Abstract
Objective
In Parkinson disease (PD) patients, deep brain stimulation (DBS) of the subthalamic nucleus (STN) may contribute to certain impulsive behavior during high-conflict decisions. A neurocomputational model of the basal ganglia has recently been proposed that suggests this behavioral aspect may be related to the role played by the STN in relaying a “hold your horses” signal intended to allow more time to settle on the best option. The aim of the present study was 2-fold: 1) to extend these observations by providing evidence that the STN may influence and prevent the execution of any response even during low-conflict decisions; and 2) to identify the neural correlates of this effect.
Methods
We measured regional cerebral blood flow during a Go/NoGo and a control (Go) task to study the motor improvement and response inhibition deficits associated with STN-DBS in patients with PD.
Results
Although it improved Unified Parkinson Disease Rating Scale motor ratings and induced a global decrease in reaction time during task performance, STN-DBS impaired response inhibition, as revealed by an increase in commission errors in NoGo trials. These behavioral effects were accompanied by changes in synaptic activity consisting of a reduced activation in the cortical networks responsible for reactive and proactive response inhibition.
Interpretation
The present results suggest that although it improves motor functions in PD patients, modulation of STN hyperactivity with DBS may tend at the same time to favor the appearance of impulsive behavior by acting on the gating mechanism involved in response initiation.
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves motor functions in patients with Parkinson disease (PD).1,2 However, growing evidence suggests that STN-DBS also causes executive inhibitory deficits and impulsive behavior under high-conflict conditions.3–7
Abnormal basal ganglia activity in PD produces a series of downstream effects affecting thalamic and cortical areas.8 Within the basal ganglia, the STN plays a major role in motor control, and operating as a relay, influences response suppression by inhibiting thalamocortical programs. The fact that a large part of the STN receives motor inputs from the primary motor cortex (M1), the premotor cortex (PMC), the supplementary motor area (SMA), and the pre-SMA strongly supports its important motor contribution.9,10 However, the STN also receives several projections from the anterior cingulate cortex (ACC) and inferior frontal cortex (IFC), as well as the medial prefrontal cortex (mPFC) and dorsolateral prefrontal cortex, accounting for a more cognitive aspect of motor control.10,11
Within this framework, Frank and colleagues have proposed a neurocomputational model7,12 in which the STN provides a dynamic “hold your horses” signal temporarily preventing the execution of any response in face of high-conflict decisions. According to their model, the STN would relay a global NoGo signal via excitatory projections to the pallidum with consequent inhibition of thalamocortical activity, thus allowing more time to integrate all necessary information and settle on the optimal choice. However, based on recent investigations in healthy human subjects, it appears that the function of the “hold your horses” signal may not be specific to complex decisional conflicts, but could generalize to basic sensorimotor functions in which preventing unwanted response is required.13–16 This model, known as “proactive inhibition,” assumes that the default state of the executive control involved in basic preparatory processes consists of preventing automatic responses to irrelevant stimulations, even for low-conflict decisions. This executive control would maintain inhibition over movement-triggering processes as long as the uncertainty lasts and release the brake when appropriate. Although accounting for simple sensorimotor reactions, this gating model relies on a network of cognitive structures, where the mPFC, the precuneus/posterior cingulate cortex (Prec/PCC), and the inferior parietal cortex (IPC) play a central role.14,15
The hypotheses underlying the “hold your horses” and “proactive inhibition” models are not mutually exclusive. Both models predict attenuation of thalamocortical activity and assume a global modulatory signal suppressing all responses rather than modulating the execution of any particular response. However, they differ in respect to 2 main aspects. First, the effects of the global modulatory signal supported by the STN are expected to apply at different times in the 2 models. In the “hold your horses” model, suppression is supposed to be implemented as response selection processes evolve. Thus, the global NoGo signal would not apply before the stimulus is presented; it would rather be triggered by it.12 Conversely, in the “proactive inhibition” model, inhibition of movement-triggering processes is supposed to apply well in advance of stimulus presentation and to be released by the identification of the Go signal. In other words, the former model assumes a phasic, reactive inhibitory process, whereas the latter rather refers to a tonic, proactive inhibitory process. Second, the 2 models predict that STN-DBS may functionally affect different STN connected cortical areas. The “hold your horses” model predicts specific changes in PMC, pre-SMA and ACC,7,10,12 whereas the “proactive inhibition” hypothesis predicts possible activation changes in mPFC, Prec/PCC, and IPC.15,16
A way to investigate basic mechanisms of response inhibition and motor impulsivity is to use a Go/NoGo (GNG) paradigm,17,18 which can examine response inhibition using only minor working memory load and without the requirement of learning complex stimulus-response associations. The aim of the present study was to identify STN-DBS–induced changes in brain activation in a simple reaction time (RT) task that required withholding response execution, within the framework of the 2 models accounting for gating motor responses. To this end, we assessed reactivity and impulsivity by measuring reaction times and response inhibition failures in a GNG task and a Go task in PD patients with stimulators bilaterally implanted in the STN. We compared behavioral performances between ON and OFF stimulation in both tasks and used H215O positron emission tomography (PET) to measure the neural correlates of performance changes induced by STN-DBS. Although PET is an ideal technique to study patients with implanted stimulators, it does not allow event-related analysis to separate out in-time different brain activations associated with the proactive and retroactive inhibition. Nevertheless, keeping this limitation in mind, we reasoned that STN-DBS–induced modulation of GNG task performance would influence to varying degrees the activity of the different cortical areas predicted by 2 models.
Materials and Methods
Participants
We studied 7 right-handed patients with PD and bilateral DBS of the STN (6 men, 1 woman; mean age, 59 ± 6 years). They were treated with bilateral STN stimulation for at least 47 months (range, 19–94 months). The implantation of the electrodes (Model 3387; Medtronic, Minneapolis, MN) was performed under local anesthesia, guided by stereotactic magnetic resonance imaging (MRI) and microelectrode recordings. The accurate placement of the electrodes was confirmed on postoperative MRI. The electrodes were connected to a pulse generator (Kinetra or Soletra, Medtronic). The technique used to localize the electrode contacts used for stimulation on postoperative images has been previously described in detail.19 The main demographic and clinical characteristics of the patients, the stimulation parameters used, and the effects of STN stimulation on Unified Parkinson Disease Rating Scale (UPDRS) motor scores are presented in Table 1.
Table 1.
Demographics of the Patients With Parkinson Disease
| Patient | Sex | Age | Disease Duration, y | UPDRS III Score OFF Med |
Time Since Surgery, mo | Stimulation Parameters |
LEDD, mg/day | ||
|---|---|---|---|---|---|---|---|---|---|
| STN-DBS ON | STN-DBS OFF | Left Side | Right Side | ||||||
| 1 | M | 65 | 15 | 26 | 40 | 29 | 3–/case +/185Hz/ 60μs/3.6V |
3–/case +/185Hz/ 60μs/3.5V |
1,370 |
| 2 | M | 61 | 21 | 14.5 | 47.5 | 69 | 1–2–/case +/180Hz/ 60μs/3.1V |
1–2–/case +/180Hz/ 90μs/3.5V |
800 |
| 3 | M | 52 | 10 | 19.5 | 33 | 29 | 1–/case +/185Hz/ 60μs/3.8V |
1–/case +/185Hz/ 60μs/3.3V |
750 |
| 4 | M | 58 | 18 | 35 | 42.5 | 33 | 1–/185Hz/ 60μs/2V |
2–1+/185Hz/ 60μs/3V |
1,000 |
| 5 | M | 52 | 24 | 29 | 37 | 94 | 1–/case+/185Hz/ 60μs/3.5V |
2–/185Hz/ 60μs/2.5V |
1,750 |
| 6 | M | 59 | 25 | 16.5 | 27.5 | 42 | 3–/case +/185Hz/ 90μs/3.1V |
2–/case +/185Hz/ 60μs/3V |
350 |
| 7 | F | 67 | 16 | 21 | 39 | 19 | 1–/185Hz/ 60μs/2.4V |
2–/185Hz/ 60μs/3.8V |
100 |
| Mean (SD) | 59 (6) | 17 (5) | 23 (7) | 38 (6) | 45 (27) | 874 (566) | |||
UPDRS = Unified Parkinson Disease Rating Scale; Med = medication; STN = subthalamic nucleus; DBS = deep brain stimulation; LEDD = levodopa equivalent daily dose; M = male; F = female; SD = standard deviation.
PET experiments were approved by the local research ethics committees. Prior to the PET experiment, the subjects gave informed written consent after the aims of the study and the nature of the procedures had been fully explained.
Activation Tasks
Patients performed 2 motor tasks: 1) a GNG task and 2) a Go task (simple RT task). The GNG task involved 2 stimuli: a Go stimulus (a white circle) and a NoGo stimulus (a white X). Patients were instructed to press a button with their right thumb at the occurrence of the circle symbol but to withhold a response when the letter X was presented. The Go task involved only 1 stimulus (the white circle). Stimulus presentation duration was 500 milliseconds, and interstimulus interval was variable (between 1,500 and 3,500 milliseconds). Each scan contained 20 trials. For the GNG task, 40% of the trials were the NoGo stimulus. RT and errors (anticipation or commission error) were recorded. Errors of anticipation were defined as responses to the Go stimulus <120 milliseconds. Errors of commission were defined as a response to the NoGo stimulus in the GNG session. Before data acquisition, patients performed a practice block consisting of 50 trials and were instructed to respond as quickly and as accurately as possible.
PET Scanning Procedure
Patients were imaged after overnight withdrawal of their antiparkinsonian medications. Subjects were positioned supine on the PET scanner bed. The head was maintained in a fixed position using a thermoformed mask. PET measurements were obtained using a whole body PET camera system, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN) operating in 3-dimensional mode with an in-plane resolution of approximately 4.6mm full width at half-maximum. To measure regional cerebral blood flow (rCBF), 10mCi H215O were administered intravenously over 50 seconds with a semibolus injection using an infusion pump. Scanning of emission data was started 10 seconds after the injection and lasted for 90 seconds. The behavioral task was started parallel to the acquisition of the frame. The interval between successive H215O administrations was 11 minutes for adequate decay of radioactivity. Before the first emission scan, a scout view was obtained to determine accurate positioning of the subject and a low-dose (0.2mSv) computed tomography (CT) scan was acquired to correct for attenuation. Images were reconstructed by 2-dimensional filtered back-projection, resulting in 81 slices with a 256 × 256 pixel matrix (pixel size, 2mm).
Twelve emission scans were obtained with both stimulators OFF and ON (6 scans each) in a block design randomized among patients. After the first block, patients were removed from the PET scanner for UPDRS III rating. Patients’ stimulator settings were then changed, and patients were replaced in the scanner after 30 minutes using identical settings. The interval of 30 minutes between blocks was chosen to maximize differences between stimulation conditions. A second CT scan and a second set of emission scans were then performed. The following conditions were studied: 1) Go-ON and GNG-ON with bilateral STN-DBS ON; and 2) Go-OFF and GNG-OFF with bilateral STN-DBS OFF. In each block, motor tasks were performed with the right hand and each condition was performed up to 3 times in randomized order.
PET Image Transformation and Statistical Analysis
Image and statistical analysis were performed in MATLAB version 7.4 (Mathworks Inc., Natick, MA) using statistical parametric mapping software (SPM2; Wellcome Department of Imaging Neuroscience, London, UK)20 to implement the following steps: 1) a mean image was generated; 2) all images were then realigned to the mean image to correct for inter-scan head movement; 3) the mean image was normalized to the standard SPM2 PET Montreal Neurological Institute space template; 4) the resulting normalization parameter set was used to spatially normalize the individual images; and 5) normalized images were thereafter smoothed using an isotropic, Gaussian kernel of 14mm to reduce the variance due to individual anatomical variability and to improve the signal/noise ratio. Variations in global flow across subjects and scans were removed by proportionally scaling each image to have an arbitrary level of 50ml/100ml/min.
To identify voxels associated with the effect of STN-DBS on Go and GNG tasks, a multisubjects × conditions and covariates model was used. To account for potential effect of disease severity, we entered the motor UPDRS scores and the disease duration as nuisance variables in the analyses. The following analyses were performed:
MAIN EFFECT OF STIMULATION
The contrast (Go-ON + GNG-ON) − (Go-OFF + GNG-OFF) assessed the increase in rCBF related to stimulation, independently of the task. The reversed contrast (Go-OFF + GNG-OFF) − (Go-ON + GNG-ON) assessed the decrease in rCBF.
MAIN EFFECT OF TASK
The contrast (GNG-ON + GNG-OFF) − (Go-ON + Go-OFF) assessed the increase in rCBF during GNG compared with Go, irrespective of stimulation condition. The reversed contrast (Go-ON + Go-OFF) − (GNG-ON + GNG-OFF) assessed the decrease in rCBF during the GNG compared with Go, across the 2 stimulation conditions.
INTERACTION
The contrast (GNG-ON − Go-ON) − (GNG-OFF − Go-OFF) assessed the increase in rCBF during the GNG task relative to the Go task with DBS ON compared with DBS OFF. The reversed contrast (GNG-OFF − Go-OFF) − (GNG-ON − Go-ON) assessed the decrease in rCBF during the GNG task relative to the Go condition with DBS ON compared with DBS OFF stimulation.
In all analyses, statistical maps were thresholded at a level of p < 0.001 uncorrected at the voxel level, and a restricted volume analysis was used to test specific a priori hypotheses.21,22 This analysis considered all voxels within a mask image that included regions of interest (ROIs). This mask image was created anatomically using the WFU-Pick Atlas tool (http://www.fmri.wfubmc.edu),23 based on the automated anatomical labeling (AAL) atlas.24 On the basis of the “pro-active inhibition” model,15 the ROIs were defined as follows: 1) mPFC, consisting of the superior medial part of the frontal lobe from the AAL template; 2) the left inferior parietal lobe; and 3) the midline cingulate posterior (PCC). On the basis of the “hold your horses” model,7,12 the ROIs were defined as follow: 1) the left motor cortical areas (M1/PMC), using the precentral gyrus in the AAL template; 2) the pre-SMA using the SMA region (with y > 0); 3) the midline cingulate anterior (ACC); and 4) the right IFC consisting of combined pars opercularis and pars triangularis from the AAL template.
Resulting clusters in a priori regions were reported as significant only if they included voxels with z values of >3.30, corresponding to the 2-tailed p < .001 level, uncorrected for multiple comparisons and an extent threshold of 20 contiguous voxels. All coordinates reported are based on the Talairach atlas and were derived from procedures developed by M. Brett (http://www.mrc-bu.cam.ac.uk/Imaging). These coordinates refer to the location of maximal activation in a particular anatomical structure, which is also indicated in Brodmann areas (BA).
Correlation Analysis
To identify brain regions where rCBF correlated with poor inhibitory control during the GNG task, the percentage of commission errors for each GNG scan were used as a covariate in a covariate only model. The negative covariation of rCBF with this covariate was determined independently of the stimulation condition.
Results
Effect of STN Stimulation on PD Motor Symptoms
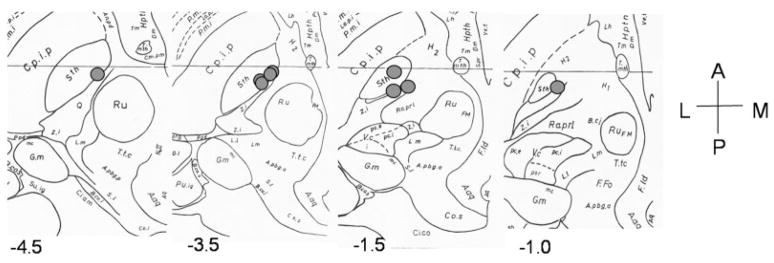
Stimulation significantly improved motor symptoms, with a mean of 61% reduction of the UPDRS motor scores (p < 0.004). This improvement in motor symptoms was observed individually in all our PD patients (Table 1). The location of the electrode contacts stimulated during the tasks and PET imaging is shown in Figure 1.
Fig 1.
Schematic representation of the approximate location of the electrode contacts used for stimulation during the study over a series of 4 axial sections of the Schaltenbrand & Wahren atlas (A = anterior; M = medial; P = posterior; L = lateral). Numbers on the graphs represent millimeters from the midcommissural point (MCP). Negative values are inferior to the midcommissural point. A.aq = Annulus aquaeductus; Aq = Aquaductus mesencephali; B.cj = Brachium conjuctivum; B.co.i = Brachium colliculi inferioris; Co.s = Colliculi superioris; Cp.i.p = Internal capsula lenticular fascicularis; G.m = corpus geniculatum mediale; H1/H2 = forel’s field; Hpth = hypothalamus; L.1 = lemniscus lateralis; L.m = lemniscus medialis; P.m = medial pallidus; Pp.d = nucleus peripeduncularis; Pu.ig = nucleus pulvinaris intergeniculartus; Q = fasciculus Q; Ra.pr1 = preliminiscal radiation; Ru = red nucleus; Sth = subthalamic nucleus; Tmth = mammillo-thalamic tract; T.t.c = central tegmental tract; v.ci = nucleus ventrocaudalis internus; v.c.pc.e = ventrocaudalis parvocellularis externus; v.c.pc.i = ventrocaudalis parvocellularis internus; v.por = nucleus ventralis portae; z.i. = zona incerta.
Effect of STN Stimulation on the Behavioral Tasks
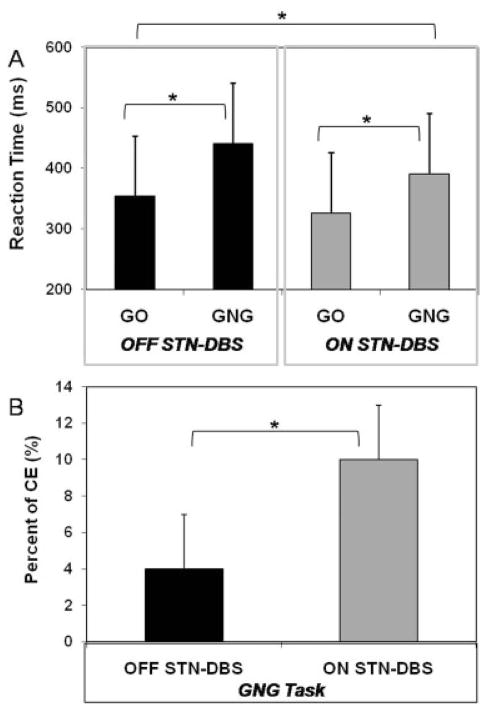
Significant effects of the stimulation (F1,6 = 18,760; p = 0.004) and task (F1,6 = 16,09; p = 0.007) factors were found on the RT variable, without interaction (F1,6 = 1,22; p = 0.31). The stimulation effect indicated that RT was shorter when DBS stimulators were ON (mean ± standard deviation [SD] = 359 ± 45 milliseconds) than when they were OFF (mean ± SD = 398 ± 60 milliseconds). The task effect revealed that RT was longer in the GNG task (mean ± SD = 416 ± 45 milliseconds) than during the Go condition (mean ± SD = 340 ± 60 milliseconds). As shown by the absence of stimulation × task interaction, the latter effect was similar in OFF and ON DBS conditions (Fig 2A). However, DBS impaired response inhibition during the GNG task, as patients made more commission errors in the ON (10%) than in the OFF (4%) condition (t = 2.66; p = 0.036) (Fig 2B).
Fig 2.
(A) Variations of reaction time as a function of the experimental condition. (B) Percentage of commission error (CE) during the Go/NoGo (GNG) task as a function of the stimulation condition (subthalamic nucleus deep brain stimulation, STN-DBS). *Statistically significant effect.
Changes of rCBF
The main effect of stimulation revealed that STN-DBS induced both an increase and decrease in rCBF in cortical areas defined in our a priori hypothesis (Fig 3). A significant increase in rCBF was observed in the subgenual ACC (BA 24/32). In contrast, a reduced activation was detected in the medial PCC (BA 29/30), pre-SMA (BA 6), and dorsal ACC (BA 24/32), as well as in the left primary motor cortex (BA 4), inferior parietal lobe (BA 40), dorsal PMC (BA 6), right ventral PMC (BA 6), and inferior frontal cortex (BA 44). The location, coordinates, and peak z score of activated and deactivated areas are detailed in Table 2. For the main effect of task, no significant effect of task was observed. The interaction did not disclose any specific effect of STN stimulation during the GNG task at the threshold selected for the analyses. In the correlation analysis, the percentage of commission errors revealed a significant negative covariation with rCBF in the right precuneus (BA 7). The location, coordinates, and peak z score of activated areas are detailed in Table 3.
Fig 3.
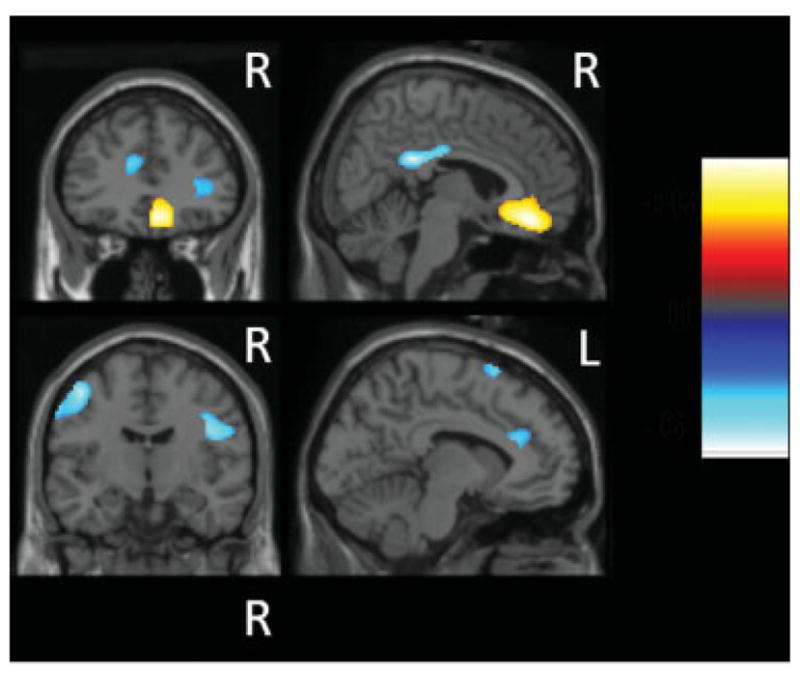
Statistical parametric maps of brain regions showing common significant changes with subthalamic nucleus (STN) stimulation during both Go and Go/NoGo tasks at a statistical threshold of p < 0.001 (uncorrected) at the single-voxel level. Areas of increased regional cerebral blood flow (rCBF) with STN stimulation involving the ventral anterior cingulate cortex (ACC) are in yellow. Areas of decreased rCBF during STN stimulation involving the left motor and premotor areas, the medial dorsal ACC, posterior cingulate cortex, and pre–supplementary motor area are in blue. These areas are superimposed on sagittal (right column) and coronal (left column) sections of a single subject’s brain magnetic resonance imaging from SPM2. R = right; L = left.
Table 2.
Main Effect of Stimulation
| Areas | Left/Right | BA | Stereotactic Coordinates (Regional Maximal) | z Score | Cluster Size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increased activation induced by STN stimulation | |||||||
| Ventral ACC | Midline | 24/32 | 8 | 28 | −8 | 4.62 | 271 |
| Decreased activation induced by STN stimulation | |||||||
| PCC | Midline | 29/30 | 3 | −44 | 20 | 5.44 | 164 |
| Primary motor cortex | L | 4 | −41 | −11 | 47 | 4.76 | 215 |
| PMC dorsal | L | 6 | −40 | −3 | 52 | 4.50 | |
| Inferior parietal lobe | L | 40 | −45 | −38 | 42 | 4.47 | 576 |
| Pre-SMA | Midline | 6 | −10 | 10 | 60 | 4.33 | 77 |
| Dorsal ACC | Midline | 24/32 | −10 | 28 | 21 | 3.88 | 117 |
| Inferior frontal cortex | R | 45 | 34 | 28 | 6 | 3.62 | 87 |
BA = Brodmann areas; STN = subthalamic nucleus; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; L = left; PMC = premotor cortex; SMA = supplementary motor area; R = right.
Table 3.
Negative Covariation of rCBF With the Percentage of Commission Errors
| Area | Left/Right | BA | Stereotactic Coordinates (Regional Maximal) | z Score | Cluster Size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Precuneus | R | 7 | 15 | −69 | 37 | 3.57 | 36 |
rCBF = regional cerebral blood flow; BA = Brodmann areas.
Discussion
Choosing between different possible responses requires substantial monitoring and inhibition to prevent incorrect reactions.25,26 The ability of PD patients to slow down when faced with high-conflict decisions is impaired under STN-DBS.7 Based on a recent proposal that withholding automatic response to external stimuli is the default state of sensorimotor reactivity in healthy subjects (ie, not restricted to high-conflict decisions but generalized to simple sensorimotor actions),14,15 we hypothesized that the executive control consisting of switching from controlled proactive tonic inhibition to automatic sensorimotor processing (ie, in releasing the brake) may also play a certain role in the pathogenesis of akinetic symptoms. Because the STN is placed in a neuroanatomical position that may allow it to be an important operating relay in the modulation of the inhibitory network that prevents erroneous responses,27 we assumed that STN-DBS effects in PD patients may exert a strong influence on the ability to control this basic inhibitory mechanism.
As expected, STN-DBS produced a significant improvement in the motor symptoms of PD patients. Consistent with the clinical evaluation, behavioral data indicate that STN stimulation yields faster generation of motor responses irrespective of the task. The present findings also demonstrated that STN stimulation induced significant increase of commission errors during the Go/NoGo task, that is, a greater difficulty inhibiting responses to NoGo signals. It is worth noting that commission error rate in a Go/NoGo task is usually considered as the primary measure of impulsivity.5,28,29 Accordingly, the pattern of behavioral results observed in the present study suggests that STN-DBS improved motor functions at the expense of response inhibition in simple sensorimotor tasks. The cerebral patterns of brain activity were found to be consistent with the behavioral outcome. In fact, STN-DBS influenced different cortical areas associated with the predictions of both reactive and proactive models of response inhibition.
The broad effect of STN stimulation was associated with a reduced activation in the left PMC and pre-SMA, as well as the dorsal ACC and IFC. As proposed in the “hold you horses” model, these interconnected structures are known to be activated during inhibitory tasks, detection and integration of response conflict, and error-related processing.10,30–32 They are particularly engaged when preventing the execution of any response in face of high-conflict decisions.7,12 The recent observation that STN-DBS reduces coupling between cingulate and basal ganglia output6 supports our finding of reduced activation of the dorsal ACC. This cortical area is well known to be activated by a wide range of higher cognitive functions, including tasks in which a prepotent or over-learned response tendency has to be overcome (such as the Stroop and the GNG task) and tasks that typically elicit response conflict. Thus, the impaired activation observed in the dorsal ACC may be accountable for the reduced ability of PD patients to inhibit responses during the GNG task. Finally, the deactivation of the right IFC, known to be involved in the active inhibitory process of ongoing responses,10,33 supports the hypothesis that STN-DBS may influence motor impulsivity by also compromising directly reactive inhibitory commands.
STN stimulation was also associated with a reduced activation in key structures supporting the proactive inhibitory control of movement-triggering mechanisms, such as the precuneus, PCC, and left inferior parietal cortex. In particular, the negative correlation observed between the amount of activation in the area of the precuneus and the number of commission errors strongly emphasizes our hypothesis. The precuneus is crucial in the process of preparing an individual to engage the inhibitory circuitry34 along with the left inferior parietal cortex, a structure involved in the initiation of a motor program35–36 that may act as a possible relay in movement initiation and modulations of the tonic inhibitory state.15 Because no effect was observed in the mPFC, the acknowledged source of the proactive inhibitory command,15,34 we could speculate that STN-DBS may compromise only its integration within posterior sensorimotor networks. This hypothesis is consistent with the observed deactivation of the precuneus/PCC node, which plays a pivotal role within the default network, acting as a convergence node where cognitive information arising from different subsystems interact.37 Thus a deactivation of this node would have a significant detrimental effect on the process monitoring of different cognitive subsystems (eg, executive).
An interesting observation was represented by the increased rCBF in the subgenual limbic ACC,38–40 a region reported to be abnormally activated in diseases associated with impulsive behavior.41,42 Thus, we could speculate that the increased activity observed in the subgenual ACC may reflect an elevated motivational drive43 related to impulsivity in PD patients with STN-DBS. Overall, the cerebral patterns of activation and deactivation observed in the ACC suggest that although STN-DBS may enhance the ventral emotional circuit, it alters the dorsal cognitive circuit involved in the performance of various executive functions.
These findings favor the hypothesis that modulation of STN hyperactivity with DBS, although it improves parkinsonian features in PD patients, induces a global impairment of response inhibition pattern that tends to increase impulsivity. Consistent with these observations, STN lesioning studies in experimental animals with dopaminergic degeneration have shown that, although they improve motor performance, they also produce selective nonmotor deficits such as increased premature response during both simple and complex RT tasks.44–47 Our results and conclusions find their rationale in the strategic position that the STN holds within the corticobasal ganglia circuitry,9,11,48 providing it with a pivotal role as a brake in the motor network.27 Importantly, the present study suggests that this deficit in response inhibition likely applies to both tonic (proactive) and phasic (reactive) inhibitory processes. Based on these observations, we propose that the impairment of the response inhibition network may play an important role in both akinesia (which may be viewed as global difficulty in releasing proactive inhibition OFF stimulation, ie, to “release the horses”) and impulsivity (which may conversely be considered as global difficulty in locking movement-triggering processes ON stimulation, ie, to “hold the horses”). In other words, akinesia and impulsivity could represent opposite sides of the same coin. We believe that this new theoretical approach may provide novel insights and better understanding of movement disorders. Further work is needed to extend this observation and to explore the hypothesis that this role of the STN may be generalized to other cognitive and behavioral aspects, as suggested by the other forms of inhibitory deficits observed with STN-DBS.3,4,6,48
Acknowledgments
This work was funded by a Canadian Institutes of Health Research (CIHR) grant to A.P.S. (MOP-64423). A.P.S. is supported by the CIHR New Investigator Research Award. A.M.L. is a Canada Research Chair (tier 1) in Neuroscience.
We thank the staff of the Centre for Addiction and Mental Health PET Imaging Centre and the Movement Disorder Unit, Toronto Western Hospital for their assistance in carrying out the studies.
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Limousin P, Pollak P, Benazzouz A, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(suppl 14):S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 3.Jahanshahi M, Ardouin CM, Brown RG, et al. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000;123(pt 6):1142–1154. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder U, Kuehler A, Haslinger B, et al. Subthalamic nucleus stimulation affects striato-anterior cingulate cortex circuit in a response conflict task: a PET study. Brain. 2002;125:1995–2004. doi: 10.1093/brain/awf199. [DOI] [PubMed] [Google Scholar]
- 5.Hershey T, Revilla FJ, Wernle A, et al. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- 6.Thobois S, Hotton GR, Pinto S, et al. STN stimulation alters pallidal-frontal coupling during response selection under competition. J Cereb Blood Flow Metab. 2007;27:1173–1184. doi: 10.1038/sj.jcbfm.9600425. [DOI] [PubMed] [Google Scholar]
- 7.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 8.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 9.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 10.Aron AR, Behrens TE, Smith S, et al. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benarroch EE. Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70:1991–1995. doi: 10.1212/01.wnl.0000313022.39329.65. [DOI] [PubMed] [Google Scholar]
- 12.Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Boulinguez P, Jaffard M, Granjon L, Benraiss A. Warning signals induce automatic EMG activations and proactive volitional inhibition: evidence from analysis of error distribution in simple RT. J Neurophysiol. 2008;99:1572–1578. doi: 10.1152/jn.01198.2007. [DOI] [PubMed] [Google Scholar]
- 14.Jaffard M, Benraiss A, Longcamp M, et al. Cueing method biases in visual detection studies. Brain Res. 2007;1179:106–118. doi: 10.1016/j.brainres.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Jaffard M, Longcamp M, Velay JL, et al. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008;42:1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Boulinguez P, Ballanger B, Granjon L, Benraiss A. The paradoxical effect of warning on reaction time: demonstrating proactive response inhibition with event-related potentials. Clin Neurophysiol. 2009;120:730–737. doi: 10.1016/j.clinph.2009.02.167. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev. 2004;3:261–275. doi: 10.1177/1534582305276838. erratum 200;4:137. [DOI] [PubMed] [Google Scholar]
- 18.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 19.Hamani C, Moro E, Zadikoff C, et al. Location of active contacts in patients with primary dystonia treated with globus pallidus deep brain stimulation. Neurosurgery. 2008;62:217–223. doi: 10.1227/01.neu.0000317396.16089.bc. discussion 223–225. [DOI] [PubMed] [Google Scholar]
- 20.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapp. 1994;2:189–210. [Google Scholar]
- 21.Friston KJ, Holmes A, Poline JB, Price CJ, Frith C. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 22.Worsley KJ, Marrett S, Neelin P, et al. A unified statistical approach for determining significant voxels in image of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Stuss DT, Alexander MP, Shallice T, et al. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dougherty DM, Moeller FG, Steinberg JL, et al. Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- 29.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Severity of bipolar disorder is associated with impairment of response inhibition. J Affect Disord. 2009;116:30–36. doi: 10.1016/j.jad.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botvinick M, Nystrom LE, Fissell K, et al. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 33.Aron AR, Fletcher PC, Bullmore ET, et al. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 34.Arce E, Leland DS, Miller DA, et al. Individuals with schizophrenia present hypo- and hyperactivation during implicit cueing in an inhibitory task. Neuroimage. 2006;32:704–713. doi: 10.1016/j.neuroimage.2006.04.189. [DOI] [PubMed] [Google Scholar]
- 35.de Jong BM, Willemsen AT, Paans AM. Brain activation related to the change between bimanual motor programs. Neuroimage. 1999;9:290–297. doi: 10.1006/nimg.1998.0410. [DOI] [PubMed] [Google Scholar]
- 36.Mattingley JB, Husain M, Rorden C, et al. Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature. 1998;392:179–182. doi: 10.1038/32413. [DOI] [PubMed] [Google Scholar]
- 37.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 38.Allman JM, Hakeem A, Erwin JM, et al. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 39.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 40.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 41.Swann AC, Pazzaglia P, Nicholls A, et al. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 42.Strakowski SM, Fleck DE, DelBello MP, et al. Characterizing impulsivity in mania. Bipolar Disord. 2009;11:41–51. doi: 10.1111/j.1399-5618.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:190–191. 220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 44.Baunez C, Nieoullon A, Amalric M. In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J Neurosci. 1995;15:6531–6541. doi: 10.1523/JNEUROSCI.15-10-06531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur J Neurosci. 1997;9:2086–2099. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 46.Florio T, Capozzo A, Cellini R, et al. Unilateral lesions of the pedunculopontine nucleus do not alleviate subthalamic nucleus-mediated anticipatory responding in a delayed sensorimotor task in the rat. Behav Brain Res. 2001;126:93–103. doi: 10.1016/s0166-4328(01)00248-0. [DOI] [PubMed] [Google Scholar]
- 47.Phillips JM, Brown VJ. Reaction time performance following unilateral striatal dopamine depletion and lesions of the subthalamic nucleus in the rat. Eur J Neurosci. 1999;11:1003–1010. [PubMed] [Google Scholar]
- 48.Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 49.Schroeder U, Kuehler A, Lange KW, et al. Subthalamic nucleus stimulation affects a frontotemporal network: a PET study. Ann Neurol. 2003;54:445–450. doi: 10.1002/ana.10683. [DOI] [PubMed] [Google Scholar]