Abstract
Hepatocyte nuclear factor-4 (HNF4) regulates gene expression by binding to direct repeat motifs of the RG(G/T)TCA sequence separated by one nucleotide (DR1). In this study we demonstrate that endogenous HNF4 present in rat liver nuclear extracts, as well as purified recombinant HNF4, activates transcription from naked DNA templates containing multiple copies of the DR1 element linked to the adenovirus major late promoter. Recombinant HNF4 also activates transcription from the rat cellular retinol binding protein II (CRBPII) promoter in vitro. The region between –105 and –63 bp of this promoter is essential for HNF-mediated transactivation. The addition of a peptide containing the LXXLL motif abolished HNF4-mediated transactivation in vitro suggesting that LXXLL-containing protein factor(s) are involved in HNF4-mediated transactivation in rat liver nuclear extracts. This is the first report on transactivation by HNF4 in a cell-free system derived from rat liver nuclei.
INTRODUCTION
Transcription factors belonging to the steroid/nuclear hormone receptor superfamily regulate gene expression in response to a variety of signaling molecules (such as steroids, thyroid hormone, retinoids, vitamin D3 and lipid metabolites) by binding to specific response elements (REs) of their target genes (1–3). These REs include direct repeats of RG(G/T)TCA with 0–5 base spacing, palindromic RG(G/T)TCA without any spacing or single half sites (2,3). Of these REs, direct repeats of RG(G/T)TCA with one base spacing (DR1) appear to be highly promiscuous, as they bind a number of nuclear receptors including RXR, hepatocyte nuclear factor-4 (HNF4), COUP-TFI, apolipoprotein regulatory protein 1 (ARP-1/COUP-TFII) and the peroxisome proliferator-activated receptor (PPAR) (3,4). These transcription factors bind to DR1 as homodimers (RXR, COUP-TFI, ARP-1 and HNF4) or heterodimers (PPAR–RXR, RAR–RXR, COUP-TFI–RXR and ARP-1–RXR) and, as a result, genes harboring the DR1 element in their promoters can be activated constitutively by HNF4, repressed by COUP-TFI and ARP-1 or activated by the ligands of PPAR and RXR (5–10). Nucleotides at base positions 1, 2 and 4 of the core motif, the spacer sequence and the location of the element within a proper promoter context were shown to determine the selective binding of these transcription factors (10). In addition, relative abundance of these transcription factors in a given tissue also plays a major role in the regulation of their target genes (5,7).
Several studies demonstrate that protein–protein and protein–DNA interactions of the nuclear receptors binding to the DR1 element result in positive or negative regulation of several hepatic genes (5,6,11). For example, HNF4, ARP-1 and COUP-TFI compete for binding to a DR1 element within the apolipoprotein CIII promoter and while HNF4 stimulates transcription, ARP-1 and COUP-TFI function as transcriptional repressors (5,6). Although COUP-TFI is generally known as a transcriptional repressor, it has been shown to activate transcription from the SV40 promoter as well as from a DR1 element present in the promoter of the gene encoding ornithine transcarbamylase (12). In the case of the gene encoding murine cellular retinol binding protein II (CRBPII), an HNF4/ARP-1 RE was shown to function as a promiscuous binding site for RXR and is inducible by retinoic acid only in cells expressing low levels of HNF4/ARP-1 (7). Co-expression of PPARα and HNF4 results in suppression of HNF4-mediated transactivation from the acyl CoA oxidase promoter and only HNF4 but not PPARα can activate transcription from the apolipoprotein CIII promoter (11). Similarly, ARP-1 or COUP-TFI repress HNF4-mediated transactivation from the human factor IX promoter and impaired HNF4 binding is one of the factors responsible for down-regulation of factor IX expression (13). These studies suggest that cross-talk amongst DR1-binding nuclear receptors can lead to significant differences in the level of expression of their target genes in different tissues. The fact that almost all the receptors that bind to the DR1 element are expressed in liver (7) suggests that transcription of their target genes will ultimately depend on the concentration and affinity of these receptors for their recognition sites, as well as protein–protein interactions with other factors binding to proximal and distal regulatory elements. Thus, regulation of hepatic gene expression by these DR1-binding nuclear receptors appears to be a complex process.
Most of the data on cross-talk amongst receptors binding to the DR1 element is generated from studies involving co-transfection of reporter plasmids and plasmids expressing nuclear receptors. However, many control mechanisms that operate in differentiated tissues do not operate in the rapidly dividing cells in culture and, even when appropriate cell lines exist, all the regulation exerted on endogenous genes may not be reproduced on transfected DNA (14). This is especially true in the case of liver cells, which rapidly lose transcription factors involved in the regulation of liver-specific genes such as albumin when cultured in vitro (14). One of the alternative methods involves the study of transcriptional regulation of genes in vitro using nuclear extracts prepared from differentiated tissues. Cell-free extracts prepared from rat liver nuclei have been extensively used for the study of tissue-specific regulation of several hepatic genes (15–17). However, there has been no report on transactivation by nuclear receptors binding to the DR1 element in liver nuclear extracts despite the fact that several of these receptors are expressed in high levels in liver and are involved in the regulation of expression of hepatic genes (7,18–21). As a first step towards understanding the mechanism of hepatic gene regulation by DR1 element-binding nuclear receptors, we developed a cell-free transcription system utilizing nuclear extracts derived from rat liver and examined DR1-dependent transactivation by HNF4 in vitro. We demonstrate that endogenous HNF4 present in rat liver nuclear extracts and purified recombinant HNF4 activate transcription from DR1 elements linked to the adenovirus major late promoter (AdMLP) and the native rat CRBPII promoter. Addition of a peptide containing the LXXLL motif abolished HNF4-mediated transactivation in vitro, indicating that LXXLL containing co-activator(s) may be involved in HNF4-mediated transactivation in rat liver nuclear extracts.
MATERIALS AND METHODS
Construction of plasmid templates for in vitro transcription
Plasmids containing the minimal AdMLP linked to a 260 (pAdMLP260) or 380 bp (pAdMLP380) G-free cassette were used as control templates for in vitro transcription as described previously (22,23). A consensus DR1 element was synthesized as complementary oligonucleotides 5′-gatcAAGGTCAAAGGTCACTCGC-3′ and 5′-gatcGCGAGTGACCTTTGACCTT-3′. The RXRE from the rat CRBPII promoter (8) present between –639 and –605 bp was synthesized by complementary oligonucleotides 5′-gatcGCTGTCACAGGTCACAGGTCACAG GTCACAGTTCA-3′ and 5′-gatcTGAACTGTGACCTGTGACCTGTGACCTGTGACCTGC-3′. The half-site sequence in each of the complementary oligonucleotides is indicated in bold. These oligonucleotides with gatc overhangs were phosphorylated at their 5′-ends. Equimolar amounts were annealed as previously described (24) and cloned upstream of the AdMLP at the BamHI site of pAdMLP380. Clones containing four copies of the DR1 element or two copies of CRBPII-RXRE oriented in the same direction were identified by dideoxynucleotide sequencing and designated pDR1-AdMLP380 and pRXRE-AdMLP380, respectively. The CRBPII promoter region between –987 and +1 bp was amplified from pCRBPIICAT (8) in a PCR reaction using the primer pair 5′-GCCAAGCTTGGATCCGTCTGAGCTGAA-3′ (P1) and 5′-GGGAAATATAGGGGAGACTG-3′ (P2). The G-free cassette was PCR-amplified from pAdMLP380 using the primers 5′-TATATTTCCCAAATCTATC-3′ (P3) and 5′-AGTCGAATTCCCCGG GAGTGGAATGAGAAATGA-3′ (P4). The PCR products obtained from these two reactions were purified and used as templates in another PCR reaction in which P1 and P4 were used as primers. The final PCR product consisting of 987 bp of the rat CRBPII promoter linked to a 300 bp G-free cassette was digested with BamHI and EcoRI and cloned into the BamHI- and EcoRI-digested pUC19 plasmid. The nucleotide sequence of the final construct (p987CRBPII300) was verified by dideoxynucleotide sequencing. The region between –987 and –746 bp of the CRBPII promoter was deleted by digesting p987CRBPII300 with SphI. The resulting vector was purified on agarose gels and religated to generate p747CRBPII300. Similarly, the construct p313CRBPII300 containing 313 bp of the CRBPII promoter was generated by PstI digestion of p987CRBPII300 followed by ligation of the purified vector. For the construction of p105CRBPII300, a PCR reaction was carried out with the primers 5′-ATGCCGGGATCCAAGGGCAATTCCTTTGAGTTGGG-3′ and P4 using p987CRBPII300 as template. The PCR product was digested with BamHI and EcoRI and cloned into the BamHI- and EcoRI-digested pUC19 plasmid. p53CRBPII300 was constructed by a similar strategy except that the primers 5′-AAGCCCGGGATCCTTTATACACCTGGTCCACTAA-3′ and P4 were used for PCR amplification.
Overexpression and purification of receptors
The cDNA encoding HNF4 was amplified from pLEN4S-rHNF4 (20) using the primers 5′-CGCGGATCCATGCATCACCATCACCATCACGACATGGCTGACTACAGT-3′ and 5′-CCGGAATTCCTAGATGGCTTCCTG-3′; the histidine codons are underlined. The PCR product was restricted with BamHI and EcoRI and cloned into BamHI- and EcoRI-digested pThioC vector (Invitrogen) to generate pThioHNF4. The plasmids were transformed in Escherichia coli BL21 (DE3) (pLysS) (Novagen). Recombinant protein expression was induced with 0.5 mM isopropylthiogalactoside (Sigma) and cells were resuspended in lysis buffer (25 mM Tris–HCl pH 7.5, 100 mM NaCl, 0.1% Triton X-100, 0.1 mM PMSF and 10 mM β-mercaptoethanol). Cells were lysed by repeated freezing and thawing and the cell lysate was obtained after centrifugation at 30 000 r.p.m. in an ultracentrifuge (Beckman) for 30 min at 4°C using a Ti50 rotor. The lysate obtained from a 1 l culture was loaded onto a 10 ml heparin–agarose (Sigma) column equilibrated with lysis buffer. After washing, the bound protein was eluted with a buffer containing 25 mM Tris–HCl pH 7.4, 600 mM NaCl, 10% glycerol, 0.1 mM PMSF and 10 mM β-mercaptoethanol. The eluate was incubated with 100 µl of Ni2+-nitrilotriacetic acid (Ni2+-NTA) agarose beads (Qiagen) for 1 h at 4°C. After washing with a buffer containing 25 mM Tris–HCl pH 7.5, 600 mM NaCl, 20 mM imidazole, 0.5% Triton X-100, 0.1 mM PMSF and 10 mM β-mercaptoethanol, the bound protein was eluted with the same buffer containing 250 mM imidazole. The eluate was dialyzed against a buffer containing 25 mM HEPES–KOH pH 7.9, 40 mM KCl, 0.1 mM EDTA, 10 mM β-mercaptoethanol, 10% glycerol and 0.2 mM PMSF and the protein was stored at –80°C in aliquots.
Preparation of rat liver nuclear extracts and cell-free transcription
Rat liver nuclear extracts were prepared essentially as described previously (15–17). The final preparation was stored in aliquots at –80°C for up to 6 months. Transcription reactions were performed in a buffer containing 25 mM HEPES pH 7.4, 25 mM KCl, 6 mM MgCl2 and 3% glycerol supplemented with 2.0–3.0 mg/ml of rat liver nuclear extract, 20 U of RNasin (Pharmacia), 100 µM 3′-O-methyl-GTP (Pharmacia), 10 µCi of [α-32P]UTP (800 Ci/mmol; NEN-DuPont), 35 µM UTP, 0.625 mM ATP, 0.625 mM CTP (Pharmacia), 1.0 µg of test DNA template and/or 0.5 µg of control DNA template in a final reaction volume of 20 µl. In experiments involving addition of recombinant receptors, the recombinant proteins were first incubated with the plasmid DNA templates for 15 min at 30°C and then added to the reaction mixture containing the rest of the components. Transcription reactions were carried out at 30°C for 45 min. Reactions were stopped by the addition of 280 µl of a solution containing 250 mM NaCl, 1% SDS, 20 mM Tris–HCl pH 7.8, 5 mM EDTA, 20 µg of yeast tRNA and 30 µg of Proteinase K. After incubation at 37°C for 30 min, samples were extracted twice with 250 µl of phenol–chloroform–isoamyl alcohol (25:24:1) by vortexing. The RNA in the aqueous phase was precipitated with 2 vol of ethanol and pelleted by microcentrifugation. The pellet was dried and resuspended in 10 µl of 80% formamide and the products were separated by electrophoresis in 5% denaturing polyacrylamide (acrylamide–bisacrylamide, 20:1; 7 M urea) gels for 2 h at 200 V in 1× Tris–borate–EDTA buffer. The gels were dried and exposed to Biomax-MR autoradiography film (Kodak) at –80°C with an intensifying screen for 8 h. Quantitation was performed by densitometric scanning of the autoradiograms or by exposure of the dried gels to phosphorimager screens, gathering data on a Fijix BAS-1000 phosphorimager and analysis by MacBAS version 2.0 bioimaging software. All the cell-free transcription experiments were repeated at least twice with two different batches of rat liver nuclear extract preparations and the representative data is presented in this paper.
DR1 oligonucleotide affinity chromatography of rat liver nuclear extracts
Complementary oligonucleotides correponding to the CRBPII-RXRE (see above) were kinased, annealed, concatamerized and coupled to cyanogen bromide-activated Sepharose 4B (Sigma), essentially as described previously (24). The Sepharose beads (1 g), corresponding to ∼3 ml of swollen resin, were coupled to 200 µg of the oligonucleotides. For the depletion of HNF4 and other DR1-binding receptors present in rat liver nuclear extracts, the extract was incubated with a given quantity of the oligonucleotide affinity resin for 30 min at 4°C. After microcentifugation at 4°C, the supernatant was transferred to a fresh tube and used directly in cell-free transcription assays. Aliquots of each batch of nuclear extract were subjected to western blot analysis using antibodies against HNF4 (α445; a kind gift of Frances Sladek, University of California, Riverside, CA) (20) before and after binding to the oligonucleotide resin. Extracts that showed a substantial decrease in HNF4 levels (>90%) were used in in vitro transcription reactions.
Peptide synthesis
Peptides corresponding to amino acids 685–699 of SRC-1 of the sequence ERHKILHRLLQEGSP that can interact with nuclear receptors in a ligand-dependent manner as well as a mutant peptide of the sequence ERHKILHRAAAEGSP that has been shown to be incapable of interacting with nuclear receptors (25,26) were purchased from Xcyton (India).
RESULTS
DR1-dependent transcriptional activation in rat liver nuclear extracts
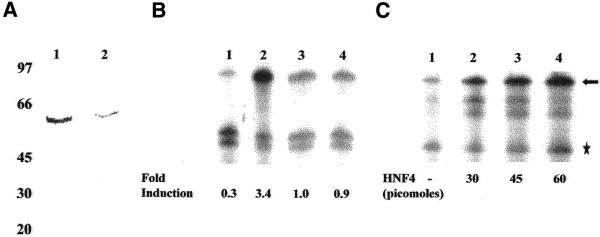
Liver is enriched in several DR1 binding nuclear receptors that have been shown to be involved in the regulation of several hepatic genes. However, the ability of these receptors to activate transcription in a cell-free system derived from rat liver nuclear extracts has not been reported. In this study, we first examined transactivation by endogenous DR1-binding hepatic nuclear receptors from naked DNA templates in a homologous cell-free system derived from rat liver nuclear extracts. A series of plasmid templates were constructed for use in cell-free transcription (see Materials and Methods). Plasmids containing the minimal AdMLP linked to a 260 (pAdMLP260) or 380 bp (pAdMLP380) G-free cassette were used as control templates. The pDR1-AdMLP380 and pRXRE-AdMLP380 test templates contain four copies of the consensus DR1 element or two copies of the CRBPII-RXRE upstream of the AdMLP-linked to 380 bp G-free cassette, respectively (Fig. 1A). Transcription reactions contained both specific and control templates and fold activation was calculated as the ratio of 32P-labeled RNA obtained from the specific template to that obtained from the control template. Rat liver nuclear extracts capable of accurate transcription initiation by RNA polymerase II were prepared (15–17) and cell-free transcription assays were carried out with pDR1-AdMLP380 and pRXRE-AdMLP380 as test templates along with pAdMLP380 or pAdMLP260 as control templates. The results presented in Figure 1B indicate that pDR1-AdMLP380 (lanes 2 and 3) and pRXRE-AdMLP380 (lanes 4 and 5) are transcribed 3- and 5-fold higher than the control templates, respectively. Transcription from pDR1-AdMLP380 and pRXRE-AdMLP380 was always higher than that from the control templates and varied from 3- to 8-fold in different batches of extract. Thus, the endogenous DR1-binding transcription factors present in rat liver nuclear extracts can selectively activate transcription from these templates.
Figure 1.
Transactivation by DR1-binding proteins present in rat liver nuclear extracts. (A) Schematic representation of plasmid templates used in cell-free transcription. Plasmids containing AdMLP linked to G-free cassettes 260 or 380 bp in length were used as control templates. Four copies of DR1 element or two copies of the CRBPII-RXRE were cloned upstream of the AdMLP linked to a G-free cassette of 380 bp length. (B) Cell-free transcription assays were performed (as described in Materials and Methods) with the control templates pAdMLP380 and pAdMLP260 (lane 1), pDR1-AdMLP380 and pAdMLP260 (lanes 2 and 3) or pRXRE-AdMLP380 and pAdMLP260 (lanes 4 and 5). The arrow and asterisk represent transcripts generated from 380 and 260 bp G-free cassettes, respectively. Addition of 9-cis-RA (10–5 M) had no significant effect on transactivation from pDR1-AdMLP380 (lane 3) or pRXRE-AdMLP380 (lane 5).
Transactivation by recombinant HNF4 in rat liver nuclear extracts
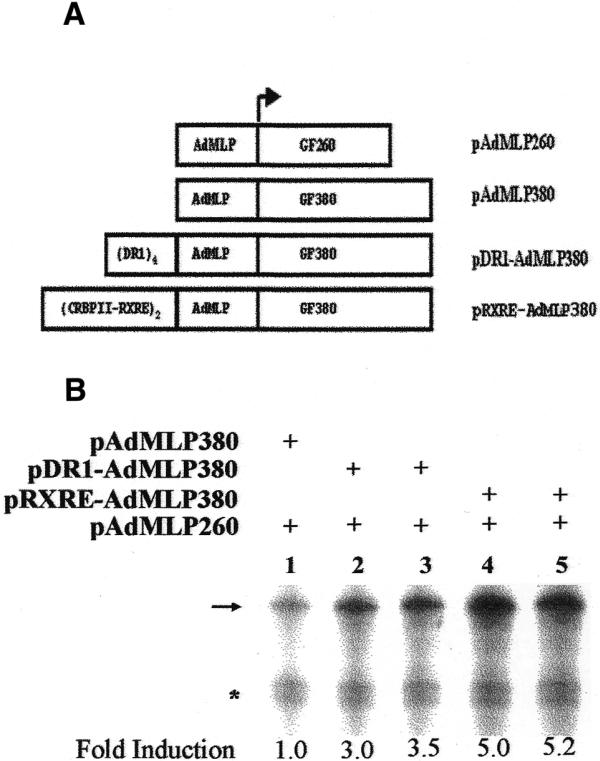
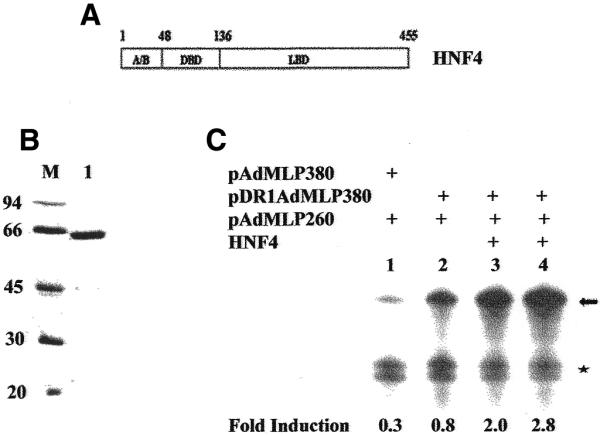
It has been reported that recombinant HNF4 expressed and purified from bacterial extracts can activate transcription in nuclear extracts derived from HeLa cells, a cell type that does not express HNF4 (27). The fact that liver nuclear extracts can support DR1-dependent transcription in vitro encouraged us to study transactivation by recombinant HNF4. We overexpressed full-length HNF4 (Fig. 2A) in E.coli and purified the protein by chromatography on a heparin–agarose column followed by chromatography on an Ni2+-NTA column (Fig. 2B, lane 1). Addition of recombinant HNF4 to rat liver nuclear extracts resulted in activation of transcription from pDR1-AdMLP380 (Fig. 2C, lanes 3 and 4). Thus, both the endogenous HNF4 present in rat liver nuclear extracts and the recombinant HNF4 can activate transcription from pDR1-AdMLP380 (Fig. 2C, compare lane 2 with lanes 3 and 4). We then examined the ability of recombinant HNF4 to activate transcription from rat liver nuclear extracts with depleted levels of the endogenous DR1-binding proteins. Incubation of liver nuclear extracts with DR1-oligonucleotide affinity resin resulted in significant depletion of HNF4 levels as evident from western blotting of extracts before and after depletion (Fig. 3A; compare lane 1 with lane 2). A similar decrease was also observed in the case of RXRα, which also binds to the DR1 element (data not shown). The ability of such an extract to activate transcription from pDR1-AdMLP380 was examined in an in vitro transcription reaction. The results indicate that transactivation from pDR1-AdMLP380 is drastically reduced in the extracts with depleted levels of endogenous DR1-binding proteins (Fig. 3B, compare lane 2 with lane 4). Addition of recombinant HNF4 to such extracts resulted in a dose-dependent increase in transcription from pDR1-AdMLP380 but not from pAdMLP260 (Fig. 3C, compare lane 1 with lanes 2, 3 and 4). Taken together, the results presented in Figures 2C and 3C indicate that recombinant HNF4 can activate transcription in the presence or absence of endogenous DR1-binding proteins, including HNF4.
Figure 2.
Transactivation by recombinant HNF4 in rat liver nuclear extracts. (A) Schematic representation of HNF4 consisting of 455 amino acids. (B) SDS–PAGE analysis of recombinant His-tagged, thio-HNF4 overexpressed and purified from bacterial extracts. Lane M, molecular weight markers; lane 1, recombinant HNF4. (C) Cell-free transcription reactions were carried out in the absence (lanes 1 and 2) or presence of 30 (lane 3) or 45 pmol (lane 4) of recombinant HNF4. The arrow and asterisk represent transcripts generated from 380 and 260 bp G-free cassettes, respectively.
Figure 3.
Depletion of endogenous DR1-binding proteins such as HNF4 in rat liver nuclear extracts. (A) Western blot analysis of rat liver nuclear extract with anti-HNF4 antibodies before (lane 1) or after (lane 2) incubation with DR1-oligonucleotide resin. (B) Cell-free transcription from pDR1-AdMLP380 and pAdMLP260 templates before (lanes 1 and 2) or after (lanes 3 and 4) depletion of endogenous DR1-binding proteins in rat liver nuclear extract. (C) Cell-free transcription from pDR1-AdMLP380 and pAdMLP260 templates in extracts depleted of endogenous DR1-binding proteins in the absence (lane 1) or presence (lanes 2–4) of recombinant HNF4. The arrow and asterisk represent transcripts generated from 380 and 260 bp G-free cassettes, respectively.
Transactivation from native rat CRBPII promoter by recombinant HNF4
The plasmid templates used thus far contain multiple copies of the DR1 element or CRBPII-RXRE cloned upstream of a heterologous promoter (AdMLP) and thus may not reflect the regulation from a DR1 element in the context of a native promoter. We therefore examined transactivation from a native promoter containing DR1 elements. We selected the rat CRBPII promoter since it has been shown to contain promiscuous binding sites for HNF4, ARP-1, RXRα and COUP-TFI at –639 (RE1), –105 (RE2) and –62 bp (RE3) (7,8) (Fig. 4A). Different regions of the CRBPII promoter were cloned upstream of a 300 bp G-free cassette (Fig. 4A) and transactivation from these templates by recombinant HNF4 was examined in rat liver nuclear extracts depleted of endogenous DR1-binding proteins. The results indicate that recombinant HNF4 activates transcription from plasmid DNA templates containing –987, –747, –313 and –105 bp (Fig. 4B, lanes 2, 4, 6 and 8, respectively) of rat CRBPII promoter. A drastic reduction in HNF4-mediated transactivation was observed in the case of p53CRBPII300, which contains –53 bp of CRBPII promoter (Fig. 4B, lane 10). Thus, a region between –105 and –54 bp is essential for HNF4-mediated transactivation of rat CRBPII promoter in the cell-free system.
Figure 4.
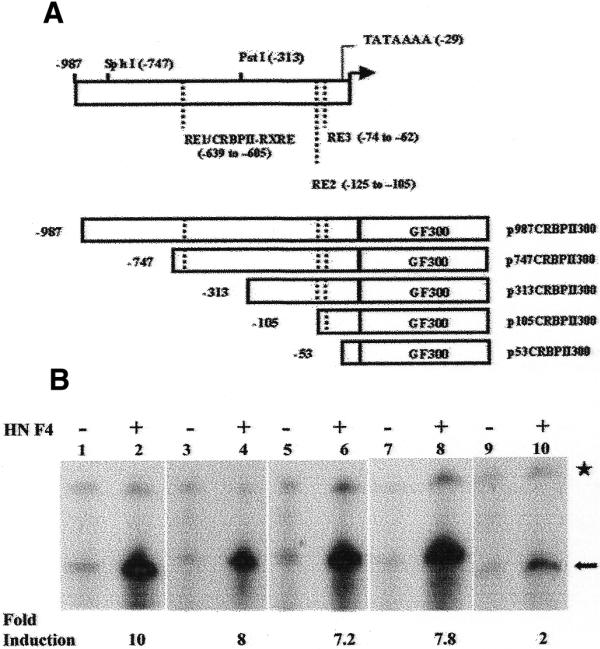
HNF4 activates transcription from rat CRBPII promoter in the cell-free system. (A) Schematic representation of the rat CRBPII promoter containing putative RA REs (RE1, RE2 and RE3) as previously described (7,8). The internal restriction sites in the CRBPII promoter are indicated. The plasmid p987CRBPII300 containing 987 bp of rat CRBPII promoter linked to a G-free cassette 300 bp in length was constructed by PCR using pCRBPIICAT (8) as template. All other constructs were generated from this plasmid either by making use of the internal restriction enzyme sites in the CRBPII promoter or by PCR using appropriate primers (see Materials and Methods). (B) Cell-free transcription reactions were carried out with rat liver nuclear extracts depleted of endogenous DR1-binding proteins in the presence of p987CRBPII300 (lanes 1 and 2), p747CRBPII300 (lanes 3 and 4), p313CRBPII300 (lanes 5 and 6), p105CRBPII300 (lanes 7 and 8) or p53CRBPII300 (lanes 9 and 10). Recombinant HNF4 (45 pmol) was added as indicated. pAdMLP380 was used as control template in all the reactions. The arrow and asterisk indicate transcripts arising from 380 and 300 bp G-free cassettes, respectively.
Addition of a peptide containing the LXXLL motif inhibits HNF4-mediated transactivation in the cell-free system
Recent studies indicate that coactivators such as RIP-140, TIF-1α, SRC-1, CBP and GRIP-1 are involved in transactivation by nuclear receptors (28,29). Amongst these, SRC-1, CBP and GRIP-1 have been shown to play a role in HNF4-mediated transactivation (30–32). These coactivators contain a short sequence motif, LXXLL, that is necessary and sufficient to mediate the binding of these proteins to liganded nuclear receptors. Short peptides containing this motif have been shown to compete with these coactivators for nuclear receptor binding (25,28–33). We therefore examined the effect of addition of a peptide containing the LXXLL motif on HNF4-mediated transactivation in the cell-free system. We synthesized a peptide containing the LXXLL motif and another one containing mutations in and around the LXXLL motif (Fig. 5A). When equimolar amounts of these peptides were added to the cell-free transcription reactions, HNF4-mediated transactivation from p105CRBPII300 (Fig. 5B, lane 2) and pDR1AdMLP380 (Fig. 5C, lane 2) was inhibited by the wild-type peptide containing the LXXLL motif (Fig. 5B and C, lanes 6–8) but not by the mutant peptide (Fig. 5B and C, lanes 3–5).
Figure 5.
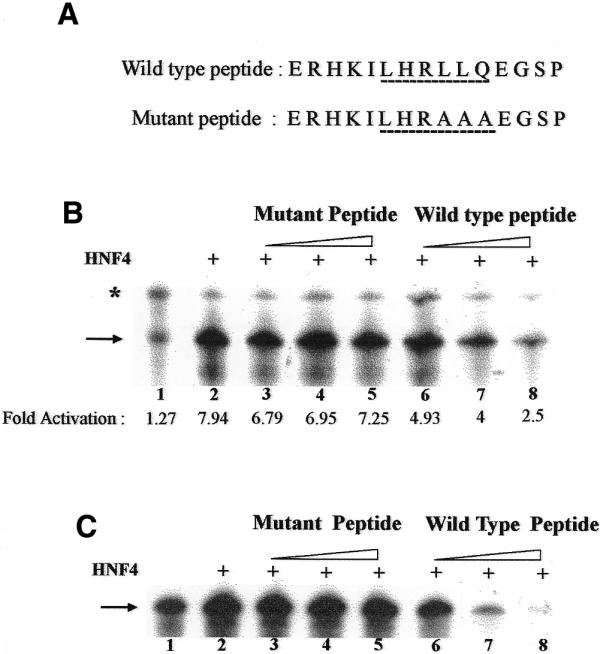
Addition of a peptide containing the LXXLL motif results in the repression of HNF4-mediated transactivation in rat liver nuclear extracts. (A) Sequences of the peptides added to the cell-free transcription reactions. The amino acids corresponding to the LXXLL motif are underlined. (B) HNF4-mediated transactivation from p105CRBPII300 template (lane 2) is repressed by the wild-type peptide (lanes 6–8) but not by the mutant peptide (lanes 3–5). All the tubes contained pAdMLP380 as did the control template. Rat liver nuclear extract with depleted levels of endogenous DR1-binding proteins was used in this experiment. The arrow and asterisk indicate transcripts arising from 380 and 300 bp G-free cassettes, respectively. (C) HNF4-mediated transactivation (lane 2) from pDR1-AdMLP380 is repressed by the wild-type peptide (lanes 6–8) but not by the mutant peptide (lanes 3–5). Rat liver nuclear extract without depletion of endogenous DR1-binding proteins was used in this experiment.
DISCUSSION
One of the characteristic features of nuclear receptors is their ability to regulate gene expression by binding to unique REs in the promoters of their target genes (1). However, certain REs are recognized by more than one receptor and in such cases co-existence of these receptors in the same cell type results in cross-coupling of nuclear receptor signaling (2,3). For example, promiscuous binding of HNF4, RXRα, COUP-TFI, ARP-1 and PPAR-RXRα to DR1 elements present in the promoters of several genes can alter their gene expression patterns in different tissues (4–21). Liver is an ideal tissue to study cross-talk amongst DR1-binding nuclear receptors since a majority of such receptors are expressed in liver and are implicated in the regulation of several hepatic genes (34). So far, there has been no report on the study of DR1-mediated in vitro transcription using cell-free extracts derived from rat liver. In this study, we demonstrate that endogenous DR1-binding transcription factors present in rat liver nuclear extracts can activate transcription in vitro. Addition of recombinant HNF4 to this cell-free system results in enhancement of DR1-mediated transcription over and above that observed with endogenous HNF4. Depletion of endogenous DR1-binding proteins abrogates DR1-dependent transactivation and addition of recombinant HNF4 to such extracts restores DR1-dependent transactivation. These observations indicate that HNF4 is the major transcription factor involved in DR1-dependent transactivation in this cell-free transcription system. HNF4 activates transcription from the rat CRBPII promoter as well, which harbors promiscuous binding sites (RE1, RE2 and RE3) for several DR1-binding transcription factors (7). Deletion of a region containing RE1 and RE2 has no effect on HNF4 activation of the CRBPII promoter. However, deletion of a region containing RE3 results in a drastic decrease in HNF4-mediated transactivation. Thus, RE3 present between –105 and –52 bp is the major cis-acting element involved in HNF4-mediated transactivation of the rat CRBPII promoter. These results are significant in view of the conflicting reports on murine CRBPII gene regulation (7,8). While RE1 was shown to mediate 9-cis-retinoic acid (RA)-dependent transactivation of the rat CRBPII gene by RXRα in F9 cells (8), RE2 and RE3 were identified as the major regulatory elements of the mouse CRBPII gene (7). Our results indicate that RE1 and RE2 are dispensable and RE3 is the major regulatory element through which HNF4 activates rat CRBPII gene transcription. While our results are in agreement with those reported by Nakshatri and Chambon (7), we wish to point out that the CRBPII gene is expressed only in the neonatal liver and adult intestine but not in the adult liver. Thus, while HNF4 is able to activate CRBPII gene transcription in rat liver nuclear extracts, tissue-specific gene silencing mechanisms that are operational in vivo may prevent HNF4 from activating CRBPII gene transcription in the adult rat liver. The fact that DR1-dependent transactivation can be restored in liver nuclear extracts with depleted levels of endogenous DR1-binding proteins by the addition of recombinant HNF4 indicates that this cell-free system can be used to further dissect the mechanism of hepatic gene regulation by HNF4 without interference from other DR1-binding receptors. To this end, we examined the role of coactivators in HNF4-mediated transactivation in rat liver nuclear extracts. Research in the last decade has led to the identification of several nuclear receptor coactivators (NCoAs) as well as nuclear receptor corepressors (29). Analysis of domains involved in the interaction between NCoAs and nuclear receptors indicated that an LXXLL motif is both necessary and sufficient for ligand-dependent interaction of several NCoAs with nuclear receptors (25,28–33). Since several NCoAs contain an LXXLL motif, we examined the involvement of such protein factors in HNF4-mediated transactivation in rat liver nuclear extracts. Our study indicates that a peptide containing the LXXLL motif inhibits HNF4-mediated transactivation in vitro. It is logical to conclude that the repression is due to competition between an endogenous LXXLL-containing coactivator(s) and the peptide for binding to HNF4. Thus, an LXXLL-containing coactivator(s) appears to be involved in HNF4-mediated transactivation in rat liver nuclear extracts. These results are significant in view of the reports that LXXLL-containing coactivators, such as CBP, SRC-1 and GRIP-1, have been shown to be involved in HNF4 function in vivo (30–32). Whether these coactivators are involved in transactivation by HNF4 in rat liver nuclear extracts is not clear. Our attempts to characterize the coactivator involved in transactivation by HNF4 in rat liver nuclear extracts have not been successful thus far. Addition of antibodies to CBP or SRC-1 did not affect HNF4-mediated transactivation in this cell-free system under conditions where addition of HNF4 antibodies readily inhibited transactivation from pDR1-AdMLP380 (data not shown). Thus, further studies are needed to characterize the coactivator(s) involved in HNF4 activation in rat liver nuclear extracts.
This study clearly demonstrates that the cell-free system derived from rat liver nuclear extracts can be a useful tool for the study of regulation of hepatic gene expression by DR1-binding nuclear receptors in general and HNF4 in particular. While we have focussed our attention on HNF4 activation in this study, this cell-free system can also be used to study transactivation by other DR1-binding nuclear receptors such as RXR, ARP-1, COUP-TF, PPAR-RXR etc. We have used only naked DNA templates in this study but it will be interesting to study transcriptional regulation by HNF4 from chromatin templates in rat liver nuclear extracts. The ligand-independent activation of HNF4 demonstrated in this study also opens up new opportunities to study the role of ligands in modulating HNF4 function since long-chain fatty acyl-CoA thioesters were shown to modulate HNF4 function in HeLa nuclear extracts (35). The cell-free system described in this study differs from that derived from HeLa cells in that the latter does not express HNF4. Since HNF4 is a major regulator of several hepatic genes, a cell-free system derived from liver is well suited for the study of hepatic gene regulation by HNF4 and identification of its ligands.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Ronald M.Evans for providing the cDNA clones of HNF4, RXRα, COUP-TFI and the rat CRBPII promoter, Frances Sladek for the HNF4 antibodies and G.Padmanaban for his help and advice. This work was supported by a research grant from the Department of Science and Technology, New Delhi, India.
References
- 1.Evans R.M. (1988) The steroid and thyroid hormone receptor superfamily. Science, 240, 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J., 10, 940–954. [PubMed] [Google Scholar]
- 3.Mangelsdorf D.J. and Evans,R.M. (1995) The RXR heterodimers and orphan receptors. Cell, 83, 841–850. [DOI] [PubMed] [Google Scholar]
- 4.Leblanc B.P. and Stunnenberg,H.G. (1995) 9-cis retinoic acid signaling: changing partners causes some excitement. Genes Dev., 9, 1811–1816. [DOI] [PubMed] [Google Scholar]
- 5.Mietus-Snyder M., Sladek,F.M., Ginsburg,G.S., Kuo,C.F., Ladias,J.A.A., Darnell,J.E.,Jr and Karathanasis,S.K. (1992) Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol. Cell. Biol., 12, 1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladias J.A.A., Hadzopoulou-Cladaras,M., Kardassis,D., Cardot,P., Cheng,J., Zannis,V. and Cladaras,C. (1992) Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J. Biol. Chem., 267, 15849–15860. [PubMed] [Google Scholar]
- 7.Nakshatri H. and Chambon,P. (1994) The directly repeated RG(G/T)TCA motifs of the rat and mouse cellular retinol-binding protein II genes are promiscuous binding sites for RAR, RXR, HNF-4, and ARP-1 homo- and heterodimers. J. Biol. Chem., 269, 890–902. [PubMed] [Google Scholar]
- 8.Mangelsdorf D.J., Umesono,K., Kliewer,S., Borgmeyer,U., Ong,E.S. and Evans,R.M. (1991) A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell, 66, 555–561. [DOI] [PubMed] [Google Scholar]
- 9.Kliewer S.A., Umesono,K., Noonan,K., Heyman,R.A. and Evans,R.M. (1992) Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature, 358, 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakshatri H. and Bhat-Nakshatri,P. (1998) Multiple parameters determine the specificity of transcriptional response by nuclear receptors HNF-4, ARP-1, PPAR, RAR and RXR through common response elements. Nucleic Acids Res., 26, 2491–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiyama C., Hi,R., Osada,S. and Osumi,T. (1998) Functional interactions between nuclear receptors recognizing a common sequence element, the direct repeat motif spaced by one nucleotide (DR-1). J. Biochem., 123, 1174–1179. [DOI] [PubMed] [Google Scholar]
- 12.Kimura A., Nishiyori,A., Murakami,T., Sukamoto,T., Hata,S., Okamura,R., Mori,M. and Takiguchi,M. (1993) Chicken ovalbumin upstream promoter-transcription factor (COUP-TF) represses transcription from the promoter of the gene for ornithine transcarbamylase in a manner antagonistic to hepatocyte nuclear factor-4 (HNF-4). J. Biol. Chem., 268, 11125–11133. [PubMed] [Google Scholar]
- 13.Naka H. and Brownlee,G.G. (1996) Transcriptional regulation of the human factor IX promoter by the orphan receptor superfamily factor, HNF4, ARP1 and COUP/Ear3. Br. J. Haemotol., 92, 231–240. [DOI] [PubMed] [Google Scholar]
- 14.Wasylyk B. (1988) Transcription elements and factors of RNA polymerase B promoters of higher eukaryotes. CRC Crit. Rev. Biochem., 23, 77–105. [DOI] [PubMed] [Google Scholar]
- 15.Gorski K., Carnerio,M. and Schibler,U. (1986) Tissue-specific in vitro transcription from the mouse albumin promoter. Cell, 47, 767–776. [DOI] [PubMed] [Google Scholar]
- 16.Lichtsteiner S. and Schibler,U. (1989) A glycosylated liver-specific transcription factor stimulates transcription of the albumin gene. Cell, 57, 1179–1187. [DOI] [PubMed] [Google Scholar]
- 17.Pao C.I., Lin,K.W., Zhu,J., Wu,G., Farmer,P.K. and Phillips,L.S. (1996) In vitro transcription of the rat insulin-like growth factor-I gene. J. Biol. Chem., 271, 8667–8674. [DOI] [PubMed] [Google Scholar]
- 18.Lucas P.C., Forman,B.M., Samuels,H.H. and Granner,D.K. (1991) Specificity of a retinoic acid response element in the phosphoenolpyruvate carboxykinase gene promoter: consequences of both retinoic acid and thyroid hormone receptor binding. Mol. Cell. Biol., 11, 5164–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo C.J., Couley,P.B., Chen,L., Sladek,F.M., Darnell,J.E.,Jr and Crabtree,G.R. (1992) A transcriptional hierarchy involved in mammalian cell-type specification. Nature, 355, 457–461. [DOI] [PubMed] [Google Scholar]
- 20.Sladek F.M., Zhong,W.M., Lai,E. and Darnell,J.E.,Jr (1990) Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev., 4, 2353–2365. [DOI] [PubMed] [Google Scholar]
- 21.Vu-Dac N., Schoonjans,K., Kosykh,V., Dallongeville,J., Heyman,R.A., Staels,B. and Anwerx,J. (1996) Retinoids increase human apolipoprotein A-11 expression through activation of the retinoid X receptor but not the retinoic acid receptor. Mol. Cell. Biol., 16, 3350–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawadogo M. and Roeder,R.G. (1985) Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc. Natl Acad. Sci. USA, 82, 4394–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani S.A., Harish,S., Vatsala,P.G., Rangarajan,P.N. and Padmanaban,G. (2000) Receptor-mediated gene delivery approach demonstrates the role of 5′-proximal DNA region in conferring phenobarbitone responsiveness to CYP2B2 gene in rat liver in vivo. Biochem. Biophys. Res. Commun., 268, 734–739. [DOI] [PubMed] [Google Scholar]
- 24.Kadonaga J.T and Tjian,R. (1986) Affinity purification of sequence-specific DNA binding proteins. Proc. Natl Acad. Sci. USA, 83, 5889–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heery D.M., Kalkhoven,E., Hoare,S. and Parker,M.G. (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature, 387, 733–736. [DOI] [PubMed] [Google Scholar]
- 26.Xu L., Glass,C.K. and Rosenfeld,M.G. (1999) Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev., 9, 140–147. [DOI] [PubMed] [Google Scholar]
- 27.Malik S. and Karathanasis,S.K. (1996) TFIIB-directed transcriptional activation by the orphan nuclear receptor hepatocyte nuclear factor 4. Mol. Cell. Biol., 16, 1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass C.K. and Rosenfield,M.G. (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev., 14, 121–141. [PubMed] [Google Scholar]
- 29.McKenna N.J., Lanz,R.B. and O’Malley,B.W. (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev., 20, 321–344. [DOI] [PubMed] [Google Scholar]
- 30.Dell H. and Hadzopoulou-Cladaras,M. (1999) CREB-binding protein is a transcriptional coactivator for hepatocyte nuclear factor-4 and enhances apolipoprotein gene expression. J. Biol. Chem., 274, 9013–9021. [DOI] [PubMed] [Google Scholar]
- 31.Wang,J.-C., Stafford,J.M. and Granner,D.K. (1998) SRC-1 and GRIP1 coactivate transcription with hepatocyte nuclear factor 4. J. Biol. Chem., 273, 30847–30850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida E., Aratani,S., Iton,H., Miyagishi,M., Takiguchi,M., Osumu,T., Murakami,K. and Fukamazi,A. (1997) Functional association between CBP and HNF4 in trans-activation. Biochem. Biophys. Res. Commun., 241, 664–669. [DOI] [PubMed] [Google Scholar]
- 33.Makishama M., Okamoto,A.Y., Repa,J.J., Tu,H., Learned,R.M., Luk,A., Hull,M.V., Lustig,K.D., Mangelsdorf,D.J. and Shan,B. (1999) Identification of a nuclear receptor for bile acids. Science, 284, 1362–1365. [DOI] [PubMed] [Google Scholar]
- 34.Giguere V. (1999) Orphan nuclear receptors: from gene to function. Endocr. Rev., 20, 689–725. [DOI] [PubMed] [Google Scholar]
- 35.Hertz R., Magenheim,J., Berman,I. and Bar-Tana,J. (1998) Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature, 392, 512–516. [DOI] [PubMed] [Google Scholar]