Abstract
There is clear evidence that the prefrontal cortex is strongly involved in executive processes and that dopamine can influence performance on working memory tasks. Although, some studies have emphasized the role of striatal dopamine in executive functions, the role played by prefrontal dopamine during executive tasks is unknown. In order to investigate cortical dopamine transmission during executive function, we used D2-dopamine receptor ligand [11C]FLB 457 PET in healthy subjects while performing the Montreal Card Sorting Task (MCST). During the retrieval with shift task of the MCST, the subjects had to match each test card to one of the reference cards based on a classification rule (color, shape or number) determined by comparing the previously viewed cue card and the current test card. A reduction in [11C]FLB 457 binding potential in the right dorsal anterior cingulate cortex (ACC) was observed when subjects performed the active task compared to the control task. These findings may suggest that right dorsal ACC dopamine neurotransmission increases significantly during the performance of certain executive processes, e.g., conflict monitoring, in keeping with previous evidence from fMRI studies showing ACC activation during similar tasks. These results may provide some insights on the origin of cognitive deficits underlying certain neurological disorders associated with dopamine dysfunction, such as Parkinson’s disease and schizophrenia.
Keywords: FLB 457, Positron emission tomography, Executive function, Anterior cingulate cortex, Dopamine, Conflict monitoring
Introduction
There is clear evidence that damage to the prefrontal cortex impairs performance on executive function tasks (Milner, 1963; Nelson, 1976; Stuss et al., 2000) and functional neuroimaging investigations support these observations (Buchsbaum et al., 2005; Konishi et al., 2002; Lie et al., 2006; Monchi et al., 2001). In a previous fMRI study, we demonstrated that performing the Wisconsin Card Sorting Task activates prefrontal areas including the dorsolateral prefrontal cortex (DLPFC), the ventrolateral prefrontal cortex (VLPFC), and the anterior cingulate cortex (ACC) (Monchi et al., 2001). More specifically, the DLPFC is most engaged during the provision of feedback after each matching response, a fact which is consistent with the proposed role of this region in the monitoring of events in working memory (Petrides, 2000). VLPFC and ACC are more engaged during negative feedback reception and we hypothesized that these activations are related to preparation to shift set and monitor conflicts of previous versus current rule of classification, respectively. The functional specificity of different prefrontal regions has been further investigated and supported by fMRI studies that used the Montreal Card Sorting Task (MCST), a test specifically designed for the investigation of the different subcomponents of executive function, i.e., retrieval of information and set-shifting (Monchi et al., 2006b; 2007).
While fMRI studies can identify task-specific neuronal correlates with high temporal and spatial resolutions, they cannot provide information on the neurochemical bases of a given function. Identifying the type of neurotransmission involved in executive function is crucial for understanding its underlying mechanism. Since it is known that dopaminergic modulation can alleviate or worsen the performance on working memory tasks (Fournet et al., 2000; Kimberg et al., 1997; Kimberg and D’Esposito, 2003; Kulisevsky et al., 1996; Mehta et al.,1999, 2001), this neurotransmitter has received particular attention.
Changes in [11C]raclopride binding potential (BP) provide a reasonable estimate of synaptic dopamine release in the striatum (Farde et al.,1986). This method has been widely used for investigating the striatal dopaminergic transmission during various cognitive tasks (Goerendt et al., 2003; Ko et al., 2008; Monchi et al., 2006a; Ouchi et al., 2002; Zald et al., 2004). However, although [11C]raclopride may offer important insight on striatal dopamine neurotransmission during executive functions (Ko et al., 2008; Monchi et al., 2006a), its low affinity limits its application to extrastriatal regions such as the prefrontal brain (Goldman-Rakic et al., 2000).
As revealed by studies in primates, despite a lower density of dopamine receptors relative to the striatum, cortical dopamine plays a critical role in executive function (Murphy et al., 1996; Watanabe et al., 1997). In humans, converging evidence suggests that cortical dopamine is involved with high-level cognition. Performing working memory task has been shown to increase dopamine release in the frontal cortex (Aalto et al., 2005a; Sawamoto et al., 2008) and ACC dopamine receptor density has been shown to be significantly correlated with performance level on the Wisconsin Card Sorting Task in normal healthy adults (Lumme et al., 2007). To address the role of the prefrontal dopamine during set-shifting tasks (e.g. MCST) in healthy human subjects, we used [11C]FLB 457, a chemical compound with a greater affinity (Kd =20 nM) for D2 receptors which allows evaluation of extrastriatal dopamine release (Aalto et al., 2005a; Olsson et al., 1999; Sudo et al., 2001). In previous reports, Olsson et al. (2004) have shown that [11C]FLB 457 BP calculated by simplified reference tissue model (Gunn et al.,1997; Lammertsma and Hume,1996; Sudo et al., 2001) may provide a reasonable estimate of receptor densities in different extrastriatal areas (e.g. cingulate cortex, frontal cortex, thalamus, temporal cortex) consistent with postmortem study with [125I]epidepride (Kessler et al., 1993). Similarly, [11C]FLB 457 has been demonstrated to be sensitive in detecting changes in extrastriatal endogenous dopamine concentration in non-human primates (Chou et al., 2000) and humans (Aalto et al., 2005a,b; Hagelberg et al., 2004; Montgomery et al., 2007). Thus, it appears that [11C]FLB 457 is well-suited to capture binding differences in prefrontal areas.
Based on previous anatomical and functional imaging studies with card sorting tasks (Buchsbaum et al., 2005; Konishi et al., 2002; Koski and Paus, 2000; Lie et al., 2006; Monchi et al., 2001, 2007), we hypothesized that performance of the MCST would be associated with increases in dopamine release (decrease BP of [11C]FLB 457) in different prefrontal areas such as the DLPFC (BA 9/46) and ACC (BA 32/24).
Method
Subjects and experimental design
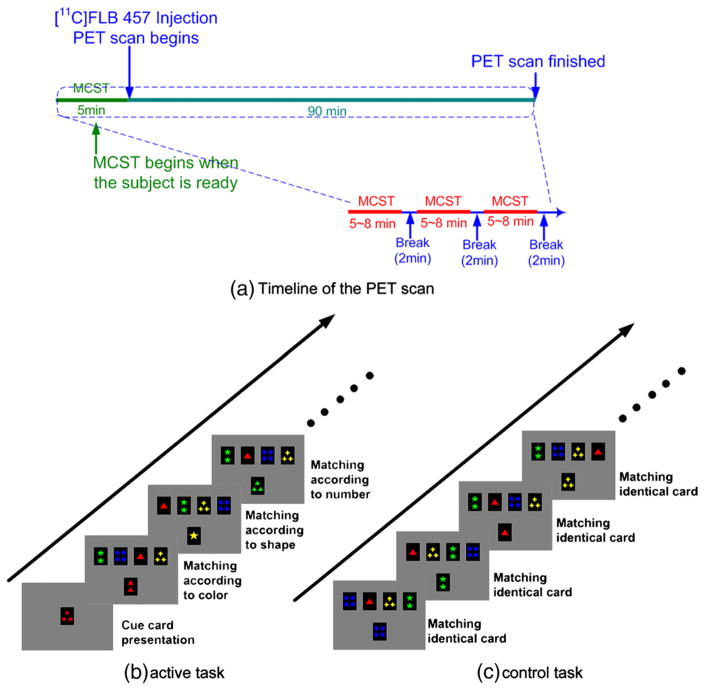
Eight healthy young right-handed adults (20–33 years, 4 males) participated in the present study after having given written informed consent. They were investigated with [11C]FLB 457 PET while performing the MCST to measure changes in cortical dopamine release. Each subject underwent two [11C]FLB 457 PET scans at the same time on two separate days while they performed either the MCST (retrieval with shift) or the control task (Fig. 1) (Ko et al., 2008). Scan order was counterbalanced across subjects. The experiments were approved by the Research Ethics Committee of the Centre for Addiction and Mental Health.
Fig. 1.
Study design. (a) Each subject underwent two [11C]FLB 457 PET scans at the same time on two separate days while performing either the MCST (retrieval with shift) or the control task (Fig. 1). Scan order was counterbalanced across subjects. Participants started the MCST 5 min before the radioligand injection and continued until the end of PET scanning with two-minute breaks between blocks; (b) active task; (c) control task.
Cognitive task
The tasks were displayed via a video eyewear (VR920; Vuzix Corporation, New York, USA) placed on the plastic thermal mask. Details of the current task have also been described in our previous studies (Ko et al., 2008). In the retrieval with shift condition of the MCST (the active task, Fig. 1b), four reference cards were displayed in a row at the top of the screen in all trials. Each one of them encompasses three kinds of characteristics, i.e., number (one to four), shape (triangle, star, cross and circle) and color (red, green, yellow and blue). Their position changed pseudo-randomly on every trial. A block of twenty classification trials was preceded by the brief presentation of a single cue card. The cue card did not reappear and had to be remembered throughout the block. On each classification trial, a new test card was presented below the reference cards and the subject had to match the test card to one of the four reference cards using one of four buttons with the right dominant hand. Matching each test card to one of the reference cards was based on a classification rule (color, shape or number) determined by making a comparison between the previously viewed cue card and the current test card (Fig. 1b). The test card and the cue card shared only one characteristic among number, shape and color. The test cards on consecutive trials never shared the same attribute with the cue card, resulting in a pseudo-random sequence which allowed for a set-shift on each trial. Each selection of the reference card was followed by a one-second positive (white) or negative (dark) feedback. Five blocks of twenty classification trials (total: 100 trials) were followed by a two-minute break. A different cue card was presented before each block. At the end of each block, the subjects were asked if they remembered the cue card.
In the control task, the test card was identical to one of the reference cards so that the subject simply selected the identical card without having to find an appropriate rule for classification as was required in the active task (Fig. 1c).
Subjects underwent a training session of the task before each PET session in order to reduce a possible learning effect. Error trials were counted as number of incorrect responses and they were averaged for each scan. The reaction time was measured from the presentation of new test card to the selection of the reference card. All values are presented as mean±SE.
Positron emission tomography
PET scans were obtained with a high resolution PET CT, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN, U.S. A.) operating in 3D mode with an in-plane resolution of approximately 4.6 mm full width at half-maximum. To minimize subject’s head movements in the PET scanner, we used a custom-made thermoplastic facemask together with a head-fixation system (Tru-Scan Imaging, Annapolis). Before each emission scan, following the acquisition of a scout view for accurate positioning of the subject, a low dose (0.2 mSv) CT scan was acquired and used for attenuation correction.
[11C]FLB 457 was injected into the left antecubital vein over 60 s and emission data were then acquired over a period of 90 min in 15 one-minute frames and 15 five-minute frames. The injected amount was 10.19±0.16 mCi for the active condition and 10.42±0.16 mCi for the control condition.
High-resolution MRI (GE Signa 1.5 T, T1-weighted images, 1 mm slice thickness) of each subject’s brain was acquired and transformed into standardized stereotaxic space (Talairach and Tournoux, 1988) using nonlinear automated feature-matching to the MNI template (Collins et al., 1994; Robbins et al., 2004).
PET frames were summed, registered to the corresponding MRI (Woods et al., 1993) and transformed into standardized stereotaxic space (Talairach and Tournoux, 1988) using the transformation parameters of the individual structural MRIs (Collins et al., 1994; Robbins et al., 2004). Voxelwise [11C]FLB 457 BP was calculated using a simplified reference tissue (cerebellum) method (Gunn et al., 1997; Lammertsma and Hume, 1996; Sudo et al., 2001) to generate statistical parametric images of change in BP (Aston et al., 2000). This method uses the residuals of the least-square fit of the compartmental model to the data at each voxel to estimate the standard deviation of the BP estimate. Parametric images of [11C]FLB 457 BP were smoothed with an isotropic Gaussian of 6 mm full width at half-maximum to accommodate for intersubject anatomical variability. A threshold level of t>4.1 was considered significant (p<0.05, 2-tailed) corrected for multiple comparisons (Friston,1997; Worsley et al.,1996) for the regions with a priori hypothesis, i.e., DLPFC and ACC and a more stringent threshold (t>4.9) when the search was extended to the entire brain. Regions within our a priori hypothesis were extracted from bilateral Brodmann areas (BA) 32/24 (ACC), 9/46 (DLPFC) using the WFU PickAtlas (SPM extension toolbox). The volume of interest included 6624 voxels and 52,992 mm3. As stated above, the reason for choosing BA 32/24 and 9/46 was based on their consistent activations during sorting task in the previous fMRI studies conducted by our and other groups (Buchsbaum et al., 2005; Konishi et al., 2002; Lie et al., 2006; Monchi et al., 2001, 2007). The functional connectivity between these regions and their contribution has been well documented in previous anatomical and functional imaging studies (for review, see Koski and Paus, 2000).
Results
MCST performance
There was no significant difference in task performance; subjects performed with a mean accuracy of 96.68±0.95% in the active task and 98.49±0.53% in the control task (paired t(7)=1.76, p>0.1). Depending on individual speed, subjects completed a mean of 1471± 45 classification trials for the active task and 1429±36 trials for the control task (p>0.05). The mean reaction time was 1199±141 ms in the active task and 844±97 ms in the control task (p>0.05). Thus, we can safely assume that the observed dopamine release could not be the consequence of different motor performances.
PET results
Performing the active task of MCST decreased [11C]FLB 457 BP in the right ACC (X=6, Y=26, Z=40) (t=4.3; p<0.05, corrected for multiple comparison) compared to the control task (Fig. 2). The mean BP of [11C]FLB 457 extracted from a spherical region of interest (r=3 mm) centered at the statistical peak revealed by the parametric map was 0.292±0.042 during control task and 0.199±0.049 during active task (paired-t test, t(7)=3.85, p=0.006, Fig. 3).
Fig. 2.
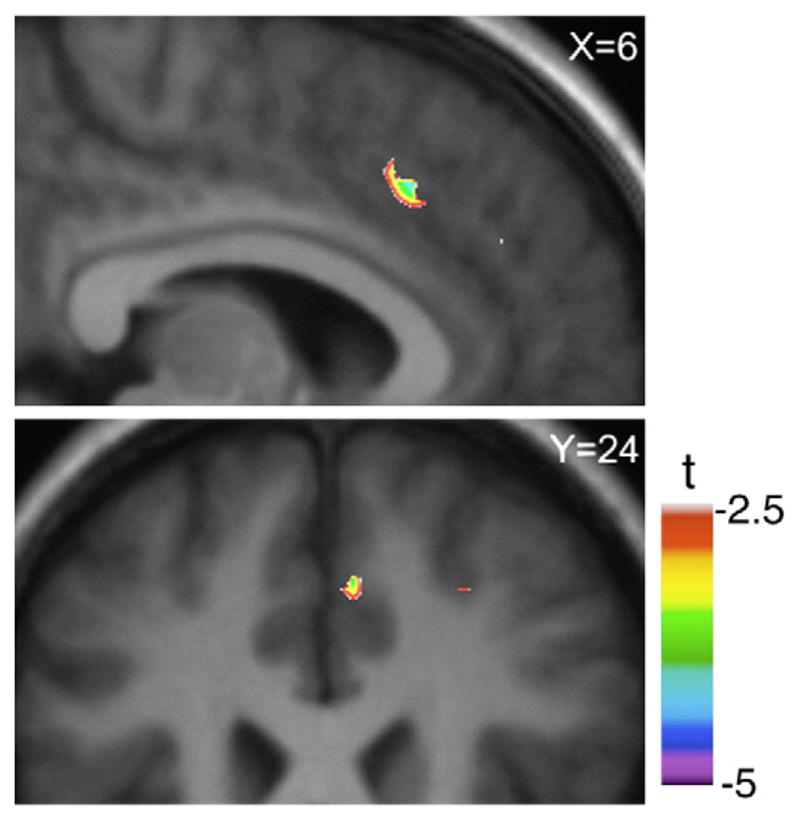
Active versus control tasks condition. Sagittal (X=6) and coronal (Y=24) section of the statistical parametric map of the change in [11C]FLB 456 BP overlaid upon the average MRI of all subjects in standardized stereotaxic space. The figure displays the significant area of dopamine changes during active task performance compared to the control task at the level of dorsal ACC.
Fig. 3.
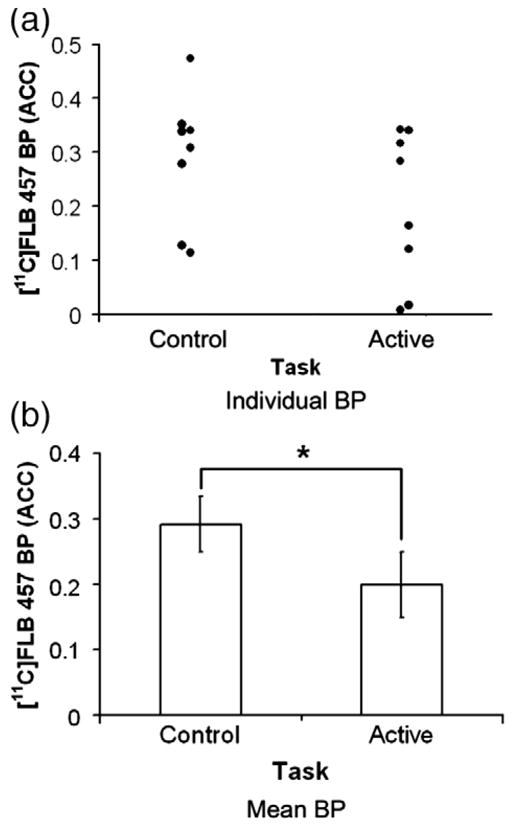
(a) Individual ACC-[11C]FLB 457 BP and (b) mean±SE of ACC-[11C]FLB 457 BP during control and active task extracted from a spherical region of interest (r=3 mm) centered at the x, y and z coordinates of the statistical peak (X=6, Y=26, Z=40) revealed by the parametric map (paired-t test, t(7)=3.85, *p=0.006).
While at more stringent threshold, voxel-based analysis did not reveal changes in other prefrontal areas defined in our a priori hypothesis, when using a less conservative threshold (uncorrected for multiple comparisons) a change in binding was observed in the left DLPFC (X=−22, Y=20, Z=44; t=3.7). The mean BP of [11C]FLB 457 extracted from a spherical region of interest (r=3 mm) centered at the statistical peak revealed by the parametric map was 0.229± 0.037 during the control task and 0.171±0.046 during the active task (paired-t test, t(7)=3.16, p=0.016).
When the search was extended to the entire brain, to areas not defined by our a priori hypothesis, a significant area of decrease in [11C]FLB 457 binding was identified at the level of the left occipital cortex (OCC) (X=−10, Y=−98, Z=−10) (t=5.1; p<0.05, corrected for multiple comparison). The mean BP of [11C]FLB 457 in this region was 0.323±0.049 during the control task and 0.255±0.046 during the active task (paired-t test, t(7)=2.81, p=0.026).
Correlation analyses did not reveal any relationship between extrastriatal [11C]FLB 457 BP and performance measures such as error trials and reaction times.
Discussion
In the present study, performing the active task of the MCST decreased [11C]FLB 457 BP in the right dorsal ACC compared to the control task. This finding confirms our previous observation that ACC is functionally involved during performance of the MCST (Monchi et al., 2007) and further extends our initial working hypothesis that ACC dopamine may play a relevant role during executive functioning.
A distinction there exists in the literature between the functions of the supracallosal (i.e. dorsal), rostral and subcallosal regions of the ACC (Devinsky et al., 1995; Koski and Paus, 2000; Mayberg, 1997; Vogt et al., 1995). It has been proposed that dorsal regions of the ACC are involved in cognition while rostral and subcallosal portions of the ACC are engaged in emotional behavior (Devinsky et al., 1995; Koski and Paus, 2000).
There is a consensus that dorsal ACC is one of the core components associated with executive function, but its precise role is still a matter of debate (Bush et al., 2000). In a meta-analysis of neuroimaging studies of executive function, dorsal ACC was activated during task-switching, response suppression, and the Wisconsin Card Sorting Task (Buchsbaum et al., 2005). Stuss and Alexander (2007) reported that lesions of frontal medial cortex that comprise ACC impairs several cognitive task performances including simple and choice reaction time, feature integration, verbal fluency and Stroop task (naming color patches and incongruent interference) as well as some tasks measuring sustained attention. Botvinick et al. (2004) also argued that dorsal ACC is involved in several cognitive tasks that engage response override, underdetermined responding and error commission. Other authors have emphasized the role of ACC in detecting and processing error signals (Debener et al., 2005; Luu et al., 2000). The common underlying feature of the aforementioned tasks and our MCST is that the subject has to monitor conflicts because previous rule classification and current response-rule are different. Our findings suggest that dopamine neurotransmission in ACC may play an important role in this type of executive function often described as “conflict monitoring” (Botvinick et al., 1999; Carter et al., 1998; MacDonald et al., 2000).
However, while ACC may be involved in detecting and processing error signals (Debener et al., 2005; Luu et al., 2000), we did not find a significant correlation between observed changes of [11C]FLB 457 BP in the right ACC and error trials on the MCST. This may be explained by the functionally distinct anatomy of the ACC. In fact, while the dorsal ACC (where our peak is located) is prevalently engaged during conflict monitoring (Kerns et al., 2004), the more rostral ACC is involved in error-signal processing (Lie et al., 2006; Taylor et al., 2006). Therefore, it is likely that the observed dopamine release in the right dorsal ACC was triggered when conflict monitoring was required and that it was unrelated to error-signal processing.
This interpretation is in keeping with other fMRI studies manipulating error-likelihood and conflict level (van Eimeren et al., 2006) which showed an increased right dorsal ACC BOLD signal as conflict load increases and error-likelihood decreases. Thus, it is likely that dopamine release may be involved during conflict monitoring rather than in error-signal processing or prediction of error-likelihood. However, while these observations may find some evidence in previous literature, it is also true and important to keep in mind that we cannot exclude the possibility that other aspects of cognitive function of MCST may have played a role in the observed dopamine release. In fact, a number of other executive functions such as monitoring information held in working memory, rule extraction, subsequent rule application and inhibition of response conflict induced by the non-relevant stimulus features may have contributed to this dopaminergic changes.
An interesting finding of the present study is the unilateral release of dopamine in the right ACC. We and others have observed this in previous fMRI studies. We showed that only the right ACC was activated when comparing retrieval with shift (active task in the present study) versus continuous shift (Monchi et al., 2007) during the MCST. Similarly, Lutcke and Frahm (2008) reported that while the right ACC was activated for correct inhibitions of go–no go task implicating conflict monitoring, error-related processes activated ACC bilaterally. This is also in agreement with MacDonald et al. (2000) who reported that only the right ACC was activated during response to the incongruent stimuli of the Stroop task. These observations seem to suggest that right ACC may play an important role in this type of executive function described as “conflict monitoring”.
The lack of a strong significant effect in other prefrontal areas other than ACC should be interpreted carefully since MCST has been previously shown to be involved with other lateral prefrontal cortices (Monchi et al., 2007). In fact, while voxel-based analysis corrected for multiple comparisons did not reveal significant changes, with a less stringent threshold (uncorrected for multiple comparisons) changes in binding could be observed in one the areas defined by our a-priori hypothesis, i.e. the DLPFC. The causality of left DLPFC in set-shifting has been recently confirmed in a transcranial magnetic stimulation–intervention study (Ko et al., 2008). A possible explanation on why DLPFC did not survive correction for multiple corrections may have multiple explanations. In fact, in demonstrating relationships between prefrontal areas, Koski and Paus (2000) have described that increases in activity within a particular subdivision of the cingulate occur most often along with increases in activity in specific regions of the frontal cortex. In particular, the relationship between supracallosal (i.e. dorsal) cingulate and the middle frontal gyrus is significantly stronger when the difficulty level of the task is greater. Thus, more difficult tasks may demand the joint efforts of both supracallosal cingulate and middle frontal cortex areas. Although our subjects during the active task appeared to take more time to respond than in control task due to the higher cognitive demand (1199±141 ms versus 844±97 ms), the lack of significant difference between these two conditions and the high accuracy of their performance during the MCST (active task: 96.68%; control task: 98.49%) suggest that the training session of the MCTS (before PET) may have significantly reduced the task challenge for them and possibly produced a ceiling effect preventing the detection of reasonable correlations between behavior and imaging. In alternative, another possible explanation could be methodological and linked to the different density of D2 and D1 receptors in the cortex where there are 20-fold more D1 receptors than D2 receptors (Goldman-Rakic et al., 2000). This agrees with the fact that in primates, performance on a working memory task has been shown to be impaired by D1 receptor antagonist administration to DLPFC, but not by D2 receptor antagonist (Brozoski et al., 1979; Sawaguchi and Goldman-Rakic, 1991, 1994; Seamans et al.,1998). Since [11C]FLB 457 is mainly a D2-receptor antagonist, it is possible that this radiotracer may have not been sensitive enough to pick-up significant dopaminergic changes over certain areas of the prefrontal cortex (i.e. DLPFC) that were not significantly engaged.
When we extended the search to the entire brain, outside PFC regions, the left OCC (BA 17/18) also showed a significant increase in dopamine release during the active task. Although this region has been consistently reported to present increased activation during imaging studies associated with sorting tasks (Buchsbaum et al., 2005) and it is known that visual stimulation can induce detectable changes in dopamine activity in the OCC (Muller and Huston, 2007), the relationship between dopamine and sorting tasks at the level of this occipital region is unclear at the moment. One possible explanation could be a greater attentional effect due to the higher task demands.
In conclusion, the present study showed that performing the MCST increased dopamine release in selective cortical areas. We propose that the dopaminergic transmission in the right ACC may be related to conflict monitoring during set-shifting processes. These results may provide some insights on the origin of cognitive deficits underlying certain neurological and psychiatric disorders associated with dopamine dysfunction, such as Parkinson’s disease and schizophrenia.
Acknowledgments
We wish to thank all the staff of the CAMH-PET imaging center for their assistance in carrying out the studies. This work was funded by the Canadian Institutes of Health Research to APS (MOP-64423). APS is supported by the CIHR New Investigator Research Award.
References
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005a;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Nagren K, Vilkman H, Gustafsson LL, Syvalahti E, Hietala J. Cortical glutamate–dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005b;182:375–383. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- Aston JA, Gunn RN, Worsley KJ, Ma Y, Evans AC, Dagher A. A statistical method for the analysis of positron emission tomography neuroreceptor ligand data. Neuroimage. 2000;12:245–256. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chou YH, Halldin C, Farde L. Effect of amphetamine on extrastriatal D2 dopamine receptor binding in the primate brain: a PET study. Synapse. 2000;38:138–143. doi: 10.1002/1098-2396(200011)38:2<138::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt. 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986;231:258–261. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J. Working memory functioning in medicated Parkinson’s disease patients and the effect of withdrawal of dopaminergic medication. Neuropsychology. 2000;14:247–253. doi: 10.1037//0894-4105.14.2.247. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5:133–136. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Goerendt IK, Messa C, Lawrence AD, Grasby PM, Piccini P, Brooks DJ. Dopamine release during sequential finger movements in health and Parkinson’s disease: a PET study. Brain. 2003;126:312–325. doi: 10.1093/brain/awg035. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Aalto S, Kajander J, Oikonen V, Hinkka S, Nagren K, Heitala J, Scheinin H. Alfentanil increases cortical dopamine D2/D3 receptor binding in healthy subjects. Pain. 2004;109:86–93. doi: 10.1016/j.pain.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Whetsell WO, Ansari MS, Votaw JR, de Paulis T, Clanton JA, Schmidt DE, Mason NS, Manning RG. Identification of extrastriatal dopamine D2 receptors in post mortem human brain with [125I]epidepride. Brain Res. 1993;609:237–243. doi: 10.1016/0006-8993(93)90878-q. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M. Cognitive effects of the dopamine receptor agonist pergolide. Neuropsychologia. 2003;41:1020–1027. doi: 10.1016/s0028-3932(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[(11)C]raclopride PET study. Eur J Neurosci. 2008;28:2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y. Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci U S A. 2002;99:7803–7808. doi: 10.1073/pnas.122644899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A. Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain. 1996;119 (Pt. 6):2121–2132. doi: 10.1093/brain/119.6.2121. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Lumme V, Aalto S, Ilonen T, Nagren K, Heitala J. Dopamine D2/D3 receptor binding in the anterior cingulate cortex and executive functioning. Psychiatry Res. 2007;156:69–74. doi: 10.1016/j.pscychresns.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Lutcke H, Frahm J. Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cereb Cortex. 2008;18:508–515. doi: 10.1093/cercor/bhm090. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. J Neurosci. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson’s disease. Psychopharmacology (Berl) 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of brain lesions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: a [(11)C] raclopride PET study. Neuroimage. 2006a;33:907–912. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006b;59:257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130:233–244. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab. 2007;27:369–377. doi: 10.1038/sj.jcbfm.9600339. [DOI] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Dopamine activity in the occipital and temporal cortices of rats: dissociating effects of sensory but not pharmacological stimulation. Synapse. 2007;61:254–258. doi: 10.1002/syn.20366. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Farde L. Differentiation of extrastriatal dopamine D2 receptor density and affinity in the human brain using PET. Neuroimage. 2004;22:794–803. doi: 10.1016/j.neuroimage.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Futatsubashi M, Okada H, Torizuka T, Sakamoto M. Effect of simple motor performance on regional dopamine release in the striatum in Parkinson disease patients and healthy subjects: a positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:746–752. doi: 10.1097/00004647-200206000-00013. [DOI] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004;8:311–323. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striatofrontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal–prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond, B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Sudo Y, Suhara T, Inoue M, Ito H, Suzuki K, Saijo T, Halldin C, Farde L. Reproducibility of [11 C]FLB 457 binding in extrastriatal regions. Nucl Med Commun. 2001;22:1215–1221. doi: 10.1097/00006231-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren T, Wolbers T, Munchau A, Buchel C, Weiller C, Siebner HR. Implementation of visuospatial cues in response selection. Neuroimage. 2006;29:286–294. doi: 10.1016/j.neuroimage.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24:4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]