590 | −0.1180 | 0.0416 | 0.159* | |
| H37B | 0.2233 | −0.0650 | −0.0641 | 0.159* | |
| H37C | 0.1158 | 0.0119 | −0.0206 | 0.159* | |
| C38 | −0.0057 (3) | 0.4495 (3) | 0.3365 (3) | 0.0771 (10) | |
| H38A | 0.0523 | 0.4502 | 0.3875 | 0.116* | |
| H38B | −0.0752 | 0.5102 | 0.3341 | 0.116* | |
| H38C | 0.0433 | 0.4662 | 0.2743 | 0.116* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O2A | 0.065 (3) | 0.046 (3) | 0.046 (2) | 0.014 (2) | −0.0035 (18) | −0.0042 (19) |
| C16A | 0.073 (4) | 0.081 (5) | 0.073 (4) | 0.025 (4) | 0.012 (3) | −0.004 (3) |
| O2B | 0.049 (6) | 0.053 (5) | 0.056 (5) | 0.001 (4) | −0.002 (4) | −0.011 (4) |
| C16B | 0.080 (11) | 0.056 (9) | 0.144 (14) | 0.010 (8) | −0.032 (10) | −0.037 (10) |
| O1 | 0.0649 (12) | 0.0613 (12) | 0.0418 (11) | 0.0228 (10) | −0.0135 (9) | −0.0206 (9) |
| O3 | 0.0831 (16) | 0.0953 (17) | 0.0435 (12) | 0.0396 (13) | −0.0134 (11) | −0.0153 (11) |
| O4 | 0.0627 (13) | 0.0872 (16) | 0.0391 (12) | 0.0051 (11) | 0.0069 (9) | −0.0210 (11) |
| O5 | 0.0621 (12) | 0.0675 (14) | 0.0502 (12) | 0.0222 (10) | −0.0046 (10) | −0.0231 (11) |
| O6 | 0.0674 (13) | 0.0582 (13) | 0.0559 (12) | 0.0258 (11) | −0.0103 (10) | −0.0198 (10) |
| O7 | 0.0533 (11) | 0.0500 (11) | 0.0460 (11) | 0.0072 (9) | −0.0111 (9) | −0.0081 (9) |
| C1 | 0.0457 (15) | 0.0435 (16) | 0.0430 (16) | 0.0035 (13) | −0.0115 (12) | −0.0116 (13) |
| C2 | 0.0491 (16) | 0.0544 (17) | 0.0439 (17) | 0.0087 (13) | −0.0065 (13) | −0.0178 (14) |
| C3 | 0.0642 (19) | 0.064 (2) | 0.0463 (18) | 0.0266 (16) | −0.0108 (14) | −0.0185 (15) |
| C4 | 0.0522 (17) | 0.064 (2) | 0.0446 (17) | 0.0107 (15) | −0.0066 (14) | −0.0086 (15) |
| C5 | 0.0432 (15) | 0.0645 (19) | 0.0382 (16) | 0.0014 (14) | −0.0005 (12) | −0.0140 (14) |
| C6 | 0.0389 (14) | 0.0518 (17) | 0.0488 (17) | 0.0053 (13) | −0.0018 (12) | −0.0160 (14) |
| C7 | 0.0401 (14) | 0.0438 (15) | 0.0434 (16) | 0.0012 (12) | −0.0061 (12) | −0.0122 (12) |
| C8 | 0.0414 (15) | 0.0434 (16) | 0.0510 (17) | 0.0056 (13) | −0.0070 (12) | −0.0138 (13) |
| C9 | 0.0442 (15) | 0.0423 (15) | 0.0421 (16) | 0.0009 (12) | −0.0117 (12) | −0.0092 (12) |
| C10 | 0.0368 (14) | 0.0470 (16) | 0.0407 (15) | 0.0022 (12) | −0.0034 (11) | −0.0135 (13) |
| C11 | 0.0503 (16) | 0.0504 (17) | 0.0556 (18) | −0.0009 (13) | 0.0001 (13) | −0.0138 (14) |
| C12 | 0.0611 (19) | 0.058 (2) | 0.085 (3) | −0.0021 (15) | 0.0023 (17) | −0.039 (2) |
| C13 | 0.072 (2) | 0.088 (3) | 0.061 (2) | 0.0083 (18) | 0.0002 (17) | −0.045 (2) |
| C14 | 0.072 (2) | 0.079 (2) | 0.0424 (18) | 0.0060 (17) | 0.0007 (15) | −0.0148 (16) |
| C15 | 0.0513 (16) | 0.0505 (17) | 0.0456 (17) | 0.0030 (13) | −0.0058 (13) | −0.0128 (14) |
| C17 | 0.084 (2) | 0.124 (3) | 0.077 (3) | 0.006 (2) | −0.018 (2) | −0.058 (2) |
| C18 | 0.0559 (19) | 0.082 (2) | 0.066 (2) | 0.0076 (17) | −0.0229 (16) | −0.0194 (17) |
| O11 | 0.0491 (10) | 0.0463 (11) | 0.0465 (11) | 0.0086 (9) | −0.0069 (8) | −0.0144 (9) |
| O12 | 0.0581 (12) | 0.0522 (11) | 0.0445 (11) | 0.0141 (9) | −0.0080 (9) | −0.0037 (9) |
| O13 | 0.0733 (13) | 0.0622 (13) | 0.0485 (12) | 0.0293 (11) | −0.0090 (10) | −0.0188 (10) |
| O14 | 0.0605 (13) | 0.0839 (15) | 0.0515 (13) | 0.0176 (11) | −0.0041 (10) | −0.0227 (11) |
| O15 | 0.0735 (15) | 0.0762 (15) | 0.0498 (12) | 0.0290 (11) | −0.0159 (11) | −0.0095 (11) |
| O17 | 0.0416 (10) | 0.0568 (13) | 0.0751 (14) | 0.0130 (9) | −0.0060 (9) | −0.0251 (11) |
| O16 | 0.0634 (13) | 0.0673 (14) | 0.0600 (13) | 0.0266 (11) | −0.0135 (10) | −0.0120 (11) |
| C21 | 0.0351 (14) | 0.0437 (16) | 0.0577 (18) | 0.0010 (12) | −0.0053 (13) | −0.0165 (14) |
| C22 | 0.0413 (14) | 0.0433 (15) | 0.0439 (16) | −0.0026 (12) | −0.0056 (12) | −0.0112 (13) |
| C23 | 0.0447 (15) | 0.0409 (15) | 0.0426 (16) | 0.0045 (12) | −0.0101 (12) | −0.0066 (12) |
| C24 | 0.0456 (16) | 0.0443 (16) | 0.0504 (18) | 0.0052 (13) | −0.0058 (13) | −0.0079 (13) |
| C25 | 0.0519 (17) | 0.0545 (18) | 0.0427 (17) | 0.0053 (14) | −0.0034 (13) | −0.0121 (14) |
| C26 | 0.0464 (16) | 0.0540 (17) | 0.0452 (17) | 0.0060 (14) | −0.0107 (13) | −0.0075 (13) |
| C27 | 0.0398 (14) | 0.0418 (15) | 0.0468 (16) | 0.0014 (12) | −0.0069 (12) | −0.0078 (12) |
| C28 | 0.0404 (15) | 0.0457 (16) | 0.0547 (18) | 0.0037 (13) | −0.0122 (13) | −0.0088 (14) |
| C29 | 0.0354 (14) | 0.0453 (16) | 0.0653 (19) | 0.0042 (12) | −0.0078 (13) | −0.0200 (14) |
| C30 | 0.0413 (15) | 0.0505 (17) | 0.0528 (18) | −0.0055 (13) | −0.0010 (13) | −0.0194 (14) |
| C31 | 0.0537 (18) | 0.070 (2) | 0.067 (2) | 0.0045 (15) | −0.0012 (15) | −0.0253 (17) |
| C32 | 0.061 (2) | 0.086 (2) | 0.065 (2) | −0.0013 (18) | 0.0100 (17) | −0.0343 (19) |
| C33 | 0.070 (2) | 0.084 (2) | 0.053 (2) | −0.0173 (19) | 0.0077 (17) | −0.0248 (18) |
| C34 | 0.075 (2) | 0.077 (2) | 0.048 (2) | 0.0015 (18) | −0.0066 (16) | −0.0123 (17) |
| C35 | 0.0560 (18) | 0.061 (2) | 0.061 (2) | 0.0049 (15) | −0.0029 (15) | −0.0196 (16) |
| C36 | 0.067 (2) | 0.067 (2) | 0.074 (2) | 0.0077 (17) | −0.0307 (17) | −0.0090 (17) |
| C37 | 0.077 (3) | 0.174 (4) | 0.091 (3) | 0.020 (3) | −0.019 (2) | −0.077 (3) |
| C38 | 0.070 (2) | 0.050 (2) | 0.111 (3) | 0.0058 (17) | 0.0011 (19) | −0.0235 (19) |
Geometric parameters (Å, °)
| O2A—C3 | 1.404 (5) | C18—H18B | 0.9599 |
| O2A—C16A | 1.416 (7) | C18—H18C | 0.9599 |
| C16A—H16A | 0.9599 | O11—C21 | 1.362 (3) |
| C16A—H16B | 0.9599 | O11—C22 | 1.369 (3) |
| C16A—H16C | 0.9599 | O12—C23 | 1.378 (3) |
| O2B—C16B | 1.425 (13) | O12—C36 | 1.431 (3) |
| O2B—C3 | 1.444 (8) | O13—C24 | 1.343 (3) |
| C16B—H16D | 0.9599 | O13—H1 | 0.8200 |
| C16B—H16E | 0.9599 | O14—C25 | 1.386 (3) |
| C16B—H16F | 0.9599 | O14—C37 | 1.391 (4) |
| O1—C1 | 1.372 (3) | O15—C26 | 1.361 (3) |
| O1—C2 | 1.378 (3) | O15—H2 | 0.8200 |
| O3—C4 | 1.356 (3) | O17—C29 | 1.370 (3) |
| O3—H3 | 0.8200 | O17—C38 | 1.435 (3) |
| O4—C5 | 1.368 (3) | O16—C28 | 1.262 (3) |
| O4—C17 | 1.423 (4) | C21—C29 | 1.363 (4) |
| O5—C6 | 1.352 (3) | C21—C30 | 1.473 (4) |
| O5—H5 | 0.8200 | C22—C23 | 1.376 (3) |
| O6—C8 | 1.243 (3) | C22—C27 | 1.395 (4) |
| O7—C9 | 1.373 (3) | C23—C24 | 1.389 (3) |
| O7—C18 | 1.423 (3) | C24—C25 | 1.404 (4) |
| C1—C9 | 1.346 (3) | C25—C26 | 1.367 (4) |
| C1—C10 | 1.473 (3) | C26—C27 | 1.414 (4) |
| C2—C3 | 1.381 (4) | C27—C28 | 1.429 (4) |
| C2—C7 | 1.387 (3) | C28—C29 | 1.450 (4) |
| C3—C4 | 1.390 (4) | C30—C35 | 1.388 (4) |
| C4—C5 | 1.398 (4) | C30—C31 | 1.396 (4) |
| C5—C6 | 1.379 (4) | C31—C32 | 1.378 (4) |
| C6—C7 | 1.404 (3) | C31—H31 | 0.9300 |
| C7—C8 | 1.456 (4) | C32—C33 | 1.367 (5) |
| C8—C9 | 1.441 (3) | C32—H32 | 0.9300 |
| C10—C15 | 1.386 (4) | C33—C34 | 1.382 (4) |
| C10—C11 | 1.390 (4) | C33—H33 | 0.9300 |
| C11—C12 | 1.378 (4) | C34—C35 | 1.380 (4) |
| C11—H11 | 0.9300 | C34—H34 | 0.9300 |
| C12—C13 | 1.376 (4) | C35—H35 | 0.9300 |
| C12—H12 | 0.9300 | C36—H36A | 0.9599 |
| C13—C14 | 1.372 (4) | C36—H36B | 0.9599 |
| C13—H13 | 0.9300 | C36—H36C | 0.9599 |
| C14—C15 | 1.375 (4) | C37—H37A | 0.9599 |
| C14—H14 | 0.9300 | C37—H37B | 0.9599 |
| C15—H15 | 0.9300 | C37—H37C | 0.9599 |
| C17—H17A | 0.9599 | C38—H38A | 0.9599 |
| C17—H17B | 0.9599 | C38—H38B | 0.9599 |
| C17—H17C | 0.9599 | C38—H38C | 0.9599 |
| C18—H18A | 0.9599 | ||
| C3—O2A—C16A | 112.2 (6) | H18B—C18—H18C | 109.5 |
| C16B—O2B—C3 | 102.2 (11) | C21—O11—C22 | 121.8 (2) |
| O2B—C16B—H16D | 109.5 | C23—O12—C36 | 114.4 (2) |
| O2B—C16B—H16E | 109.5 | C24—O13—H1 | 109.5 |
| H16D—C16B—H16E | 109.5 | C25—O14—C37 | 115.6 (2) |
| O2B—C16B—H16F | 109.5 | C26—O15—H2 | 109.5 |
| H16D—C16B—H16F | 109.5 | C29—O17—C38 | 113.8 (2) |
| H16E—C16B—H16F | 109.5 | O11—C21—C29 | 119.9 (2) |
| C1—O1—C2 | 119.78 (19) | O11—C21—C30 | 110.4 (2) |
| C4—O3—H3 | 109.5 | C29—C21—C30 | 129.7 (2) |
| C5—O4—C17 | 113.5 (2) | O11—C22—C23 | 116.5 (2) |
| C6—O5—H5 | 109.5 | O11—C22—C27 | 120.7 (2) |
| C9—O7—C18 | 113.8 (2) | C23—C22—C27 | 122.8 (2) |
| C9—C1—O1 | 121.8 (2) | C22—C23—O12 | 119.7 (2) |
| C9—C1—C10 | 127.0 (2) | C22—C23—C24 | 118.1 (2) |
| O1—C1—C10 | 111.2 (2) | O12—C23—C24 | 121.9 (2) |
| O1—C2—C3 | 116.3 (2) | O13—C24—C23 | 117.6 (2) |
| O1—C2—C7 | 121.0 (2) | O13—C24—C25 | 121.8 (2) |
| C3—C2—C7 | 122.6 (2) | C23—C24—C25 | 120.6 (2) |
| C2—C3—C4 | 117.8 (2) | C26—C25—O14 | 122.8 (2) |
| C2—C3—O2A | 124.0 (3) | C26—C25—C24 | 120.5 (2) |
| C4—C3—O2A | 117.1 (3) | O14—C25—C24 | 116.7 (2) |
| C2—C3—O2B | 119.9 (4) | O15—C26—C25 | 119.7 (2) |
| C4—C3—O2B | 115.5 (4) | O15—C26—C27 | 120.2 (2) |
| O3—C4—C3 | 122.4 (2) | C25—C26—C27 | 120.0 (2) |
| O3—C4—C5 | 116.5 (2) | C22—C27—C26 | 117.9 (2) |
| C3—C4—C5 | 121.2 (3) | C22—C27—C28 | 119.6 (2) |
| O4—C5—C6 | 120.0 (2) | C26—C27—C28 | 122.5 (2) |
| O4—C5—C4 | 120.2 (2) | O16—C28—C27 | 122.0 (3) |
| C6—C5—C4 | 119.8 (2) | O16—C28—C29 | 121.6 (2) |
| O5—C6—C5 | 119.3 (2) | C27—C28—C29 | 116.4 (2) |
| O5—C6—C7 | 120.7 (2) | C21—C29—O17 | 120.7 (3) |
| C5—C6—C7 | 120.0 (2) | C21—C29—C28 | 121.4 (2) |
| C2—C7—C6 | 118.6 (2) | O17—C29—C28 | 117.8 (2) |
| C2—C7—C8 | 120.3 (2) | C35—C30—C31 | 117.8 (3) |
| C6—C7—C8 | 121.1 (2) | C35—C30—C21 | 119.1 (2) |
| O6—C8—C9 | 122.6 (2) | C31—C30—C21 | 123.1 (3) |
| O6—C8—C7 | 122.4 (2) | C32—C31—C30 | 120.7 (3) |
| C9—C8—C7 | 115.0 (2) | C32—C31—H31 | 119.7 |
| C1—C9—O7 | 119.2 (2) | C30—C31—H31 | 119.7 |
| C1—C9—C8 | 122.0 (2) | C33—C32—C31 | 120.7 (3) |
| O7—C9—C8 | 118.8 (2) | C33—C32—H32 | 119.7 |
| C15—C10—C11 | 119.5 (2) | C31—C32—H32 | 119.7 |
| C15—C10—C1 | 120.0 (2) | C32—C33—C34 | 119.7 (3) |
| C11—C10—C1 | 120.5 (2) | C32—C33—H33 | 120.1 |
| C12—C11—C10 | 119.5 (3) | C34—C33—H33 | 120.1 |
| C12—C11—H11 | 120.2 | C35—C34—C33 | 119.8 (3) |
| C10—C11—H11 | 120.2 | C35—C34—H34 | 120.1 |
| C13—C12—C11 | 120.5 (3) | C33—C34—H34 | 120.1 |
| C13—C12—H12 | 119.8 | C34—C35—C30 | 121.2 (3) |
| C11—C12—H12 | 119.8 | C34—C35—H35 | 119.4 |
| C14—C13—C12 | 120.1 (3) | C30—C35—H35 | 119.4 |
| C14—C13—H13 | 120.0 | O12—C36—H36A | 109.5 |
| C12—C13—H13 | 120.0 | O12—C36—H36B | 109.5 |
| C13—C14—C15 | 120.2 (3) | H36A—C36—H36B | 109.5 |
| C13—C14—H14 | 119.9 | O12—C36—H36C | 109.5 |
| C15—C14—H14 | 119.9 | H36A—C36—H36C | 109.5 |
| C14—C15—C10 | 120.2 (3) | H36B—C36—H36C | 109.5 |
| C14—C15—H15 | 119.9 | O14—C37—H37A | 109.5 |
| C10—C15—H15 | 119.9 | O14—C37—H37B | 109.5 |
| O4—C17—H17A | 109.5 | H37A—C37—H37B | 109.5 |
| O4—C17—H17B | 109.5 | O14—C37—H37C | 109.5 |
| H17A—C17—H17B | 109.5 | H37A—C37—H37C | 109.5 |
| O4—C17—H17C | 109.5 | H37B—C37—H37C | 109.5 |
| H17A—C17—H17C | 109.5 | O17—C38—H38A | 109.5 |
| H17B—C17—H17C | 109.5 | O17—C38—H38B | 109.5 |
| O7—C18—H18A | 109.5 | H38A—C38—H38B | 109.5 |
| O7—C18—H18B | 109.5 | O17—C38—H38C | 109.5 |
| H18A—C18—H18B | 109.5 | H38A—C38—H38C | 109.5 |
| O7—C18—H18C | 109.5 | H38B—C38—H38C | 109.5 |
| H18A—C18—H18C | 109.5 | ||
| C2—O1—C1—C9 | 0.5 (4) | C12—C13—C14—C15 | 1.6 (5) |
| C2—O1—C1—C10 | −179.9 (2) | C13—C14—C15—C10 | −1.1 (4) |
| C1—O1—C2—C3 | 178.1 (3) | C11—C10—C15—C14 | −0.6 (4) |
| C1—O1—C2—C7 | −2.9 (4) | C1—C10—C15—C14 | −179.5 (2) |
| O1—C2—C3—C4 | 179.7 (3) | C22—O11—C21—C29 | −2.0 (4) |
| C7—C2—C3—C4 | 0.8 (5) | C22—O11—C21—C30 | 178.8 (2) |
| O1—C2—C3—O2A | 12.1 (5) | C21—O11—C22—C23 | 179.2 (2) |
| C7—C2—C3—O2A | −166.8 (4) | C21—O11—C22—C27 | −2.2 (3) |
| O1—C2—C3—O2B | −30.4 (6) | O11—C22—C23—O12 | 2.0 (4) |
| C7—C2—C3—O2B | 150.6 (5) | C27—C22—C23—O12 | −176.6 (2) |
| C16A—O2A—C3—C2 | −70.4 (7) | O11—C22—C23—C24 | 175.4 (2) |
| C16A—O2A—C3—C4 | 121.9 (5) | C27—C22—C23—C24 | −3.1 (4) |
| C16A—O2A—C3—O2B | 25.1 (6) | C36—O12—C23—C22 | −113.0 (3) |
| C16B—O2B—C3—C2 | 90.0 (11) | C36—O12—C23—C24 | 73.8 (3) |
| C16B—O2B—C3—C4 | −119.5 (9) | C22—C23—C24—O13 | −177.3 (2) |
| C16B—O2B—C3—O2A | −17.8 (8) | O12—C23—C24—O13 | −4.0 (4) |
| C2—C3—C4—O3 | 178.0 (3) | C22—C23—C24—C25 | 2.7 (4) |
| O2A—C3—C4—O3 | −13.5 (5) | O12—C23—C24—C25 | 176.0 (2) |
| O2B—C3—C4—O3 | 26.8 (6) | C37—O14—C25—C26 | −70.2 (4) |
| C2—C3—C4—C5 | −1.3 (5) | C37—O14—C25—C24 | 111.0 (3) |
| O2A—C3—C4—C5 | 167.2 (3) | O13—C24—C25—C26 | 178.8 (3) |
| O2B—C3—C4—C5 | −152.5 (5) | C23—C24—C25—C26 | −1.2 (4) |
| C17—O4—C5—C6 | 92.7 (3) | O13—C24—C25—O14 | −2.4 (4) |
| C17—O4—C5—C4 | −87.7 (3) | C23—C24—C25—O14 | 177.6 (2) |
| O3—C4—C5—O4 | 2.6 (4) | O14—C25—C26—O15 | 1.3 (4) |
| C3—C4—C5—O4 | −178.1 (3) | C24—C25—C26—O15 | −180.0 (3) |
| O3—C4—C5—C6 | −177.8 (3) | O14—C25—C26—C27 | −178.7 (2) |
| C3—C4—C5—C6 | 1.6 (4) | C24—C25—C26—C27 | 0.0 (4) |
| O4—C5—C6—O5 | −1.4 (4) | O11—C22—C27—C26 | −176.5 (2) |
| C4—C5—C6—O5 | 178.9 (2) | C23—C22—C27—C26 | 2.0 (4) |
| O4—C5—C6—C7 | 178.4 (2) | O11—C22—C27—C28 | 2.6 (4) |
| C4—C5—C6—C7 | −1.2 (4) | C23—C22—C27—C28 | −178.9 (2) |
| O1—C2—C7—C6 | −179.4 (2) | O15—C26—C27—C22 | 179.6 (2) |
| C3—C2—C7—C6 | −0.5 (4) | C25—C26—C27—C22 | −0.3 (4) |
| O1—C2—C7—C8 | 3.2 (4) | O15—C26—C27—C28 | 0.5 (4) |
| C3—C2—C7—C8 | −177.9 (3) | C25—C26—C27—C28 | −179.5 (2) |
| O5—C6—C7—C2 | −179.5 (2) | C22—C27—C28—O16 | −179.1 (2) |
| C5—C6—C7—C2 | 0.7 (4) | C26—C27—C28—O16 | 0.0 (4) |
| O5—C6—C7—C8 | −2.1 (4) | C22—C27—C28—C29 | 0.8 (4) |
| C5—C6—C7—C8 | 178.1 (2) | C26—C27—C28—C29 | 179.9 (2) |
| C2—C7—C8—O6 | 179.1 (2) | O11—C21—C29—O17 | −179.2 (2) |
| C6—C7—C8—O6 | 1.7 (4) | C30—C21—C29—O17 | −0.1 (4) |
| C2—C7—C8—C9 | −1.1 (4) | O11—C21—C29—C28 | 5.6 (4) |
| C6—C7—C8—C9 | −178.5 (2) | C30—C21—C29—C28 | −175.3 (2) |
| O1—C1—C9—O7 | −178.1 (2) | C38—O17—C29—C21 | 101.0 (3) |
| C10—C1—C9—O7 | 2.4 (4) | C38—O17—C29—C28 | −83.6 (3) |
| O1—C1—C9—C8 | 1.6 (4) | O16—C28—C29—C21 | 175.0 (3) |
| C10—C1—C9—C8 | −177.9 (2) | C27—C28—C29—C21 | −4.9 (4) |
| C18—O7—C9—C1 | −100.6 (3) | O16—C28—C29—O17 | −0.3 (4) |
| C18—O7—C9—C8 | 79.7 (3) | C27—C28—C29—O17 | 179.8 (2) |
| O6—C8—C9—C1 | 178.5 (3) | O11—C21—C30—C35 | 7.0 (3) |
| C7—C8—C9—C1 | −1.2 (4) | C29—C21—C30—C35 | −172.1 (3) |
| O6—C8—C9—O7 | −1.8 (4) | O11—C21—C30—C31 | −171.4 (2) |
| C7—C8—C9—O7 | 178.4 (2) | C29—C21—C30—C31 | 9.5 (4) |
| C9—C1—C10—C15 | −42.8 (4) | C35—C30—C31—C32 | 1.5 (4) |
| O1—C1—C10—C15 | 137.6 (2) | C21—C30—C31—C32 | 179.9 (3) |
| C9—C1—C10—C11 | 138.3 (3) | C30—C31—C32—C33 | −0.8 (5) |
| O1—C1—C10—C11 | −41.3 (3) | C31—C32—C33—C34 | −0.1 (5) |
| C15—C10—C11—C12 | 1.8 (4) | C32—C33—C34—C35 | 0.2 (5) |
| C1—C10—C11—C12 | −179.3 (2) | C33—C34—C35—C30 | 0.6 (5) |
| C10—C11—C12—C13 | −1.3 (4) | C31—C30—C35—C34 | −1.4 (4) |
| C11—C12—C13—C14 | −0.4 (5) | C21—C30—C35—C34 | −179.9 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3···O16i | 0.82 | 2.06 | 2.806 (3) | 152 |
| O3—H3···O2A | 0.82 | 2.36 | 2.772 (5) | 112 |
| O3—H3···O2B | 0.82 | 2.37 | 2.799 (9) | 114 |
| O5—H5···O6 | 0.82 | 1.86 | 2.586 (3) | 146 |
| O13—H1···O5ii | 0.82 | 2.05 | 2.825 (3) | 158 |
| O13—H1···O14 | 0.82 | 2.29 | 2.736 (3) | 115 |
| O15—H2···O16 | 0.82 | 1.88 | 2.600 (3) | 147 |
Symmetry codes: (i) −x, −y+1, −z; (ii) −x+1, −y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PK2205).
References
- Ali, E., Bagchi, D. & Pakrashi, S. C. (1979). Phytochemistry, 18, 356–357.
- Bruker (2005). SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chinese Materia Medica (2007). Chinese Materia Medica, Vol. 21, p. 643. Shanghai Science Press.
- Cubukcu, B. & Bingol, S. (1984). Plant Med. Phytother 18, 28–35.
- Guerreiro, E., Kavka, J. & Giordano, O. S. (1982). Phytochemistry, 21, 2601–2602.
- Horie, T., Kawamura, Y., Yamamoto, H. & Yamashita, K. (1995). Chem. Pharm. Bull. 43, 2054–2063.
- Jakupovic, J., Zdero, C., Grenz, M., Tsichritzis, F., Lehmann, L., Hashemi-Nejad, S. & Bohlmann, F. (1989). Phytochemistry, 28, 1119–1131.
- Lavault, M. & Richomme, P. (2004). Chem. Nat. Comp., 40, 118–121.
- Mericli, A. H., Damadtan, B. & Cubukcu, B. (1986). Sci. Pharm. 54, 363–365.
- Sheldrick, G. M. (1996). SADABS. University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tomas-Lorente, F., Iniesta-Sanmrtin, E., Tomas-Barberan, F. A., Trowitzsch-Kienast, W. & Wray, V. (1989). Phytochemistry, 28, 1613–1615.
- Torrenegra, R. D., Escarria, S., Raffelsberger, B. & Achenbach, H. (1980). Phytochemistry, 19, 2795–2796.
- Urzua, A., Torres, R., Bueno, C. & Mendoza, L. (1995). Biochem. Syst. Ecol 23, 459.
- Wollenweber, E., Fischer, R., Doerr, M., Irvine, K., Pereira, C. & Stevens, J. F. (2008). J. Biosci. 63, 731–739. [DOI] [PubMed]
- Wollenweber, E., Fritz, H., Henrich, B., Jakupovic, J., Schilling, G. & Roitman, J. N. (1993). J. Biosci. 48, 420–424.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809050715/pk2205sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809050715/pk2205Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report