Abstract
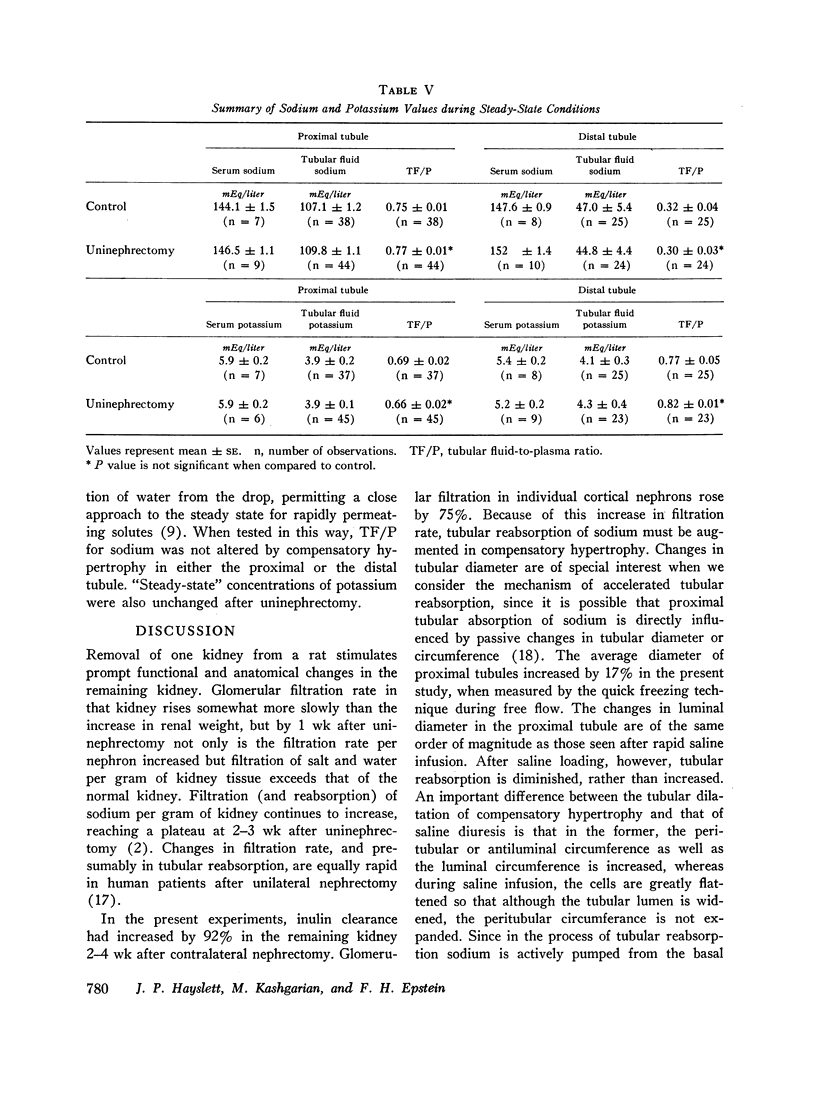
The functional correlates of compensatory renal hypertrophy were studied by micropuncture techniques in rats after the removal of one kidney. The glomerular filtration rate increased to roughly the same extent in the whole kidney and in individual surface nephrons, resulting in a greater amount of sodium delivered to the tubules for reabsorption. The fraction of the glomerular filtrate absorbed [determined from the tubular fluid-to-plasma ratio (TF/P) for inulin] remained unchanged in both proximal and distal portions of the nephron. The way in which the tubules adjusted to nephrectomy, however, differed in proximal and distal convolutions. After nephrectomy, the reabsorptive half-time, indicated by the rate of shrinkage of a droplet of saline in a tubule blocked with oil, was unchanged in the proximal tubule but significantly shortened in the distal convoluted tubule. Nevertheless, steady-state concentrations of sodium in an isolated raffinose droplet in the distal as well as the proximal tubule were the same in hypertrophied kidneys as in control animals. Possible reasons for this paradox are discussed.
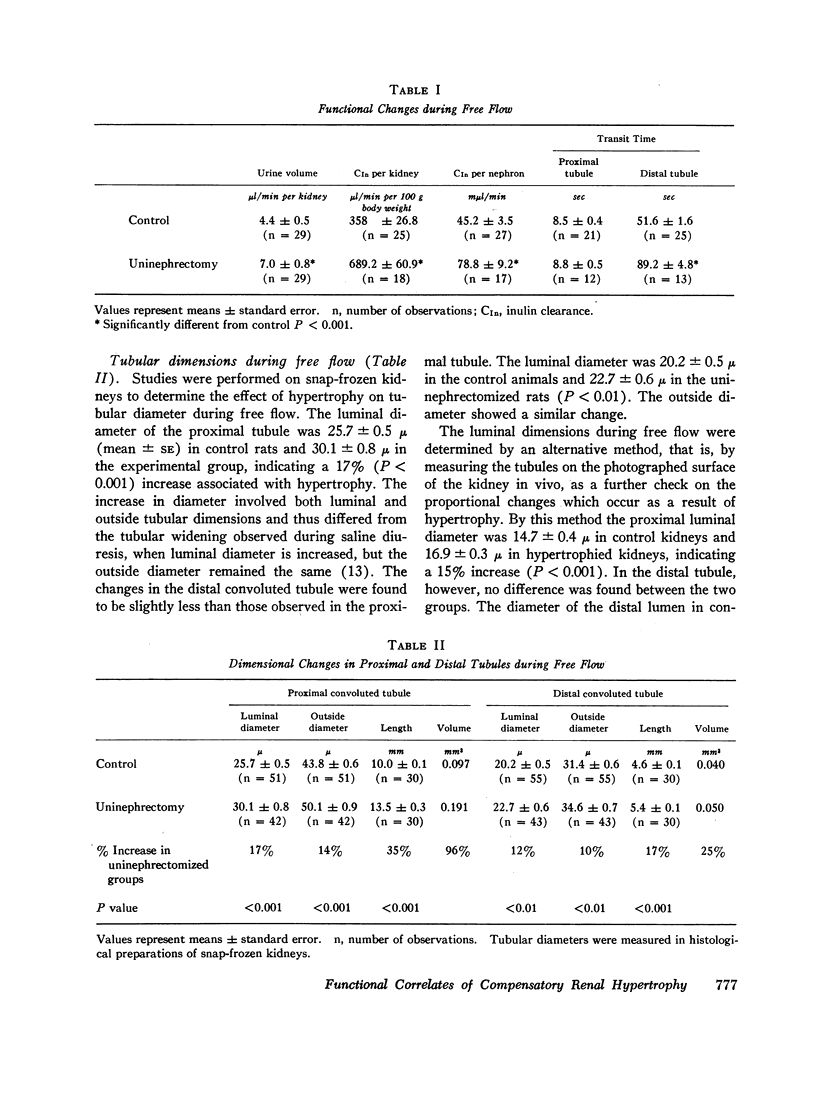
Transit time through the proximal tubules was unchanged by compensatory hypertrophy, but transit time to the distal tubules was prolonged.
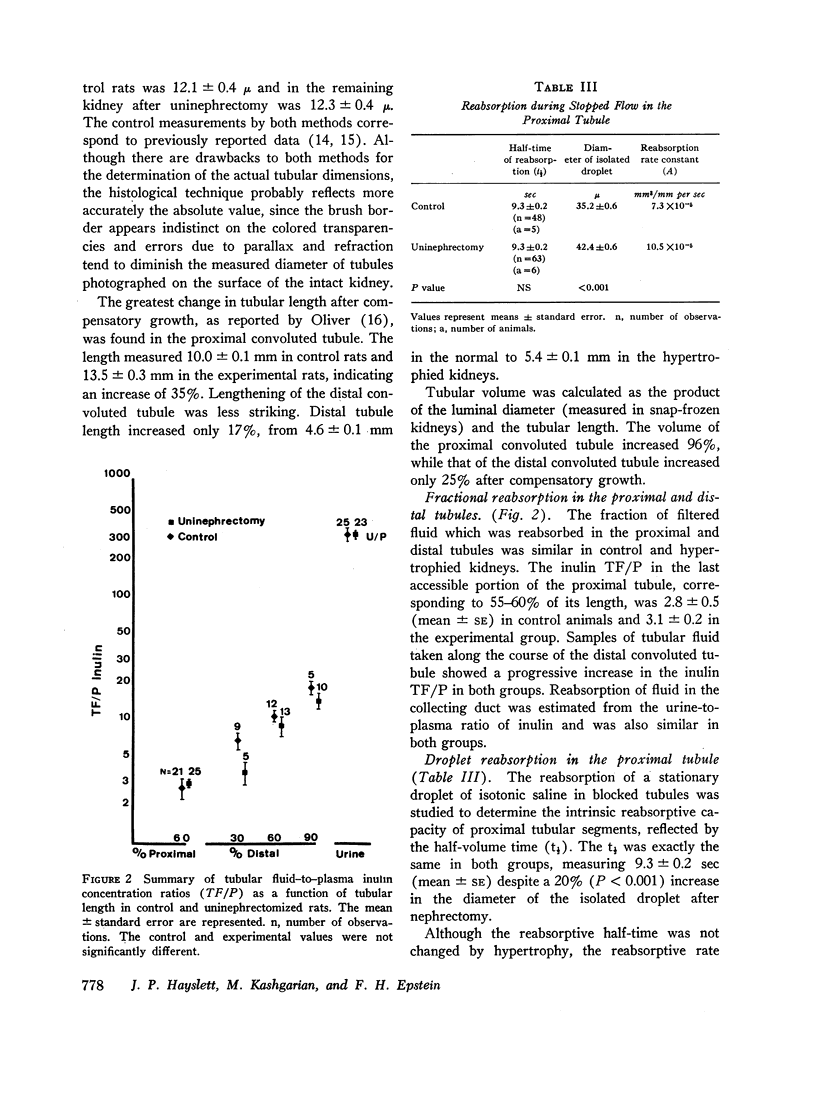
Changes in renal structure resulting from compensatory hypertrophy were also found to differ in the proximal and the distal protions of the nephron. Although tubular volume increased in both protions, the volume increase was twice as great in the proximal tubule as in the distal. In order, therefore, for net reabsorption to increase in the distal tubule, where the changes in tubular volume are not so marked, an increase in reabsorptive capacity per unit length of tubule is required. This increase is reflected in the shortening of reabsorptive half-time in the oil-blocked distal tubule that was actually observed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRICKER N. S., DEWEY R. R., LUBOWITZ H., STOKES J., KIRKENSGAARD T. Observations on the concentrating and diluting mechanisms of the diseased kidney. J Clin Invest. 1959 Mar;38(3):516–523. doi: 10.1172/JCI103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz K. H., Mangos J. A., Braun G., Pagel H. D. On the glomerular tubular balance in the rat kidney. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Sep 15;285(4):360–372. doi: 10.1007/BF00363236. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Changes in proximal and distal tubular reabsorption produced by rapid expansion of extracellular fluid. J Clin Invest. 1967 Jul;46(7):1254–1263. doi: 10.1172/JCI105618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHGARIAN M., STOCKLE H., GOTTSCHALK C. W., ULLRICH K. J. Transtubular electrochemical potentials of sodium and chloride in proximal and distal renal tubules of rats during antidiuresis and water diuresis (diabetes insipidus). Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:89–106. doi: 10.1007/BF00362394. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. The role of sodium-potassium-activated adenosine triphosphatase in the reabsorption of sodium by the kidney. J Clin Invest. 1967 Dec;46(12):1999–2011. doi: 10.1172/JCI105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn A. G., Ogden D. A., Holmes J. H. Renal function in 29 healthy adults before and after nephrectomy. JAMA. 1966 Apr 25;196(4):322–324. [PubMed] [Google Scholar]
- LEYSSAC P. P. Dependence of glomerular filtration rate on proximal tubular reabsorption of salt. Acta Physiol Scand. 1963 Jun-Jul;58:236–242. doi: 10.1111/j.1748-1716.1963.tb02644.x. [DOI] [PubMed] [Google Scholar]
- MALNIC G., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF RENAL POTASSIUM EXCRETION IN THE RAT. Am J Physiol. 1964 Apr;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Microperfusion study of distal tubular potassium and sodium transfer in rat kidney. Am J Physiol. 1966 Sep;211(3):548–559. doi: 10.1152/ajplegacy.1966.211.3.548. [DOI] [PubMed] [Google Scholar]
- Morrison A. B., Howard R. M. The functional capacity of hypertrophied nephrons. Effect of partial nephrectomy on the clearance of inulin and PAH in the rat. J Exp Med. 1966 May 1;123(5):829–844. doi: 10.1084/jem.123.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVER J., MacDOWELL M., TRACY A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951 Dec;30(121):1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLATT R., ROSCOE M. H., SMITH F. W. Experimental renal failure. Clin Sci. 1952 Aug;11(3):217–231. [PubMed] [Google Scholar]
- Rector F. C., Jr, Brunner F. P., Seldin D. W. Mechanism of glomerulotubular balance. I. Effect of aortic constriction and elevated ureteropelvic pressure on glomerular filtration rate, fractional reabsorption, transit time, and tubular size in the proximal tubule of the rat. J Clin Invest. 1966 Apr;45(4):590–602. doi: 10.1172/JCI105373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIRZ H. Der osmotische Druck in den corticalen Tubuli der Rattenniere. Helv Physiol Pharmacol Acta. 1956;14(3):353–362. [PubMed] [Google Scholar]


