Abstract
Clustered damages are formed in DNA by ionising radiation and radiomimetic anticancer agents and are thought to be biologically severe. 7,8-dihydro-8-oxoguanine (8-oxoG), a major DNA damage resulting from oxidative attack, is highly mutagenic leading to a high level of G·C→T·A transversions if not previously excised by OGG1 DNA glycosylase/AP lyase proteins in eukaryotes. However, 8-oxoG within clustered DNA damage may present a challenge to the repair machinery of the cell. The ability of yeast OGG1 to excise 8-oxoG was determined when another type of damage [dihydrothymine, uracil, 8-oxoG, abasic (AP) site or various types of single-strand breaks (SSBs)] is present on the complementary strand 1, 3 or 5 bases 5′ or 3′ opposite to 8-oxoG. Base damages have little or no influence on the excision of 8-oxoG by yeast OGG1 (yOGG1) whereas an AP site has a strong inhibitory effect. Various types of SSBs, obtained using either oligonucleotides with 3′- and 5′-phosphate termini around a gap or through conversion of an AP site with either endonuclease III or human AP endonuclease 1, strongly inhibit excision of 8-oxoG by yOGG1. Therefore, this large inhibitory effect of an AP site or a SSB may minimise the probability of formation of a double-strand break in the processing of 8-oxoG within clustered damages.
INTRODUCTION
Clustered DNA damages, in which two or more elemental lesions are induced within one to two helical turns of DNA, are formed in DNA by ionising radiation (1,2) as well as by radiomimetic anticancer agents such as bleomycin. Indeed, their formation is correlated to the ionisation density of these radiations (3) and clustered DNA damage is thought to be difficult to repair by compromising the repair machinery. For high linear energy transfer (LET) radiation, the yield of clustered damage is high with >50% of single-strand breaks (SSBs) and double-strand breaks (DSBs) having vicinal lesions (4,5). One of the health consequences of high LET radiation is that ∼10–15% of all deaths from lung cancer can be attributed to radon exposure (6). These clustered damages may be composed of multiple damaged bases or base lesions associated with abasic (AP) sites as well as SSBs or DSBs formed within a few base pairs of each other. The viability and transformation potential of the cell will depend upon the kinetics of processing of these lesions. The sequential excision of two opposite damages may convert the clustered damage into a DSB that may activate the apoptosis pathway and, if not repaired, is genotoxic. In contrast, the excision and repair of one of the lesions within two closely opposed lesions would lead to maintenance of double-stranded DNA, avoiding the formation of a DSB.
Only a few studies have assessed the way in which clustered damages are processed using prokaryotic proteins (7–10). In particular, Harrison et al. (9) and our results (M.-H.David-Cordonnier, J.Laval and P.O’Neill, unpublished data) showed that a 7,8-dihydro-8-oxoguanine (8-oxoG) positioned one base opposite to a SSB containing a nucleotide gap with 3′- and 5′-phosphate termini is a poor substrate for formamidopyrimidine DNA glycosylase (Fpg). The only study with nuclear cell extracts showed that the greatest inhibitory effect on excision of a base modification [dihydrothymine (DHT)] is the presence of an AP site on the complementary strand within 5 bp separation (10). The present study concentrates on the effect of either an AP site (normal or reduced) or SSBs with various termini on the excision of 8-oxoG, formed as a major oxidative and radiation damage (11), by the eukaryotic OGG1 DNA glycosylase/AP lyase (12–16). An 8-oxoG lesion on its own is strongly mutagenic through the propensity of DNA polymerases to insert either A or C opposite the 8-oxoG, leading to a high level of G·C→T·A transversions (reviewed in 17). The Saccharomyces cerevisiae OGG1 protein (43 kDa) is a 376 amino acid protein (18) and has a helix–hairpin–helix motif in its catalytic domain, homologous to that of the Nth (endonuclease III) protein. OGG1 does not contain any sequence homology with the bacterial Fpg protein (for reviews see 19–21). The yeast OGG1 (yOGG1) is able to remove 8-oxoG and an AP site (particularly when complemented with cytosine; 15,22) and possibly 8-oxoA (23) but not other types of oxidatively- or radiation-induced DNA damage (22,24). As with Nth, the yOGG1 protein excises its substrates by a β-elimination process, producing a SSB with 5′-phosphate and 3′-phospho-α,β-unsaturated aldehyde termini (22,25).
In this study, specific oligonucleotide constructs containing 8-oxoG base damage at precisely known sites within clustered damages (with DHT, AP site, reduced AP site or a variety of SSBs on the complementary strand) have been synthesised with the positions of the two lesions varied systematically relative to each other. The influence of the other lesion within the clustered damage on the excision of the highly mutagenic base lesion 8-oxoG by the yOGG1 protein has been assessed to gain insight into how such clustered damages are processed initially, since DNA damage clustering is thought to be biologically severe.
MATERIALS AND METHODS
Substrate oligonucleotides
All oligonucleotides were purchased from Genosys or Glen Research. The sequences of the various oligonucleotides are presented in Table 1. Strand 1 contains either DHT, 8-oxoG, uracil or the corresponding undamaged base as a control at the variable position Y. To obtain a probe containing a β–δ-SSB (see structures in Fig. 4), two oligonucleotides were used to form strand 1 with a gap at position Y. The oligonucleotides 5′ of the gap Y contain a 3′-phosphate terminus whereas the 3′ oligonucleotides contain a 5′-phosphate terminus. Strand 2, containing an 8-oxoG at the fixed position X, was 5′-32P-end-labelled using 10 U of T4 polynucleotide kinase (Gibco BRL) with 50 µCi [γ-32P]ATP (6000 Ci/mmol, 10 mCi/ml, NEN DuPont) in 25 µl of the recommended buffer for 1 h at 37°C. Following purification on a 12% denaturing polyacrylamide gel, the labelled oligonucleotides were hybridised with 1.5-fold excess of the various purified, non-radiolabelled complementary strands. That the annealing was efficient was verified by migration of the DNA samples on a native 10% polyacrylamide gel. To prepare the oligonucleotides containing an AP site at given positions, the 32P-labelled double-stranded oligonucleotides containing a uracil were treated with 1 U of uracil-DNA-glycosylase (UDG) (Gibco BRL) for 30 min at 37°C in 50 µl of buffer (10 mM Tris–HCl pH 7.5, 50 mM NaCl, 1 mM EDTA).
Table 1. Sequences of the oligonucleotides.
| Positions |
Sequence |
Strand |
| –5 | 5′-ctcttagtca ggaaYatgtc tctatgctgg gagcaaaggc-3′ | (1) |
| 3′-gagaatcagt ccttNtacaX agatacgacc ctcgtttccg-5′ | (2) | |
| –3 | 5′-ctcttagtca ggaataYgtc tctatgctgg gagcaaaggc-3′ | |
| 3′-gagaatcagt ccttatNcaX agatacgacc ctcgtttccg-5′ | ||
| –1 | 5′-ctcttagtca ggaatatgYc tctatgctgg gagcaaaggc-3′ | |
| 3′-gagaatcagt ccttatacNX agatacgacc ctcgtttccg-5′ | ||
| +1 | 5′-ctcttagtca ggaatatgtc Yctatgctgg gagcaaaggc-3′ | |
| 3′-gagaatcagt ccttatacaX Ngatacgacc ctcgtttccg-5′ | ||
| +3 | 5′-ctcttagtca ggaatatgtc tcYatgctgg gagcaaaggc-3′ | |
| 3′-gagaatcagt ccttatacaX agNtacgacc ctcgtttccg-5′ | ||
| +5 | 5′-ctcttagtca ggaatatgtc tctaYgctgg gagcaaaggc-3′ | |
| 3′-gagaatcagt ccttatacaX agatNcgacc ctcgtttccg-5′ |
X, 8-oxoG; Y, DHT, 8-oxoG, uracil, AP, reduced AP site, β-SSB, β–δ-SSB, HAP1-SSB or the normal corresponding base (T for DHT, uracil, AP and relatives, G for 8-oxoG); N, normal base complementary to the Y base (A opposite uracil, AP or DHT, C opposite 8-oxoG). –5 to –1, position on the complementary strand of the X base 3′ from the Y base. +1 to +5, position on the complementary strand of the X base 5′ from the Y base.
Figure 4.
Effect of the presence of various types of SSBs on the excision of 8-oxoG by yOGG1. The oligonucleotide containing an AP site at positions 5 bases 5′ (–5) to 5 bases 3′ (+5) opposite 8-oxoG or no damage opposite 8-oxoG were treated with either Nth (A) or HAP1 (B) as described in Materials and Methods to obtain either a β-SSB or a HAP1-SSB, respectively. The β–δ-SSB (C) was obtained by hybridisation of the radiolabelled 8-oxoG-containing oligonucleotide with two oligonucleotides corresponding to the strand 1 sequence but with a gap at the various positions Y and containing 3′P and 5′P termini around the gap. Oligonucleotides were then subjected to excision of 8-oxoG by yOGG1 and the efficiency of excision of 8-oxoG was measured after migration of the samples on a 12% denaturing polyacrylamide gel. The diagrams reflect the fold inhibition/activation by comparison to the control without damage on the non-labelled strand. The error bars represent the standard deviation from three to four different experiments. The structures of the SSB are shown schematically with an arbitrary choice of base pairs for illustrative purposes; a filled circle represents a phosphate group.
Purified proteins
The purified yOGG1 protein was extracted and purified as described in Girard et al. (22). The purified Nth and HAP1 proteins were generous gifts from Prof. Rick Wood (Imperial Cancer Research Fund, South Mimms, UK) (26) and Prof. Ian Hickson (Institute of Molecular Medicine, Oxford, UK) (27), respectively.
Preparation of a reduced AP site using NaBH4
The AP site-containing oligonucleotides were mixed 1:1 (v/v) with a freshly prepared solution of NaBH4 (Sigma) to a final concentration of 1 M and incubated at room temperature for 30 min as described by Castaing et al. (28). The oligonucleotides were then desalted by passage through a microspin G-25 column (Amersham Pharmacia Biotech) and precipitated with cold ethanol. The efficiency of reduction was checked by labelling both strands of the oligonucleotides prior to incubation with either Nth or OGG1 proteins, which do not effectively cleave a reduced AP site as compared with their ability to cleave a non-reduced AP site as control.
Preparation of a β-elimination strand break by Nth treatment
Oligonucleotides containing an AP site were treated with 10 ng of Nth protein, as described by David-Cordonnier et al. (10), to obtain, by a β-elimination process, a β-SSB with 5′-phosphate and 3′-phospho-aldehyde termini (see Fig. 4 for structure) at the various fixed positions opposite to the 8-oxoG at position X. The oligonucleotides were then precipitated with cold ethanol, washed, dried and resuspended in the appropriate amount of TE buffer. The efficiency of formation of a β-SSB was visualised after labelling both strands of these oligonucleotides and comparing the migration profiles of the DNA with or without a second treatment with either Nth (25 ng) or HAP1 (5 ng). If additional cuts are not seen, it is inferred that the amount of Nth used was sufficient to nick all the AP sites in the various oligonucleotides.
Preparation of a SSB by HAP1 treatment
Oligonucleotides containing an AP site were treated with 500 pg of human AP endonuclease 1 (HAP1) in 50 µl of buffer (20 mM HEPES pH 7.9, 100 mM KCl, 1 mM MgCl2, 20% glycerol) to obtain a SSB with 5′-phosphate and 3′-hydroxy termini at the various fixed positions opposite to the 8-oxoG at position X. The oligonucleotides were then precipitated with cold ethanol, washed, dried and resuspended in the appropriate amount of TE buffer. The efficiency of the formation of a HAP1-SSB (see Fig. 4 for structure) was visualised after labelling both strands of a sample of these oligonucleotides and comparing the migration profiles of the DNA with or without another treatment with either HAP1 (5 ng) or Nth (25 ng). If no increase in cleaved oligonucleotides is seen, it is inferred that the amount of HAP1 used was sufficient to nick all the AP site lesions in the various oligonucleotides.
Cleavage assays for SSB analysis
The double-stranded oligonucleotides (10 000 c.p.m., 200 fmol) were incubated for 30 min with the various amounts of yOGG1, as specified in the legends to the figures, in 5 µl of the incubation buffer (20 mM HEPES pH 7.9, 100 mM KCl, 0.2 mM EDTA, 20% glycerol) at 37°C. Subsequently, 5 µl of denaturing stop solution (98% formamide, 0.025% bromophenol blue, 0.025% xylene cyanol, 2 mM EDTA pH 8.0) was added to the samples, which were then subjected to electrophoresis on a 12% denaturing polyacrylamide gel containing 8 M urea in 1× TBE (89 mM Tris–HCl, 89 mM boric acid, 2 mM EDTA pH 8.3) for 90 min at 85 W. The dried gel was then exposed to a Bio-Rad PhosphorImager screen to visualise cleaved and full-length DNA fragments using phosphorimagery (Bio-Rad, Molecular Imager“ FX). Quantification was undertaken using Quantity One software (Bio-Rad) to determine the excision efficiency of each enzyme for each of the DNA sequences used. The number of nicked molecules reflects the number of modified bases excised. The efficiencies for multiple damages are compared with that for a single damage to assess the effect of the second damage, present on the unlabelled strand, on the excision of the damage on the labelled strand by the various proteins.
Measure of DSB formation induced by yOGG1
The experiments were done as described above for the analysis of SSB, except that the reactions were stopped by addition of 5 µl of a non-denaturing solution (40% sucrose, 0.025% bromophenol blue, 0.025% xylene cyanol, 5 mM EDTA pH 8.0). The samples were run on a 10% native polyacrylamide gel in 1× TBE for 3 h at 300 V, dried and quantified using the Bio-Rad PhosphorImager as described above.
RESULTS
Effect of a vicinal base lesion on the excision of 8-oxoG by yOGG1
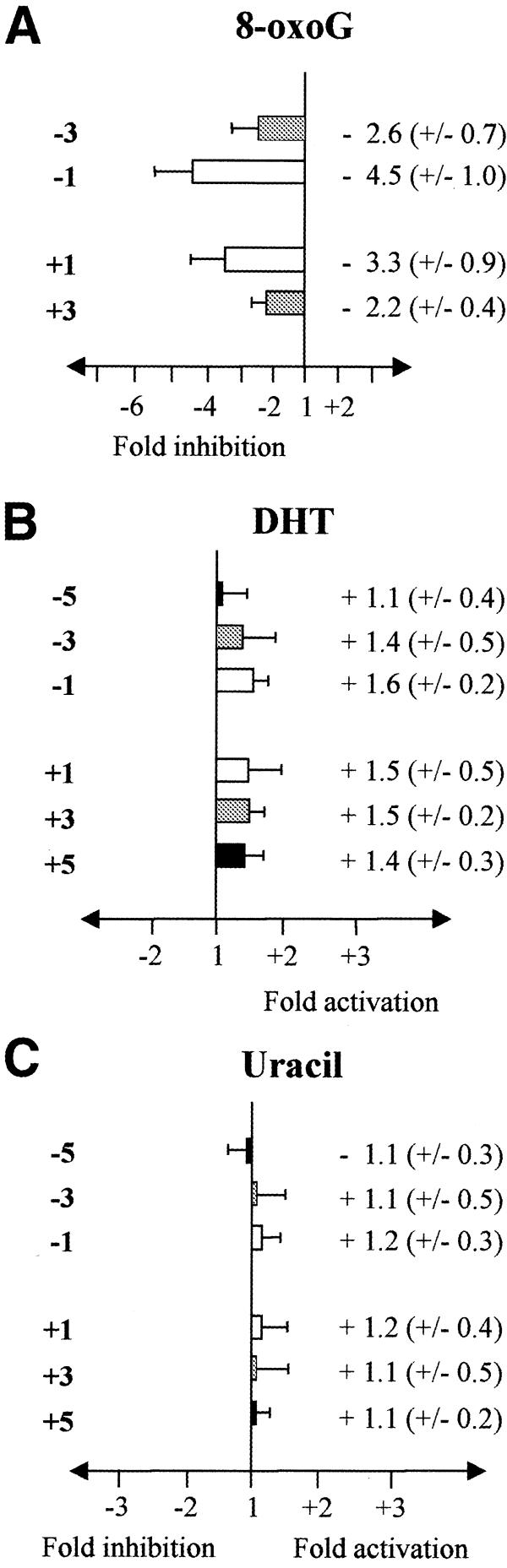
The effect of the presence of a second 8-oxoG on the non-labelled strand at position Y from –3 to +3 (1 or 3 bp 5′ or 3′, see Table 1) on the excision of 8-oxoG on the labelled strand was tested using yOGG1 protein (Fig. 1A). The major inhibitory effect on excision of 8-oxoG is seen when another 8-oxoG is 1 base either 3′ or 5′ immediately opposite to 8-oxoG on the labelled strand (Fig. 1A). In contrast, DHT situated 1, 3 or 5 bases 5′ or 3′ opposite to the 8-oxoG of interest on the complementary strand only slightly, if at all, modifies the extent of excision of 8-oxoG on strand Y, seen as a small increase of 1.6 in the efficiency of excision of 8-oxoG by yOGG1 (Fig. 1B). It is worth noting that the DHT-modified base is not recognised by yOGG1 (data not shown and 24). The presence of a uracil 1–5 bases 5′ or 3′ opposite 8-oxoG does not influence cleavage of DNA at the 8-oxoG position by yOGG1 (Fig. 1C).
Figure 1.
Effect of the presence of another opposite base damage on the excision efficiency of 8-oxoG by yOGG1. The oligonucleotide containing an 8-oxoG was labelled and hybridised to a complementary strand containing either the complementary base (control) or another 8-oxoG (A), DHT (B) or uracil (C) at various positions opposite the 8-oxoG on the labelled strand (see Table 1). Double-stranded oligonucleotides were treated with yOGG1 (100–500 pg). The fold inhibition/activation was obtained from comparison with the control without damage on the non-labelled strand. The error bars represent the standard deviation from three different experiments.
Effect of a vicinal AP site on the excision of 8-oxoG by yOGG1
To create an AP site opposite adenine at the positions defined, the oligonucleotides containing a single uracil at the various positions were treated with UDG. The efficiency of uracil removal was verified by piperidine treatment that chemically cleaves the AP site to give a SSB. Oligonucleotides containing an AP site 5, 3 or 1 base 3′ or 5′ opposite 8-oxoG on the labelled strand were tested for the ability of yOGG1 (Fig. 2A) to remove the 8-oxoG lesion. The major effect is inhibition of excision of 8-oxoG, especially when an AP site is situated 1 base 3′ opposite the 8-oxoG (position +1), with a 6.2-fold inhibition (Fig. 2A). An AP site at any of the other positions does not have a significant effect on the excision of 8-oxoG by yOGG1. As shown in Figure 3, the yOGG1 protein excises 8-oxoG 5-fold more efficiently than an AP site from DNA containing these single damages. Therefore, the vicinal AP site probably remains intact when the 8-oxoG is excised from the clustered damage described above. However, the following experiments were undertaken to address this point.
Figure 2.
Effect of the presence of an opposite AP site or reduced AP site on the efficiency of excision of 8-oxoG by yOGG1. The oligonucleotide containing an 8-oxoG was labelled and hybridised to a complementary strand containing either the complementary base (control) or another uracil opposite the positions between 5 bases 5′ (–5) and 5 bases 3′ (+5) from the 8-oxoG on the labelled strand (see Table 1). Double-stranded oligonucleotides were treated with UDG to create an AP site at the various uracil positions before (A) or after (B) treatment with NaBH4 to reduce the AP site. The oligonucleotides were then subjected to excision of 8-oxoG using yOGG1 (100–500 pg). The fold inhibition/activation was obtained from comparison with the control containing no other damage on the non-labelled strand. The error bars represent the standard deviation from three to five different experiments.
Figure 3.
Excision efficiency of 8-oxoG versus AP site by yOGG1. 8-oxoG (filled triangles) or AP site (open circles) containing oligonucleotides were treated with increasing amounts of yOGG1 protein (0.5–60 nM) for 30 min at 37°C. The percentage of excision was measured after migration on a 12% denaturing polyacrylamide gel.
Effect of a reduced AP site or of a proximal SSB on the excision of 8-oxoG
To elucidate whether the inhibition of excision of 8-oxoG within a clustered damage containing an AP site reflects the presence of an AP site or its conversion into a SSB by the AP lyase activity of yOGG1, we used oligonucleotides that contain a reduced AP site (refractory to excision by AP lyase proteins) or an AP site converted into a SSB with different end-group termini (see Fig. 4 for structures).
The yOGG1 protein is unable to cleave the strand containing a reduced AP site confirming that the AP site is fully reduced (data not shown). As shown in Figure 2B, there is no difference between the effect of a reduced AP site or a normal AP site (Fig. 2A) on the removal of 8-oxoG by yOGG1.
AP sites were converted into SSB by incision with either Nth or AP endonuclease to create either a sugar remnant with a 3′-phosphoraldehyde terminus and a 5′-phosphate terminus or 3′-hydroxy and 5′-phosphate termini, respectively. We initially focused on the effect of a β-SSB, created by the β lyase action of Nth on an AP site on the complementary strand (see Materials and Methods), on the excision of 8-oxoG. The rate of incision of an AP site by Nth is not affected by the presence of an opposite 8-oxoG at any of the positions tested (10). This β-SSB mimics the cleavage of an AP site by the AP lyase activity of yOGG1. A β-SSB present at a given position in DNA has an inhibitory effect on the excision of 8-oxoG by yOGG1, with the maximum inhibitory effect occurring when the β-SSB is at position +1 (Fig. 4A). A β-SSB 1 base 5′ or 3 bases 3′ to 8-oxoG on the complementary strand also decreases the extent of excision of 8-oxo-G by yOGG1, whereas an AP site at the equivalent positions has no effect (Fig. 2A). It is inferred that the inhibitory effect of a β-SSB extends over a larger inter-lesion distance relative to the position of 8-oxoG, compared with that of an AP site.
A HAP1-SSB was obtained by treatment of oligonucleotides containing an AP site with HAP1. Such a HAP1-SSB gives the same profile of inhibition on the excision of 8-oxoG by yOGG1 (Fig. 4B) as that for a β-SSB (Fig. 4A). However, a larger inhibitory effect was seen when an HAP1-SSB is at positions +1, +3 and –3 compared with that seen with a β-SSB at the equivalent positions.
A β–δ-SSB was obtained using various oligonucleotides (see Materials and Methods) to give 5′- and 3′-phosphate strand break termini with loss of a nucleotide, equivalent to that obtained after β–δ elimination of an AP site or by ionising radiation. As presented in Figure 4C, a β–δ-SSB at positions +1 and –1 opposite 8-oxoG has the largest inhibitory effect (23.2- and 15.4-fold, respectively) on removal of 8-oxoG for all the positions tested. This inhibitory effect on excision of 8-oxoG by yOGG1 is larger than that seen with an AP site, normal or reduced, a β-SSB or a HAP1-SSB (Figs 2A and B, 4A and B, respectively). This inhibition by a β–δ-SSB also extends over a greater inter-lesion distance, with at least a 2-fold inhibition observed from positions –5 to +3. This inhibitory effect of a β–δ-SSB on the removal of 8-oxoG by yOGG1 is comparable with that of an AP site on the excision of 8-oxoG by the bacterial Fpg protein (M.-H.David-Cordonnier, J.Laval and P.O’Neill, unpublished data).
Impact of excision of 8-oxoG within clustered damage on DSB formation
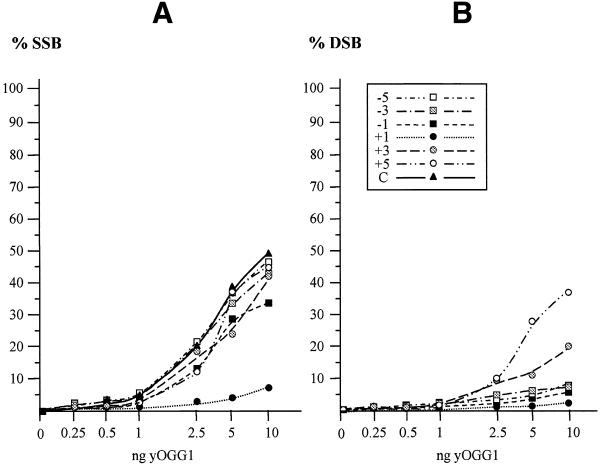
The efficiency of SSB formation resulting from removal of 8-oxoG opposite to another damage was compared with that for formation of a DSB. As shown in Figure 5, the dependence obtained for the formation of a DSB (Fig. 5B) by yOGG1, using an oligonucleotide containing a β–δ-SSB opposite 8-oxoG, is very similar to that for formation of a SSB on the 8-oxoG-containing strand (Fig. 5A). In contrast, the formation of a DSB is significantly less efficient than the formation of a SSB, produced by release of 8-oxoG by yOGG1 from an oligonucleotide containing an AP site opposite 8-oxoG (Fig. 6). This difference probably reflects the reduced efficiency for removal of an AP site in comparison with that for removal of 8-oxoG by yOGG1, as shown in Figure 3.
Figure 5.
Comparison of SSB and DSB formation for a β–δ-SSB opposite 8-oxoG. Increasing amounts of OGG1 (0.5–10 ng) were incubated with an oligonucleotide containing an 8-oxoG on the labelled strand and a β–δ-SSB with 3′P and 5′P termini at various positions on the opposite strand. The percentage of SSBs (A) and DSBs (B) induced by varying amounts of yOGG1 is shown. The curves –5 to +5 represent the relative positions of the β–δ-SSB from 5 bases 5′ to 5 bases 3′ to the 8-oxoG as described in Table 1; curve C represents the control DNA containing 8-oxoG opposite the normal complementary strand.
Figure 6.
Comparison of SSB and DSB formation by yOGG1 using oligonucleotides containing an AP site opposite to 8-oxoG. Increasing amounts of yOGG1 (0.5–10 ng) were incubated with an oligonucleotide containing an 8-oxoG on the labelled strand and an AP site at various positions on the opposite strand. The reaction conditions were as described in Figure 2A; half of the sample migrated on a 12% denaturing polyacrylamide gel to measure the percentage of SSBs (A) and the other half separated on a 10% native polyacrylamide gel to determine the level of DSBs induces by yOGG1 (B). The curves –5 to +5 represent the different positions of the β–δ-SSB relative to 8-oxoG as described in Table 1, curve C represents the control DNA containing 8-oxoG opposite the normal complementary strand.
Effect of an opposite 8-oxoG on the excision of an AP site by yOGG1
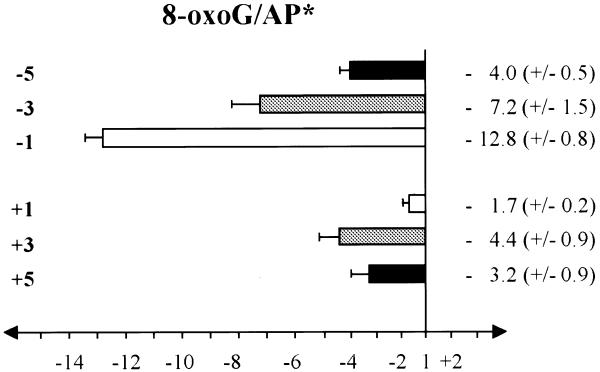
The effect of an 8-oxoG on the opposite strand on the AP lyase activity of yOGG1 is presented in Figure 7 using the same oligonucleotides as for Figure 2A but labelling the uracil-containing strand (see Table 1) before hybridisation to the 8-oxoG-containing non-labelled complementary strand. Higher amounts of protein were used than in the previous experiments (2.5–10 ng) due to the efficiency of excision of an AP site by yOGG1 being less than that for 8-oxoG (Fig. 3). The presence of an 8-oxoG at all the tested positions has an inhibitory effect on the excision of an AP site on the labelled strand. The inter-lesion distance over which the excision of the AP site by yOGG1 is affected is larger than that seen for excision of 8-oxoG within the same series of clustered damages (Fig. 2A) or in the vicinity of a variety of SSBs (Fig. 4). The maximum effect is at position –1. It is worth noting that the effect is very low when an 8-oxoG is at position +1 opposite to the AP site of interest (1.7-fold inhibition, Fig. 7).
Figure 7.
Effect of the presence of an opposite 8-oxoG on the efficiency of excision of an AP site (asterisk denotes that the AP site is on the labelled strand) by the AP lyase activity of the yOGG1 protein. The oligonucleotides containing a uracil on strand 1 at the various positions from –5 to +5 were 5′-end-labelled and hybridised to the complementary strand containing either the complementary base (used as control) or an 8-oxoG at the fixed base on strand 2 (see Table 1). Double-stranded oligonucleotides were treated with UDG to create an AP site at the various uracil positions prior to incubation with yOGG1 (100–500 pg). The fold inhibition/activation was obtained from comparison with the control containing no other damage on the non-labelled strand. The error bars represent the standard deviation from three to five different experiments.
DISCUSSION
In the present study, purified yOGG1 protein was used to determine the effect of various neighbouring DNA damages on the excision of 8-oxoG, a frequently-occurring and highly mutagenic base lesion, to gain insight into the DNA recognition/repairability of clustered DNA damage. The presence of an opposite base damage only slightly modifies the excision efficiency of 8-oxoG by yOGG1 (8-oxoG, DHT), if at all (uracil) (Fig. 1). The presence of a vicinal base lesion was previously reported to have only a slight influence on the excision of DHT by the bacterial base excision repair enzymes Nth (8,10) or Fpg and XRS5 nuclear proteins (10). In contrast, the presence of an AP site at position +1 opposite 8-oxoG has a larger inhibitory effect on 8-oxoG excision by yOGG1 (Fig. 2A). Therefore, provided that both strands of DNA remain intact, the inhibitory effect of a second lesion on excision of 8-oxoG is low except for nearest neighbours. Reduction of the AP site with sodium borohydride (28) results in inhibited excision of the AP site by β-elimination with yOGG1. The profile of inhibition of excision of 8-oxoG by yOGG1 in the presence of a reduced AP site (Fig. 2B) is similar to that for excision of 8-oxoG within cluster damage by the bacterial Fpg protein (M.-H.David-Cordonnier, J.Laval and P.O’Neill, unpublished data). If the base pair distance from 8-oxoG to the second lesion over which the inhibition of excision of 8-oxoG extends is defined as n, then n = 1 in the above situations.
This study focuses more precisely on assessing the way in which the presence of an opposite AP site or the resulting strand breaks induces a large inhibitory effect on the excision of the highly mutagenic 8-oxoG lesion. To confirm that a SSB has an inhibitory effect, even when positioned several base pairs away from 8-oxoG, a variety of oligonucleotide-containing SSBs with different end-group termini were prepared. SSBs are induced in DNA by reactive oxygen species and ionising radiation and represent a loss of a nucleotide where the strand break termini are a 5′-phosphate and either a 3′-phosphate or a 3′-phosphoglycolate. The β-SSB or β–δ-SSB-containing oligonucleotides mimic the type of SSB produced by ionising radiation as a single lesion or within clustered damage and the HAP1-SSB represents the major damage from treatment of an AP site by HAP-1, abundant in cells. It is clear that a β–δ-SSB is much more efficient than a HAP1-SSB in inhibiting the excision of 8-oxoG by yOGG1. A further important finding is the major inhibitory effect on the excision of 8-oxoG when a β–δ-SSB is one base 3′ or 5′ opposite to 8-oxoG (15- and 23-fold inhibition, respectively) and to a lesser extent at the other positions tested (1.7- to 4.3-fold inhibition, Fig. 4C). This inhibitory effect of SSBs on excision of 8-oxoG extends several base pairs away from the 8-oxoG lesion and may be very important in minimising the formation of DSBs in cells when processing clustered DNA damage containing base modifications (Figs 5 and 6). Several studies have shown that a DSB is formed when there are two SSBs, one on each strand, with n = 3–6, at a salt concentration relative to the ionic strength in the nucleus (29,30). From this finding, together with that from Figure 2A, it is suggested that, for an AP site 1–5 bases 5′ or 3–5 bases 3′ opposite 8-oxoG, the excision of 8-oxoG is initially favoured followed by the incision of the AP site. It is possible that the relative delay in the incision of an AP site by yOGG1 may permit the processing of 8-oxoG prior to incision at the AP site, thus minimising the formation of a DSB (as visualised in Fig. 6B). In the particular case of an 8-oxoG at position +1 opposite an AP site, the rate of excision of 8-oxoG is significantly reduced whereas the rate of incision of the AP site by yOGG1 is only slightly affected by the presence of 8-oxoG. It is inferred that the formation of a DSB may therefore be avoided (Fig. 6B) and possibly favour the incision of an AP site in cells by AP endonuclease, followed by repair prior to excision of the second damage (8-oxoG).
Even though Fpg and OGG1 are not homologous, the effect of a base damage or a SSB on excision of 8-oxoG is similar. The extent of the inhibitory effect of a β–δ-SSB on excision of 8-oxoG seen in the presence of Fpg (M.-H.David-Cordonnier, J.Laval and P.O’Neill, unpublished data) is very similar to that observed in the presence of yOGG1. It is suggested that an evolutionary reproduction of the same processes occurred in bacteria and in eukaryotic cells for the excision of 8-oxoG within clustered damage by the Fpg protein and the yOGG1 protein. These studies provide insights into the sequence of events that may occur during the processing of a clustered DNA damage especially if DSB formation is to be minimised.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Profs Rick Wood (Imperial Cancer Research Fund) and Ian Hickson (Institute of Molecular Medicine) for providing the purified Nth and HAP1 proteins, respectively.
References
- 1.Goodhead D.T., Munson,R.J., Thacker,J. and Cox,R. (1980) Mutation and inactivation of cultured mammalian cells exposed to beams of accelerated heavy ions. Int. J. Radiat. Biol., 37, 135–137. [DOI] [PubMed] [Google Scholar]
- 2.Ward J.F. (1985) Biochemistry of DNA lesions. Radiat. Res., 104, S103–S111. [PubMed] [Google Scholar]
- 3.Goodhead D.T. (1994) Initial events in the cellular effect of the ionizing radiations: clustered damage in DNA. Int. J. Radiat. Biol., 65, 7–17. [DOI] [PubMed] [Google Scholar]
- 4.Nikjoo H., O’Neill,P., Terrissol,M. and Goodhead,D.T. (1999) Quantitative modelling of DNA damage using Monte Carlo track structure method. Radiat. Environ. Biophys., 38, 31–38. [DOI] [PubMed] [Google Scholar]
- 5.Cunniffe S. and O’Neill,P. (1999) The complexity of radiation-induced DNA damage as revealed by exposure to cell extracts. Radiat. Res., 152, 421–427. [PubMed] [Google Scholar]
- 6. BEIR VI, Committee on health risks of exposure to radon (1999) Health Effects of Exposure to Radon. National Academy Press, Washington, DC.
- 7.Chaudhry M.A. and Weinfeld,M. (1995) The action of Escherichia coli endonuclease III on multiply damaged sites in DNA. J. Mol. Biol., 249, 914–922. [DOI] [PubMed] [Google Scholar]
- 8.Harrison L., Hatahet,Z., Purmal,A.A. and Wallace,S.S. (1998) Multiple damaged sites in DNA: interaction with Escherichia coli endonucleases III and VIII. Nucleic Acids Res., 26, 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison L., Hatahet,Z. and Wallace,S.S. (1999) In vitro repair of synthetic ionizing radiation-induced multiple damaged DNA sites. J. Mol. Biol., 290, 667–684. [DOI] [PubMed] [Google Scholar]
- 10.David-Cordonnier M.H., Laval,J. and O’Neill,P. (2000) Clustered DNA damage: influence on damage excision by XRS5 nuclear extracts and Escherichia coli Nth and Fpg proteins. J. Biol. Chem., 275, 11865–11873. [DOI] [PubMed] [Google Scholar]
- 11.Kasai H., Crain,P.F., Kuchino,Y., Nishimura,S., Ootsuyama,A. and Tanooka,H. (1986) Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis, 7, 1849–1851. [DOI] [PubMed] [Google Scholar]
- 12.Nash H.M., Bruner,S.D., Scharer,O.D., Addona,T.A., Spooner,E., Lane,W.S. and Verdine,G.L. (1996) Cloning of a yeast 8-oxoguanine DNA glycosylase reveals the existence of a base-excision DNA-repair protein superfamily. Curr. Biol., 6, 968–980. [DOI] [PubMed] [Google Scholar]
- 13.Radicella J.P., Dherin,C., Desmaze,C., Fox,M.S. and Boiteux,S. (1997) Cloning and characterization of hOGG1, a human homolog of the OGG1 gene of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 94, 8010–8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenquist T.A., Zharkov,D.O. and Grollman,A.P. (1997) Cloning and characterization of a mammalian 8-oxoguanine DNA glycosylase. Proc. Natl Acad. Sci. USA, 94, 7429–7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjoras M., Luna,L., Johnsen,B., Hoff,E., Haug,T., Rognes,T. and Seeberg,E. (1997) Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J., 16, 6314–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aburatani H., Hippo,Y., Ishida,T., Takashima,R., Matsuba,C., Kodama,T., Takao,M., Yasui,A., Yamamoto,K. and Asano,M. (1997) Cloning and characterization of mammalian 8-hydroxyguanine-specific DNA glycosylase/apurinic, apyrimidinic lyase, a functional mutM homologue. Cancer Res., 57, 2151–2156. [PubMed] [Google Scholar]
- 17.Grollman A.P. and Moriya,M. (1993) Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet., 9, 246–249. [DOI] [PubMed] [Google Scholar]
- 18.van der Kemp P.A., Thomas,D., Barbey,R., de Oliveira,R. and Boiteux,S. (1996) Cloning and expression in Escherichia coli of the OGG1 gene of Saccharomyces cerevisiae, which codes for a DNA glycosylase that excises 7,8-dihydro-8-oxoguanine and 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine. Proc. Natl Acad. Sci. USA, 93, 5197–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laval J., Jurado,J., Saparbaev,M. and Sodorkina,O. (1998) Antimutagenic role of base-excision repair enzymes upon free radical-induced DNA damage. Mutat. Res., 402, 93–102. [DOI] [PubMed] [Google Scholar]
- 20.Boiteux S. (1993) Properties and biological functions of the NTH and FPG proteins of Escherichia coli: two DNA glycosylases that repair oxidative damage in DNA. J. Photochem. Photobiol. B, 19, 87–96. [DOI] [PubMed] [Google Scholar]
- 21.Demple B. and Harrison,L. (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem., 63, 915–948. [DOI] [PubMed] [Google Scholar]
- 22.Girard P.-M., Guibourt,N. and Boiteux,S. (1997) The Ogg1 protein of Saccharomyces cerevisiae: a 7,8-dihydro-8-oxoguanine DNA glycosylase/AP lyase whose lysine 241 is a critical residue for catalytic activity. Nucleic Acids Res., 25, 3204–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girard P.-M., D’Ham,C., Cadet,J. and Boiteux,S. (1998) Opposite base-dependent excision of 7,8-dihydro-8-oxoadenine by the Ogg1 protein in Sacchromyces cerevisiae. Carcinogenesis, 19, 1299–1305. [DOI] [PubMed] [Google Scholar]
- 24.Dherin C., Radicella,J.P., Dizdaroglu,M. and Boiteux,S. (1999) Excision of oxidatively damaged DNA bases by the human alpha-hOgg1 protein and the polymorphic alpha-hOgg1(Ser326Cys) protein which is frequently found in human populations. Nucleic Acids Res., 27, 4001–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazra T.K., Izumi,T., Maidt,L., Floyd,R.A. and Mitra,S. (1998) The presence of two distinct 8-oxoguanine repair enzymes in the human cells: their potential complemantary roles in preventing mutation. Nucleic Acids Res., 26, 5116–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asahara H., Wistort,P.M., Bank,J.F., Bakerian,R.H. and Cunningham,R.P. (1989) Purification and characterization of Escherichia coli endonuclease III from the cloned nth gene. Biochemistry, 28, 4444–4449. [DOI] [PubMed] [Google Scholar]
- 27.Barzilay G. and Hickson,I.D. (1995) Structure and function of apurinic/apyrimidic endonucleases. Bioessays, 17, 713–719. [DOI] [PubMed] [Google Scholar]
- 28.Castaing B., Boiteux,S. and Zelwer,C. (1992) DNA containing a chemically reduced apurinic site is a high affinity ligand for the E.coli formamidopyrimidine-DNA glycosylase. Nucleic Acids Res., 20, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Sonntag C. (1987) The Chemical Basis of Radiation Biology. Taylor and Francis, Basingstoke, UK.
- 30.Hanai R., Yazu,M. and Heida,K. (1998) On the experimental distinction between ssbs and dsbs in circular DNA. Int. J. Radiat. Biol., 73, 475–479. [DOI] [PubMed] [Google Scholar]