Abstract
The 3,5-methoxy groups in the title compound, C16H23NO4, are almost coplanar with the aromatic ring, whereas the 4-methoxy group is bent out of this plane. The three CH3—O—C—C torsion angles are −1.51 (18), 0.73 (19) and 75.33 (15)°. The cyclohexane ring adopts a chair conformation. In the crystal, molecules are connected by intermolecular N—H⋯O hydrogen bonds into chains running along the b axis.
Related literature
For the biological activity of benzanilides, see: Olsson et al. (2002 ▶); Lindgren et al. (2001 ▶); Calderone et al. (2006 ▶). For the use of benzamides in organic synthesis, see: Zhichkin et al. (2007 ▶); Beccalli et al. (2005 ▶). For related structures, see: Bowes et al. (2003 ▶); Chopra & Guru Row (2008 ▶); Kashino et al. (1979 ▶); Saeed et al. (2008 ▶).
Experimental
Crystal data
C16H23NO4
M r = 293.35
Monoclinic,

a = 23.4539 (19) Å
b = 5.2145 (6) Å
c = 12.4559 (10) Å
β = 92.886 (6)°
V = 1521.4 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 173 K
0.37 × 0.37 × 0.33 mm
Data collection
Stoe IPDSII two-circle diffractometer
Absorption correction: none
6868 measured reflections
2823 independent reflections
2360 reflections with I > 2σ(I)
R int = 0.062
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.109
S = 1.05
2823 reflections
198 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.27 e Å−3
Δρmin = −0.22 e Å−3
Data collection: X-AREA (Stoe & Cie, 2001 ▶); cell refinement: X-AREA; data reduction: X-AREA; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: PLATON (Spek, 2009 ▶) and SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809050284/fj2261sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809050284/fj2261Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1i | 0.916 (19) | 2.153 (19) | 3.0262 (15) | 159.0 (15) |
Symmetry code: (i) 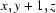 .
.
supplementary crystallographic information
Comment
N-substituted benzamides are well known anticancer compounds and the mechanism of action for N-substituted benzamide-induced apoptosis has been studied, using declopramide as a lead compound (Olsson et al., 2002). N-substituted benzamides inhibit the activity of nuclear factor- B and nuclear factor of activated T cells activity while inducing activator protein 1 activity in T lymphocytes (Lindgren et al., 2001). Heterocyclic analogs of benzanilide derivatives are potassium channel activators (Calderone et al., 2006). N-Alkylated 2-nitrobenzamides are intermediates in the synthesis of dibenzo[b,e][1,4]diazepines (Zhichkin et al., 2007) and N-Acyl-2-nitrobenzamides are precursors of 2,3-disubstitued 3H-quinazoline-4-ones (Beccalli et al., 2005).
The two m-methoxy groups the title compound are almost coplanar with the aromatic ring [CH3—O—C—C -1.51 (18)° and 0.73 (19)°] whereas the methoxy group in para position is bent out of the plane of the aromatic ring [CH3—O—C—C 75.33 (15)°]. The cyclohexyl ring adops a chair conformation. The molecules are connected by N—H···O hydrogen bonds to chains running along the b axis.
Experimental
3,4,5-Trimethoxybenzoyl chloride (1 mmol) in CHCl3 was treated with cyclohexylamine (3.5 mmol) under a nitrogen atmosphere at reflux for 3 h. Upon cooling, the reaction mixture was diluted with CHCl3 and washed consecutively with 1 M aq HCl and saturated aq NaHCO3. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. Crystallization of the residue in methanol afforded the title compound (78%) as colourless crystals: Anal. calcd. for C16H23NO4: C, 65.51; H, 9.70; N, 4.77%; found: C, 65.58; H, 9.65; N, 4.81%.
Refinement
Hydrogen atoms were located in difference syntheses, but those bonded to C were refined at idealized positions using a riding model with C–H = 0.95–1.00 Å) and with Uiso(H) = 1.2Ueq(C) or Uiso(H) = 1.5Ueq(Cmethyl). The methyl groups were allowed to rotate but not to tip. The H atom bonded to N was freely refined.
Figures
Fig. 1.
Molecular structure of title compound. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
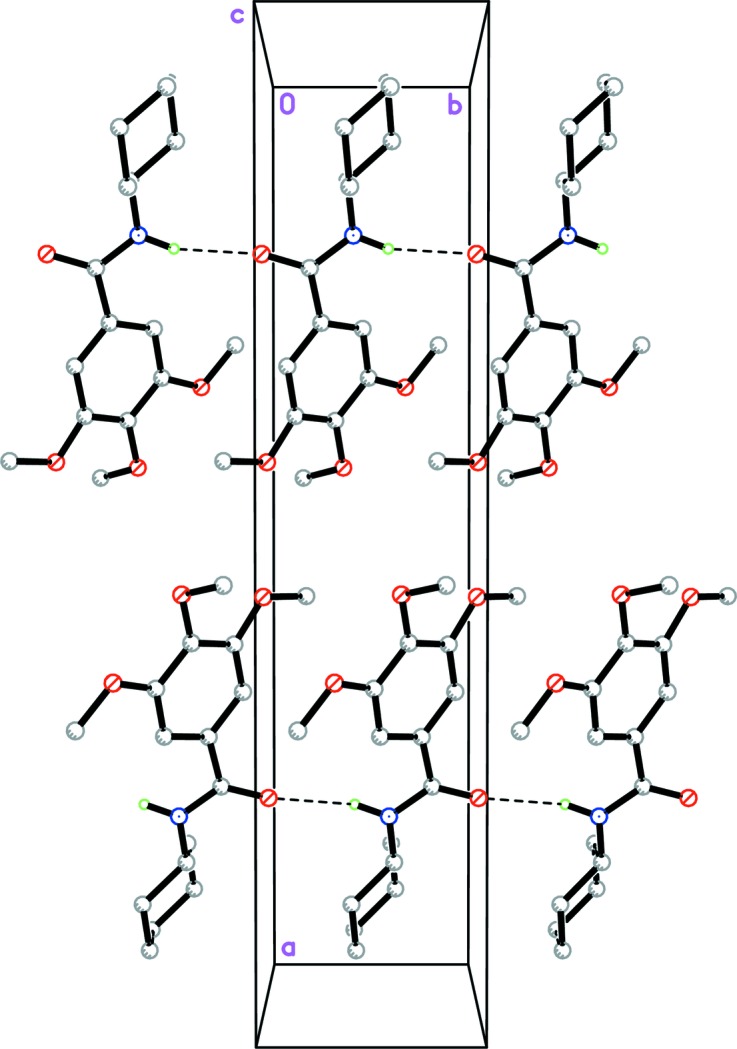
Crystal packing viewed along [001] with intermolecular hydrogen bonds indicated as dashed lines. H-atoms not involved in hydrogen bonding are omitted.
Crystal data
| C16H23NO4 | F(000) = 632 |
| Mr = 293.35 | Dx = 1.281 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 6103 reflections |
| a = 23.4539 (19) Å | θ = 3.4–26.0° |
| b = 5.2145 (6) Å | µ = 0.09 mm−1 |
| c = 12.4559 (10) Å | T = 173 K |
| β = 92.886 (6)° | Block, colourless |
| V = 1521.4 (2) Å3 | 0.37 × 0.37 × 0.33 mm |
| Z = 4 |
Data collection
| Stoe IPDSII two-circle diffractometer | 2360 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.062 |
| graphite | θmax = 25.6°, θmin = 3.4° |
| ω scans | h = −28→23 |
| 6868 measured reflections | k = −5→6 |
| 2823 independent reflections | l = −15→15 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.040 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.109 | w = 1/[σ2(Fo2) + (0.0651P)2 + 0.0617P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 2823 reflections | Δρmax = 0.27 e Å−3 |
| 198 parameters | Δρmin = −0.22 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.023 (3) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.19554 (5) | 0.4159 (2) | 0.53398 (9) | 0.0237 (3) | |
| H1 | 0.2089 (8) | 0.579 (4) | 0.5241 (13) | 0.034 (4)* | |
| O1 | 0.21507 (4) | −0.01048 (18) | 0.53804 (8) | 0.0289 (3) | |
| O2 | 0.42937 (4) | 0.00520 (19) | 0.45580 (7) | 0.0279 (3) | |
| O3 | 0.43346 (4) | 0.36344 (18) | 0.30218 (7) | 0.0242 (2) | |
| O4 | 0.34531 (4) | 0.66623 (18) | 0.25120 (7) | 0.0255 (2) | |
| C1 | 0.22841 (6) | 0.2119 (2) | 0.51372 (10) | 0.0211 (3) | |
| C11 | 0.28301 (5) | 0.2637 (2) | 0.45950 (9) | 0.0198 (3) | |
| C12 | 0.32948 (6) | 0.1047 (2) | 0.48557 (9) | 0.0213 (3) | |
| H12 | 0.3265 | −0.0265 | 0.5379 | 0.026* | |
| C13 | 0.38033 (6) | 0.1395 (3) | 0.43442 (9) | 0.0211 (3) | |
| C14 | 0.38357 (5) | 0.3267 (2) | 0.35373 (9) | 0.0202 (3) | |
| C15 | 0.33712 (6) | 0.4871 (2) | 0.32918 (9) | 0.0198 (3) | |
| C16 | 0.28646 (6) | 0.4586 (2) | 0.38262 (9) | 0.0201 (3) | |
| H16 | 0.2550 | 0.5693 | 0.3671 | 0.024* | |
| C17 | 0.42929 (6) | −0.1910 (3) | 0.53660 (10) | 0.0268 (3) | |
| H17A | 0.4199 | −0.1149 | 0.6055 | 0.040* | |
| H17B | 0.4671 | −0.2707 | 0.5438 | 0.040* | |
| H17C | 0.4008 | −0.3214 | 0.5157 | 0.040* | |
| C18 | 0.44384 (6) | 0.1648 (3) | 0.22488 (11) | 0.0294 (3) | |
| H18A | 0.4411 | −0.0034 | 0.2593 | 0.044* | |
| H18B | 0.4821 | 0.1863 | 0.1981 | 0.044* | |
| H18C | 0.4154 | 0.1767 | 0.1647 | 0.044* | |
| C19 | 0.29830 (6) | 0.8303 (3) | 0.22110 (10) | 0.0258 (3) | |
| H19A | 0.2659 | 0.7263 | 0.1939 | 0.039* | |
| H19B | 0.3095 | 0.9489 | 0.1649 | 0.039* | |
| H19C | 0.2873 | 0.9282 | 0.2839 | 0.039* | |
| C21 | 0.14322 (6) | 0.3901 (3) | 0.59287 (11) | 0.0247 (3) | |
| H21 | 0.1261 | 0.2185 | 0.5755 | 0.030* | |
| C22 | 0.15657 (7) | 0.4025 (4) | 0.71420 (11) | 0.0376 (4) | |
| H22A | 0.1758 | 0.5670 | 0.7323 | 0.045* | |
| H22B | 0.1831 | 0.2617 | 0.7357 | 0.045* | |
| C23 | 0.10231 (7) | 0.3799 (4) | 0.77699 (12) | 0.0404 (4) | |
| H23A | 0.0851 | 0.2082 | 0.7649 | 0.048* | |
| H23B | 0.1122 | 0.3984 | 0.8548 | 0.048* | |
| C24 | 0.05945 (7) | 0.5845 (3) | 0.74171 (12) | 0.0372 (4) | |
| H24A | 0.0750 | 0.7557 | 0.7612 | 0.045* | |
| H24B | 0.0239 | 0.5599 | 0.7800 | 0.045* | |
| C25 | 0.04589 (7) | 0.5735 (4) | 0.62060 (12) | 0.0376 (4) | |
| H25A | 0.0196 | 0.7153 | 0.5994 | 0.045* | |
| H25B | 0.0264 | 0.4097 | 0.6023 | 0.045* | |
| C26 | 0.10009 (6) | 0.5950 (3) | 0.55750 (11) | 0.0310 (3) | |
| H26A | 0.0901 | 0.5757 | 0.4797 | 0.037* | |
| H26B | 0.1173 | 0.7668 | 0.5692 | 0.037* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0193 (6) | 0.0209 (6) | 0.0316 (6) | −0.0013 (5) | 0.0099 (4) | 0.0005 (4) |
| O1 | 0.0248 (5) | 0.0215 (5) | 0.0413 (6) | −0.0004 (4) | 0.0099 (4) | 0.0034 (4) |
| O2 | 0.0198 (5) | 0.0365 (6) | 0.0277 (5) | 0.0075 (4) | 0.0047 (4) | 0.0091 (4) |
| O3 | 0.0189 (5) | 0.0278 (5) | 0.0266 (5) | −0.0017 (4) | 0.0083 (4) | −0.0024 (4) |
| O4 | 0.0250 (5) | 0.0272 (5) | 0.0248 (4) | 0.0026 (4) | 0.0065 (4) | 0.0070 (4) |
| C1 | 0.0188 (6) | 0.0230 (6) | 0.0215 (6) | −0.0005 (5) | 0.0011 (5) | −0.0006 (5) |
| C11 | 0.0184 (6) | 0.0221 (6) | 0.0192 (5) | −0.0026 (5) | 0.0036 (5) | −0.0048 (5) |
| C12 | 0.0218 (7) | 0.0225 (6) | 0.0197 (6) | −0.0007 (5) | 0.0030 (5) | 0.0009 (5) |
| C13 | 0.0175 (6) | 0.0248 (6) | 0.0210 (6) | 0.0015 (5) | 0.0007 (5) | −0.0025 (5) |
| C14 | 0.0172 (6) | 0.0240 (6) | 0.0197 (6) | −0.0025 (5) | 0.0039 (5) | −0.0035 (5) |
| C15 | 0.0214 (7) | 0.0205 (6) | 0.0178 (6) | −0.0012 (5) | 0.0024 (5) | −0.0011 (4) |
| C16 | 0.0176 (6) | 0.0212 (6) | 0.0215 (6) | 0.0018 (5) | 0.0009 (5) | −0.0017 (5) |
| C17 | 0.0278 (7) | 0.0269 (7) | 0.0254 (6) | 0.0054 (6) | −0.0003 (5) | 0.0037 (5) |
| C18 | 0.0293 (8) | 0.0299 (7) | 0.0301 (7) | 0.0031 (6) | 0.0125 (6) | −0.0023 (6) |
| C19 | 0.0287 (7) | 0.0255 (7) | 0.0233 (6) | 0.0040 (6) | 0.0007 (5) | 0.0033 (5) |
| C21 | 0.0190 (7) | 0.0246 (7) | 0.0312 (7) | −0.0017 (6) | 0.0086 (5) | −0.0003 (5) |
| C22 | 0.0250 (8) | 0.0572 (10) | 0.0312 (8) | 0.0053 (7) | 0.0064 (6) | 0.0073 (7) |
| C23 | 0.0338 (9) | 0.0543 (10) | 0.0342 (8) | 0.0028 (8) | 0.0124 (7) | 0.0066 (7) |
| C24 | 0.0318 (8) | 0.0385 (9) | 0.0431 (8) | −0.0008 (7) | 0.0184 (7) | −0.0058 (7) |
| C25 | 0.0218 (8) | 0.0462 (9) | 0.0456 (9) | 0.0068 (7) | 0.0085 (6) | 0.0034 (7) |
| C26 | 0.0238 (8) | 0.0352 (8) | 0.0346 (7) | 0.0050 (6) | 0.0073 (6) | 0.0057 (6) |
Geometric parameters (Å, °)
| N1—C1 | 1.3451 (17) | C18—H18B | 0.9800 |
| N1—C21 | 1.4670 (16) | C18—H18C | 0.9800 |
| N1—H1 | 0.916 (19) | C19—H19A | 0.9800 |
| O1—C1 | 1.2424 (16) | C19—H19B | 0.9800 |
| O2—C13 | 1.3615 (16) | C19—H19C | 0.9800 |
| O2—C17 | 1.4353 (16) | C21—C26 | 1.521 (2) |
| O3—C14 | 1.3761 (15) | C21—C22 | 1.529 (2) |
| O3—C18 | 1.4432 (16) | C21—H21 | 1.0000 |
| O4—C15 | 1.3681 (15) | C22—C23 | 1.532 (2) |
| O4—C19 | 1.4307 (17) | C22—H22A | 0.9900 |
| C1—C11 | 1.5020 (17) | C22—H22B | 0.9900 |
| C11—C12 | 1.3945 (19) | C23—C24 | 1.516 (2) |
| C11—C16 | 1.4015 (18) | C23—H23A | 0.9900 |
| C12—C13 | 1.3920 (18) | C23—H23B | 0.9900 |
| C12—H12 | 0.9500 | C24—C25 | 1.527 (2) |
| C13—C14 | 1.4058 (18) | C24—H24A | 0.9900 |
| C14—C15 | 1.3951 (19) | C24—H24B | 0.9900 |
| C15—C16 | 1.3988 (18) | C25—C26 | 1.5320 (19) |
| C16—H16 | 0.9500 | C25—H25A | 0.9900 |
| C17—H17A | 0.9800 | C25—H25B | 0.9900 |
| C17—H17B | 0.9800 | C26—H26A | 0.9900 |
| C17—H17C | 0.9800 | C26—H26B | 0.9900 |
| C18—H18A | 0.9800 | ||
| C1—N1—C21 | 121.53 (11) | H19A—C19—H19B | 109.5 |
| C1—N1—H1 | 120.3 (11) | O4—C19—H19C | 109.5 |
| C21—N1—H1 | 117.0 (11) | H19A—C19—H19C | 109.5 |
| C13—O2—C17 | 118.23 (10) | H19B—C19—H19C | 109.5 |
| C14—O3—C18 | 112.80 (10) | N1—C21—C26 | 110.57 (11) |
| C15—O4—C19 | 117.42 (10) | N1—C21—C22 | 110.82 (11) |
| O1—C1—N1 | 122.58 (12) | C26—C21—C22 | 110.89 (12) |
| O1—C1—C11 | 120.55 (12) | N1—C21—H21 | 108.1 |
| N1—C1—C11 | 116.87 (11) | C26—C21—H21 | 108.1 |
| C12—C11—C16 | 121.26 (11) | C22—C21—H21 | 108.2 |
| C12—C11—C1 | 117.54 (11) | C21—C22—C23 | 111.55 (13) |
| C16—C11—C1 | 121.16 (11) | C21—C22—H22A | 109.3 |
| C13—C12—C11 | 119.56 (11) | C23—C22—H22A | 109.3 |
| C13—C12—H12 | 120.2 | C21—C22—H22B | 109.3 |
| C11—C12—H12 | 120.2 | C23—C22—H22B | 109.3 |
| O2—C13—C12 | 125.32 (11) | H22A—C22—H22B | 108.0 |
| O2—C13—C14 | 114.92 (11) | C24—C23—C22 | 110.72 (13) |
| C12—C13—C14 | 119.75 (12) | C24—C23—H23A | 109.5 |
| O3—C14—C15 | 119.15 (11) | C22—C23—H23A | 109.5 |
| O3—C14—C13 | 120.54 (12) | C24—C23—H23B | 109.5 |
| C15—C14—C13 | 120.22 (11) | C22—C23—H23B | 109.5 |
| O4—C15—C14 | 115.40 (11) | H23A—C23—H23B | 108.1 |
| O4—C15—C16 | 124.30 (12) | C23—C24—C25 | 111.23 (13) |
| C14—C15—C16 | 120.29 (11) | C23—C24—H24A | 109.4 |
| C15—C16—C11 | 118.82 (12) | C25—C24—H24A | 109.4 |
| C15—C16—H16 | 120.6 | C23—C24—H24B | 109.4 |
| C11—C16—H16 | 120.6 | C25—C24—H24B | 109.4 |
| O2—C17—H17A | 109.5 | H24A—C24—H24B | 108.0 |
| O2—C17—H17B | 109.5 | C24—C25—C26 | 111.54 (13) |
| H17A—C17—H17B | 109.5 | C24—C25—H25A | 109.3 |
| O2—C17—H17C | 109.5 | C26—C25—H25A | 109.3 |
| H17A—C17—H17C | 109.5 | C24—C25—H25B | 109.3 |
| H17B—C17—H17C | 109.5 | C26—C25—H25B | 109.3 |
| O3—C18—H18A | 109.5 | H25A—C25—H25B | 108.0 |
| O3—C18—H18B | 109.5 | C21—C26—C25 | 110.91 (12) |
| H18A—C18—H18B | 109.5 | C21—C26—H26A | 109.5 |
| O3—C18—H18C | 109.5 | C25—C26—H26A | 109.5 |
| H18A—C18—H18C | 109.5 | C21—C26—H26B | 109.5 |
| H18B—C18—H18C | 109.5 | C25—C26—H26B | 109.5 |
| O4—C19—H19A | 109.5 | H26A—C26—H26B | 108.0 |
| O4—C19—H19B | 109.5 | ||
| C21—N1—C1—O1 | 4.06 (19) | C19—O4—C15—C16 | −1.51 (18) |
| C21—N1—C1—C11 | −175.93 (11) | O3—C14—C15—O4 | 2.05 (17) |
| O1—C1—C11—C12 | −32.62 (17) | C13—C14—C15—O4 | 178.53 (11) |
| N1—C1—C11—C12 | 147.36 (12) | O3—C14—C15—C16 | −178.18 (11) |
| O1—C1—C11—C16 | 145.07 (13) | C13—C14—C15—C16 | −1.70 (18) |
| N1—C1—C11—C16 | −34.94 (17) | O4—C15—C16—C11 | 178.64 (11) |
| C16—C11—C12—C13 | −0.02 (18) | C14—C15—C16—C11 | −1.11 (18) |
| C1—C11—C12—C13 | 177.67 (11) | C12—C11—C16—C15 | 1.99 (18) |
| C17—O2—C13—C12 | 0.73 (19) | C1—C11—C16—C15 | −175.62 (11) |
| C17—O2—C13—C14 | −179.46 (11) | C1—N1—C21—C26 | −150.78 (12) |
| C11—C12—C13—O2 | 177.00 (12) | C1—N1—C21—C22 | 85.85 (16) |
| C11—C12—C13—C14 | −2.81 (19) | N1—C21—C22—C23 | 179.14 (13) |
| C18—O3—C14—C15 | −108.20 (13) | C26—C21—C22—C23 | 55.95 (18) |
| C18—O3—C14—C13 | 75.33 (15) | C21—C22—C23—C24 | −56.00 (19) |
| O2—C13—C14—O3 | 0.29 (17) | C22—C23—C24—C25 | 55.59 (19) |
| C12—C13—C14—O3 | −179.89 (11) | C23—C24—C25—C26 | −55.74 (19) |
| O2—C13—C14—C15 | −176.15 (11) | N1—C21—C26—C25 | −178.60 (12) |
| C12—C13—C14—C15 | 3.68 (19) | C22—C21—C26—C25 | −55.27 (17) |
| C19—O4—C15—C14 | 178.25 (11) | C24—C25—C26—C21 | 55.42 (18) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1i | 0.916 (19) | 2.153 (19) | 3.0262 (15) | 159.0 (15) |
Symmetry codes: (i) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FJ2261).
References
- Beccalli, E. M., Broggini, G., Paladinoa, G. & Zonia, C. (2005). Tetrahedron, 61, 61–68.
- Bowes, K. F., Glidewell, C., Low, J. N., Skakle, J. M. S. & Wardell, J. L. (2003). Acta Cryst. C59, o1–o3. [DOI] [PubMed]
- Calderone, V., Fiamingo, F. L., Giorgi, I., Leonardi, M., Livi, O., Martelli, A. & Martinotti, E. (2006). Eur. J. Med. Chem. 41, 761–767. [DOI] [PubMed]
- Chopra, D. & Guru Row, T. N. (2008). CrystEngComm, 10, 54–67.
- Kashino, S., Ito, K. & Haisa, M. (1979). Bull. Chem. Soc. Jpn, 52, 365–369.
- Lindgren, H., Pero, R. W., Ivars, F. & Leanderson, T. (2001). Mol. Immunol. 38, 267–277. [DOI] [PubMed]
- Olsson, A. R., Lindgren, H., Pero, R. W. & Leanderson, T. (2002). Br. J. Cancer, 86, 971–978. [DOI] [PMC free article] [PubMed]
- Saeed, A., Abbas, N., Hussain, S. & Flörke, U. (2008). Acta Cryst. E64, o773. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2001). X-AREA. Stoe & Cie, Darmstadt, Germany.
- Zhichkin, P., Kesicki, E., Treiberg, J., Bourdon, L., Ronsheim, M., Ooi, H. C., White, S., Judkins, A. & Fairfax, D. (2007). Org. Lett. 9, 1415–1418. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809050284/fj2261sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809050284/fj2261Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



