Abstract
Hypertension represents a complex, multifactorial disease and contributes to the major causes of morbidity and mortality in industrialized countries: ischemic and hypertensive heart disease, stroke, peripheral atherosclerosis and renal failure. Current pharmacological therapy of essential hypertension focuses on the regulation of vascular resistance by inhibition of hormones such as catecholamines and angiotensin II, blocking them from receptor activation. Interaction of G-protein coupled receptor kinases (GRKs) and Regulator of G-Protein Signaling (RGS) proteins with activated G-protein coupled receptors (GPCRs) effect the phosphorylation state of the receptor leading to desensitization and can profoundly impair signalling. Defects in GPCR regulation via these modulators have severe consequences affecting GPCR-stimulated biological responses in pathological situations such as hypertension, since they fine-tune and balance the major transmitters of vessel constriction versus dilatation, thus representing valuable new targets for anti-hypertensive therapeutic strategies. Elevated levels of GRKs are associated with human hypertensive disease and are relevant modulators of blood pressure in animal models of hypertension. This implies therapeutic perspective in a disease that has a prevalence of 65 million in the United States while being directly correlated with occurrence of major adverse cardiac and vascular events. Therefore, therapeutic approaches using the inhibition of GRKs to regulate GPCRs are intriguing novel targets for treatment of hypertension and heart failure.
Introduction
G-Protein coupled receptor (GPCR) kinases (GRKs) regulate vital processes by controlling expression and function of seven-transmembrane receptors such as adrenergic and angiotensin receptors [1]. These are particularly important in widespread cardiovascular disease resulting from hypertension, where the degree of constriction of blood vessels is generated in part by elevated levels of agonists such as catecholamines and angiotensin II [2]. Vascular smooth muscle (VSM) is a key player contributing to the regulation of blood pressure by controlling the diameter of blood vessels and thereby modulating peripheral resistance. Because of the exponential relationship between diameter and resistance (resistance α1/r4), contraction of VSM has the potential to dramatically increase blood pressure. The hypercontractile state of VSM is one hallmark of essential hypertension. In turn, elevated blood pressure requires the heart to adapt to accommodate higher systolic loads which ultimately leads to ventricular hypertrophy and reduced myocardial compliance [3, 4].
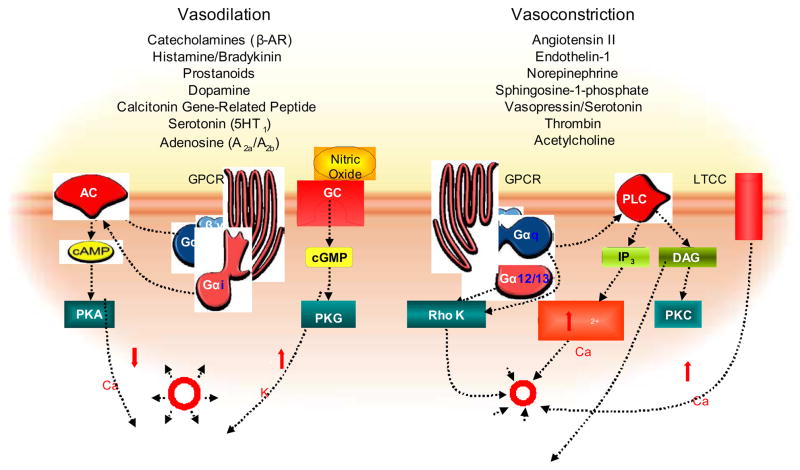
Since GPCRs expressed on VSM and endothelial cells are responsible for maintaining the balance between constriction and relaxation of vessels, their modulation has been a primary target of therapeutic advances. Agonists binding to a GPCR trigger the activation of heterotrimeric G-proteins that transduce the signal to intracellular effector molecules [5]. Upon activation, the heterotrimeric G protein complex disassembles into Gα and Gβγ subunits. There are four main classes of heterotrimeric G proteins, described based on the Gα component, and activation of different Gα subtypes lead to diverse intracellular signalling cascades eliciting contractile response by (i) activation of adenylyl cyclase (Gαs), (ii) phospholipase C initiating Ca2+ release from intracellular stores (Gαq/11), (iii) inhibition of adenylyl cyclase (Gαi), or (iv) Rho kinase (Gα12/13 and Gαq/11), which inhibits the myosin light chain phosphatase (MLCP), finally activating the myosin light chain (MLC) [6–8] and thereby initiating vasoconstriction. This can lead to complex and conflicting responses in the vasculature where catecholamines for instance are acting as vasoconstricting agonists when binding to α-adrenergic receptors in peripheral arteries but can likewise lead to vasodilation when binding to β-adrenergic receptors activating the Gαs subunit that stimulates cAMP formation and smooth muscle relaxation (Fig.1).
Figure 1.
Overview of GPCR signalling cascade resulting in vasodilation or vasoconstriction: Within the present review we have outlined activation of GPCRs via a wide range of agonists, leading to activation of adenylyl cyclase (AC) via Gαs as well as guanylate cyclase (GC), increased 2nd messenger concentrations finally leading to protein kinase A or G-mediated vasodilation. Vasoconstrictory agents lead to activation of Gq and G12/13, which mediates smooth muscle con-traction via Protein Kinase C (PKC) or Rho Kinase as well as increased Ca2+, leading to inactivation of the myosin- light-chain kinase (MLCK) by dephosphorylation.
GPCRs are tightly regulated by a family of kinases, GRKs, that upon activation phosphorylate the receptor, thereby increasing its affinity for the binding of an arrestin, which further prevents coupling of that receptor to its Gα-protein [9]. This process is termed desensitization and can occur as an immediate response to agonist binding and with persistent stimulation can result in receptor internalization and down-regulation thus attenuating signaling [10]. It is believed to protect the receptive cell from overstimulation but desensitization can be harmful in pathological conditions such as heart failure, where desensitization of the β-adrenergic receptor system marks the decline of ventricular output.
GRKs form a family of seven mammalian members (GRK1-GRK7), that share a similar basic structure with an N-terminus that bears an RGS domain, a catalytic and a C-terminal domain [11]. Distinguishing variability occurs at the carboxy-terminal sequence [12]. GRKs can be subdivided into three types: The GRK1-like retinal opsin kinases, consisting of GRK1 and GRK7, known to be involved in the pathophysiology of deleterious rhodopsin mutations that underlie several inherited retinal disorders [10]. GRK2-like-enzymes, including GRK2 and GRK3, act on a wide range of GPCRs and were first characterized in studies of the phosphorylation of agonist-occupied β2-adrenergic receptors, where GRK2 can contribute to diseases such as heart failure [13]. Kinases of this subtype share a carboxyl-terminal pleckstrin homology (PH) domain, that mediates phosphoinositol-4,5-bisphosphonate (PIP2) and G-Protein βγ-subunit specific regulation. Lastly, the GRK4-like subfamily consists of GRK4, GRK5 and GRK6, which in contrast to the other subtypes, selectively phosphorylate basic terminal amino acid residues. GRK4 has been found to play an important role in regulating the Dopamine D1 receptor within the renal proximal tubule, thus controlling blood pressure [14]. Polymorphisms in the GRK4 locus can be linked to essential hypertension in patients who have decreased D1 signaling and decreased adenylyl cyclase activity. The distribution of 3 of the GRKs is limited. GRK1/GRK7 are mainly in the ocular system whereas GRK4 is limited to the proximal tubule of the kidney and testis. This suggests these GRKs may be successful therapeutic targets because of their limited distribution and thus low potential for side-effects. However, most of the over 1000 GPCRs of the body seem to be regulated by only the four widely expressed isoforms: GRK2, GRK3, GRK5 and GRK6, and their downstream arrestins, Arrestin-2 and Arrestin-3 (β-Arrestin-1 and β-Arrestin-2, respectively).
Studies of GRKs in cardiovascular pathophysiology have mostly been performed in cellular and animal models of heart disease, where increased GRK levels are associated with hypertrophy and the progression of heart failure [15, 16]. This review will provide an overview of the GPCR-mediated signaling regulation via GRKs as well as RGS-proteins, which share homologous regions with some GRKs, in development and maintenance of high blood pressure. We will focus on the pathophysiological role of GRKs taking into account recent studies in genetically modified animals as well as a view of involvement and regulation of GRKs in human cardiovascular disease. In the current review, we focus on GRK2 and GRK5, as our focus is the role VSM plays in blood pressure regulation. Another critical regulator of renal GPCRs in hypertension, GRK4, is reviewed elsewhere (please refer to review by PA Jose in this issue).
Adenylyl Cyclase and Phospholipase C in Hypertension
Many of the earliest and consistent findings support the assumption that hypertension is due in part to dysregulation and desensitization of vasodilatory receptors. Mainly Gs-coupled receptors such as β-adrenergic, histamine and dopamine D1-receptors impart vasodilation and their alteration ultimately leads to decreased adenylyl cyclase activity and cAMP formation. This was shown to impair VSM relaxation and linked to hypertension in animal models such as the spontaneously hypertensive heart failure (SHHF) rat [17]. Activity of adenylyl cyclase has additionally been shown to be reduced in various animal models of induced hypertension in response to Gs-coupled receptor agonists [18]. In humans, isoproterenol-stimulated lymphocytes, isolated from borderline hypertensive subjects, were less responsive and showed decreased adenylyl cyclase activity compared to healthy subjects [19]. Interestingly, Gi, a major inhibitor of adenylyl cyclase is also upregulated in VSM and myocardium of hypertensive subjects in animal models as well as humans. Therefore, altered adenylyl cyclase activity may be involved in the development and maintenance of high blood pressure [20]. The potential therapeutic use of this knowledge and further studies is underlined by the emerging novel compound selectively inhibiting specific adenylyl cyclase isoforms in the heart, brain and possibly also the vasculature [21].
The role of phospholipase C (PLC) in hypertension is reciprocal to the one of adenylyl cyclase in that it is a mediator of pathways transmitting vasoconstrictive signals. Downstream of PLC, increased protein kinase C (PKC) and Ca2+ levels increase myosin light chain kinase (MLCK) activity, which is balanced by phosphorylated MLC, thus controlling constriction of smooth muscle in resistance vessels. In hypertensive rats, protein levels of both PLC and PKC isoforms are upregulated, suggesting augmented signaling via this pathway through GPCRs, perhaps additionally responsible for elevated blood pressure. Whether certain Gq-coupled constricting GPCRs are not a target of GRKs or whether GRK phosphorylation of these receptors promotes alternative signaling pathways remains to be determined.
Additionally, signaling through constrictive GPCRs such as the angiotensin II receptor also activates RhoA Kinase (ROCK) through Gα12/13,[22, 23] as well as Gαq/11 [24]via RhoGEFs (RhoGTPase nucleotide exchange factors). RhoA as well as ROCK are increased in animal models of hypertension, but also have a role in vascular remodeling and smooth muscle cell proliferation [25] Selective inhibition of ROCKs can normalize blood pressure as a result of ameliorated vascular hyper-sensitivity [26]. How this pathway may be regulated by GRKs warrants further investigation.
Ion Channels and Hypertension
GPCR activation and desensitization via GRKs affect the balance of ion channel activation in VSM. Vasoconstriction, in general, is mediated by intracellular Ca2+, governing the contractile force and maintenance of smooth muscle contraction. The open-probability of the L-type calcium channel (LTCC), Ca2+ influx and therefore the intracellular Ca2+ concentration is influenced by GPCR signaling. Angiotensin II, for instance, mediates vasoconstriction by increasing intracellular Ca2+ by activation of IP3-sensitive Ca2+ stores. Furthermore, inhibition of voltage gated K+ channels by PKC leads to decreased K+ efflux maintaining Ca2+ in the cell. GRKs regulate the GPCR signaling, therefore the interplay between ion channels and GPCRs, as well as the GRKs regulating them, is a critically important mechanism of hypertension.
In hypertension, defective relaxation induced by ion channels may be important to high blood pressure development. The hypertensive phenotype of the spontaneously hypertensive rat (SHR) is characterized by a down-regulation of the large-conductance Ca2+-activated K+ channel (BKCa2+) [27], although other groups have challenged this notion by showing increased presence of the BKCa2+ in the vessel walls of SHHF rats [28]. Angiotensin induced hypertension leads to decreased expression of the BKCa2+ channel and this was attributed to a nuclear factor of activated T-cells (NFAT)/Calcineurin dependent pathway [29, 30]. Most recent data indicate that PKC phosphorylation inhibits BKCa2+ channels in vascular smooth muscle, whereas BKCa2+ channels are activated by vasodilators coupled to PKA as shown in studies using β-adrenergic receptor agonists [31].
Regarding Ca2+ channels, expression has not been shown to change in hypertension, but one study found increased blood pressure to be the reason for elevated levels of pore forming subunit α1C subunit in the vessels of SHHF rats [32]. Taken together these results indicate that high blood pressure itself and the agonist activated GPCRs underlying it may alter ion channel expression and activity, thus constituting an input for blood pressure regulation. Whether there is a role of GRKs on ion channel function remains to be determined but it is intriguing to hypothesize that GRKs may have some direct effect.
The Role of GRKs in Hypertension
Blood pressure is regulated by alterations in heart rate, vascular resistance and fluid and electrolyte balance, all of which are GPCR-regulated and therefore largely subject to differential regulation by GRKs.
GRK2
GRK2 synthesis and degradation
GRK mRNA levels are regulated in a variety of disease states and during differentiation [33]., Vascular tone can act as a promoter of aorta smooth muscle GRK2 expression in a Gαq dependent manner [34]. Vasoconstriction led to enhanced GRK2-promotor activity whereas dilatory stimuli and cytokines inhibited it. This effect was cell-type specific as it could not be found in non-cardiovascular cell lines such as epithelial (HEK293) cells [34]. Another group found that activation of MAP Kinases (ERK1/2) increased endogenous GRK2 protein levels in primary cardiomyocyte cultures [35, 36]. In cardiovascular disease, GRK2 mRNA levels are upregulated [1] in correlation with increased catecholamine levels and β-adrenergic receptor activity [37], which suggests a reciprocal regulation that may explain fundamental alterations of GPCR signaling in many pathophysiological conditions.
Degradation of GRK2 occurs via the proteosome [33] in a process depending on its own activity, as confirmed by the slower degradation of an enzymatic-activity-deficient mutant (GRK2 K220). β-adrenergic receptor stimulation accelerates the ubiquitination and degradation of GRK2 in a feedback loop. Benovic and colleagues have found that heat-shock protein Hsp90 has an important role in maturation of GRKs where interaction between Hsp90 and GRKs may prevent GRKs from being degraded by stabilizing the correct folding of the protein [38]. Furthermore, oxidative stress, which is often increased during disease states, induces degradation of GRK2, at least in lymphocytes, possibly via tyrosine phosphorylation [39]. These studies suggest that GRK2 is intricately regulated at multiple levels through different signaling events which makes it even more interesting to determine whether changes in GRK levels concomitant with disease states are a cause or consequence and the impact changes in expression of these kinases have on GPCR signaling.
Upregulation of GRK2 in humans with hypertension and in animal models of the disease
Evidence is accumulating to suggest a role of GRK2 in hypertension. GRK2 is increased in lymphocytes derived from young hypertensive patients as well as in the VSM of animal models of hypertension [40, 41]. Increased GRK2 expression also correlated with decreased activity of adenylyl cyclase downstream of isoproterenol-stimulated β-adrenergic receptors [42]. We have recently described in a larger cohort of black American adults that lymphocyte GRK2, but not GRK5, mRNA expression and activity directly correlates with systolic blood pressure [43]. Norepinephrine levels were also increased in these subjects therefore, we proposed that a greater sympathetic nervous system activation resulting in exaggerated βAR signaling and subsequent GRK2-mediated desensitization of the receptors may represent a potential mechanism. It also suggests that GRK2 levels might be able to act as a surrogate measure of catecholamine levels and catecholamine-mediated stimulation of GPCRs. Other neurohormonal agonists are also regulated with increased blood pressure and it is possible that these ligands, such as endothelin, also elevated in hypertensive black Americans, contribute [44, 45]. endothelin-A-receptors, predominantly mediating potent vasoconstriction effects of endothelin-1 in VSM, were recently found to be desensitized only by GRK2, and not GRK3, 5 or 6 [46]. These findings would suggest a protective role of GRK2, preventing pro-hypertensive signals from prevailing and pointing towards GRK2 elevation in hypertensive subjects as an adaptive response. It also illustrates the complexity of signaling in that GRK2 may have influence over inputs mediating either dilation and/or constriction and a need to further understand the in vivo substrates for these kinases and the full impact expression changes have on cardiovascular signaling. At this point is should also be mentioned that there is a putative role for the RH domain of the n-terminal region of GRK2 acting as a regulator of G-protein function, namely as inhibitor of Gαq [47–49], in a phosphorylation-independent manner, implying an additional mechanism for GRK2-mediated inhibition of GPCR signaling to be further investigated with respect to hypertensive disease.
Interestingly, when we created transgenic mice directing VSM expression of GRK2 using a portion of the smooth muscle-specific SM22α-promotor, the mice had elevated resting blood pressure with subsequent vascular thickening and cardiac hypertrophy and exhibited reduced sensitivity to vascular relaxation afforded by the β-adrenergic receptor agonist isoproterenol [50]. Surprisingly, when these animals were challenged with an agonist such as angiotensin II, VSM GRK2 overexpression also reduced angiotensin II-mediated constriction and blood pressure elevation. These findings suggest that impaired β-adrenergic receptor signaling caused by desensitization and subsequent decreased vasorelaxation is likely a primary abnormality resulting from GRK2 overexpression in VSM. Why this effect outranks the equally desensitized vasoconstricting GPCR-linked signals such as angiotensin II warrants further investigation as do the mechanisms underlying elevated GRK2 levels found in humans with high blood pressure.
GRK2 regulates blood pressure by reducing nitric oxide production in endothelial cells
Increased GRK2 expression may also play a role in hypertension by reducing nitric oxide (NO) production [51, 52] and thereby deactivating one of the main vasodilatory response pathways in endothelial cells. GPCRs critically regulate endothelial function and the maintenance of vascular tone, but little is known as to the implications of GRKs. GRK2 has, in an animal model of portal hypertension, been shown to physically interact with Akt in endothelial cells, thereby inhibiting the Akt-dependent activation of endothelial nitric oxide synthetase (eNOS), and thus shift vascular tone towards constriction [52]. In rats with portal hypertension, GRK2 expression was increased and a restoration of Akt phosphorylation and eNOS activity as well as a normalization of portal blood pressure could be afforded by knock-down of GRK2 in vivo. Altered activity of adrenergic, angiotensin II and endothelin receptors all targeted by GRK2 might play an important role in the critical function of the endothelium in the pathophysiology of hypertension. This implies a beneficial effectiveness of similar drugs both in portal hypertension and heart failure. Therefore, it warrants further investigation to uncover the role of GRKs in endothelial cells and their impact on blood pressure regulation.
GRK2 overexpression in VSM leads to hypertension, vascular and cardiac hypertrophy
The hypertensive state results in a dynamic process in the structural aspects of blood vessels in an attempt to counterbalance the increased pressure on the lumen wall by increasing vascular wall thickness which thereby adds incrementally to narrowing of the lumen. We found that VSM GRK2 overexpression led to a 30% increase in vascular wall thickness. Further, we observed concomitant cardiac hypertrophy [50]. Recent studies done by our group suggest that Gq signaling may be implicated in this process and that they hypertrophy may be directly dependent on the increased blood pressure because inhibition of VSM Gq signaling normalized blood pressure and the vascular and cardiac hypertrophy that was produced by overexpression of GRK2 [53].
Further supporting our contention that the VSM-GRK2-mediated cardiac and vascular hypertrophy was blood pressure driven, mitogenic signaling and proliferation via endothelin-1, angiotensin II, thrombin and platelet-derived growth factor were significantly reduced when GRK2 was overexpressed [54]. Further, a reduction of neointimal hyperplasia in saphenous vein grafts through GRK2 overexpression in VSM cells was described suggesting that GRK2 in and of itself, at least under certain conditions, is anti-hypertrophic [55]. Whether this is the case remains to be further elucidated but again, it speaks to the complexity of a few kinases regulating a plethora of different GPCRs.
GRK3
GRK3 has a selectivity for the α1B-adrenergic receptor as well as for the thrombin receptor that GRK2 does not exhibit [56] GRK3 has been implicated in cardiac hypertrophy and a hypercontractile state through regulation of the α1-adrenergic receptor. Inhibition of GRK3 expression in the cardiac myocardium caused hypertension, most probably due to hypercontractiliy and increased cardiac output as a result of hypersensitivity to activation of the α1-adrenergic receptor [57]. Because α-adrenergic receptors are key regulators of vascular resistance, GRK3 remains an intriguing target to further investigate..
GRK5/6
Unlike the classical desensitization procured by GRK2, phosphorylation of GRK5 and GRK6 is now believed to also lead to recruitment of non-classical signaling pathways [58, 59]. GRK5/6 phosphorylation is thought to facilitate arrestin-mediated transactivation of receptor tyrosine kinases (RTKs) and subsequently activate PI3K and MAPK as well as Ca2+ accumulation [60–62]. The implications of these findings in regulation of contractile state of VSM through GRK5 remain to be determined, but it has potentially profound implications in these cells.
GRK5 is upregulated subsequent to hemodynamic stress and hypertension in animal models of angiotensin II and norepinephrine induced high blood pressure [63]. This first report determined that the actual increase of sheer stress in vascular tissue was causing elevated GRK5 levels, since continuous angiotensin II infusion in vivo at a subpressor dose failed to elicit a response. In addition, pretreatment with antihypertensive medications hydralazine or losartan were able to abolish the effect suggesting it was increased blood pressure per se that was increasing GRK5 expression. We also found that transgenic overexpression of VSM-GRK5 using a portion of the SM22α-promotor created transgenic mice that developed high blood pressure in a sex-specific way, where the increase in mean arterial pressure was approximately 45mmHg in male versus 17 mmHg in female mice [64]. Instead of the blood pressure being regulated by a decrease in β-adrenergic receptor dilation, we found VSM-GRK5 induced hypertension was mediated by Gi signaling. Inhibition of Gi signaling using pertussis toxin restored blood pressure to control values in both male and female mice. Plasma catecholamine levels were comparable and the lack of estrogen could not account for differences, since female mice that had undergone ovariectomy did not show higher increases in blood pressure, i.e. acquire the male phenotype. Furthermore, vascular reactivity showed a differential pattern with female but not male mice exhibiting exaggerated aortic constriction after angiotensin II stimulation. The GPCR targets of GRK5 warrant further investigation as the intricate mechanisms at play may provide helpful insight into sex specific efficacy of anti-hypertensive therapy.
On a subcellular level, GRK5 in contrast to GRK2, has a DNA-binding nuclear localization sequence and it has recently been described that GRK5 in a model of pressure overload induced heart failure traffics to the nucleus and is involved in the transcription of pro-hypertrophic signaling via MEF-2 and HDAC5 [13]. These data support an earlier study in spontaneously hypertensive heart failure rats, where GRK5 but not GRK2, was distributed to the nucleus in hypertrophied myocardial tissue obviously due to hypertension, suggesting a role in the regulation of hypertrophic gene transcription [65]. The reverse did not seem to be true since in our study the overexpression of VSM GRK5 leading to a hypertensive phenotype did not create myocardial hypertrophy or heart failure [64]. On the whole, our data suggest the involvement of GRK5 in development and progression of hypertension may originate from a unique signaling paradigm from GRK2, making its further study important to better understanding potential causes and consequences of high blood pressure.
RGS (Regulator of G-Protein Signaling) Proteins in Hypertension
Parts of GRKs, namely the N-terminal portion, can act as an RGS, and it has been shown that this can critically impact GPCR regulation of vessel reactivity and blood pressure therefore, a discussion of this family of proteins is included.
To date we have knowledge of about 35 RGS or RGS-like proteins that were discovered as novel molecules governing the already complex system of GPCR signal transduction and most are present in the cardiovascular system [66]. RGS proteins - so called GTPase activating proteins (GAPs) - negatively regulate GPCRs by accelerating Gα-dependent GTP hydrolysis to reconstitute the heterotrimeric G-protein complex, thereby inhibiting the agonist induced dissociation of the Gα from the Gβγ subunit and preventing the activation of downstream effectors [67]. RGS can also act by physically blocking G protein signaling by competing for a similar binding site. Three members of the RGS protein family have been implicated in the cardiovascular system, kidney and autonomous nervous system: RGS2, RGS4 and RGS5. Data suggest that these three RGSs have relevance in blood pressure maintenance in animal models as well as in human disease [68]. It remains intriguing how GRKs interact with or act as RGSs and this remains an important avenue of investigation.
RGS2
The RGS2−/− knock-out mouse is hypertensive and has altered GPCR signaling in a number of tissues [69]. RGS2 has selectivity for dampening Gαq signaling [70] both by regulating p63RhoGEF, an activator of gene transcription, as well as GRK2-mediated GPCR phosphorylation [71]. In vivo, it was not surprising that gene ablation of RGS2 increased blood pressure, depending a significant extent on angiotensin II [72, 73], [74]. As with most signaling molecules, upon further investigation, the mechanism is more complex and it was suggested that nitric oxide (NO) mediated mechanisms act to increase RGS2 activity and further enhance NO-mediated vasodilation [75, 76]. Binding and phosphorylation of RGS2 by PKGI-α, a mediator of VSM relaxation in response to NO, stimulates its GTPase activity and further diminishes activation of Gq [77]. These findings further promote the importance of RGSs as potential therapeutics because they could potentially terminate maladaptive signaling through Gq-coupled receptors. These features are possibly shared by GRKs carrying an RGS homology, although this potential mechanism of action remains to be elucidated.
RGS3
RGS3 proteins, a subfamily of RGS proteins, carry a characteristic extended N-terminus, which is expressed in two truncated versions that appear to be able to attenuate calcitonin gene-related peptide (CGRP) receptor signaling through inhibition of both IP3 and cAMP formation [78]. This suggests that RGS3 can regulate signaling through both Gq and Gs thus if any of the GRKs had similar binding characteristics to RGS3 they could work to further decrease GPCR signaling. RGS3 can also bind to Gβγ and decrease MAPK, as well as PI3K activity [79]. Importantly, GRK2 associates with Gβγ through the PH domain within its C-terminal portion. Whether it is also capable of interacting with Gβγ within its N-terminal RGS domain remains to be determined. There is evidence for the RGS3-isoform RGS3L to act as a molecular switch between Gβγ-mediated activation of Rac-1 and RhoA [80] which, considering the importance of the ROCK pathway in smooth muscle contractility, warrants further investigation with respect to hypertension, even more, since RGS3 is has lately been shown to be upregulated in human heart disease[81]. Recently, another isoform of RGS3 was identified, PSD95 (postsynaptic density 95), which plays a role in protein assembly by way of its N-terminal PDZ domain [82, 83]. This is of relevance because PSD95 can also form a complex with the β1-adrenergic receptor, which can be disrupted by GRK5 [84]. Interaction between GRK5, or any other GRK, and PSD95 remains to be determined. All of this taken together at least suggests that if the GRKs do not act as RGS proteins per se, they could at least affect RGS activity.
RGS4
Although it was originally characterized as a brain-specific RGS, the RGS4 isoform has been strongly implicated in regulation of cardiovascular cell signaling, namely by interference with GPCRs involved in myocyte function and hypertrophy, both closely linked to vascular function and hypertension. RGS4 can likewise inhibit both Gαq and Gαi-coupled signaling and was specifically shown to interfere with endothelin-1 mediated effects in human heart disease [85], but also is specifically upregulated in situations of pathophysiological stress, such as pressure induced hypertension and cardiac hypertrophy in an animal model of suprarenal aortic banding [86]. In this model, RGS4 was upregulated over 30 days and this effect could not be blocked by AT1 receptor blockade. This implies a receptor independent upregulation of RGS proteins in that model, which importantly leads to impaired vascular reactivity to GPCR induced contraction. It has also been shown that RGS4 in cardiac myocytes inhibits phenylephrine and endothelin-induced myofilament organization and cell growth [87, 88], and associates with the muscarinic M2 receptor and G-protein coupled inward rectifying potassium (GIRK) channels in atrial myocytes [89]. Whether GRKs interact with or act similar to RGS4 remains to be determined.
RGS5
RGS5 is expressed strongly and specifically in pericytes and VSM [90]. In arterial VSM, RGS5 is able to activate GTPase and inhibits angiotensin II and endothelin-induced intracellular Ca2+ transients mediated by Gαi and Gαq [91–93]. MAPK activation pathway is also regulated by RGS5 in VSM and MAPK was increased after knock-down of RGS5 in vitro, suggesting constitutive inhibition by endogenous levels of RGS5 [94].
RGS5−/− knockout leads to hypotension and lean body type
In contrast to the RGS2−/− mouse, mice lacking RGS5 exhibited a phenotype with persistent low blood pressure [86, 95]. This was accompanied by dilated blood vessel diameter and unchanged vessel wall thickness. Isolated aorta VSMs lacking RGS5 exhibited increased extracellular signal-regulated kinase and vasodilator-stimulated phosphoprotein activation [86]. The results suggest that RGS5 acts by inhibiting nitric oxide-mediated dilatory signaling and thus would act opposite to RGS2. Whether any of the GRKs have RGS5-like binding domains and could either act similarly or compete with RGS5 remains to be determined.
Hypertension in development and progression of heart failure
In approximately 90% of patients, heart failure is preceded by hypertension, according to the American Heart Association (AHA). Hypertension remains a critical risk factor for pathological cardiac hypertrophy, renal failure and stroke, diseases showing elevated RGK levels and activities [15]. In a model of pressure overload hypertrophy leading to heart failure, GRK-activity levels were 3-fold increased [96], suggesting that GRK upregulation may play a role in the pathophysiological basis of hypertensive heart failure. In an earlier study by our group using spontaneously hypertensive heart failure (SHHF) rats, the decline of β-adrenergic responsiveness was in fact preceded by an earlier increase in GRK2 levels [97]. This indicates that up-regulation of GRK2 may be a potential target in the prevention of hypertensive heart disease to heart failure. Whether changes in cardiac GRK levels are due to hypertension itself or a result of increased neurohormonal activation that is associated with high blood pressure remains to be determined.
GRK2 inhibition in VSM as a potential therapeutic target
The delicate balance between relaxation and constriction is swayed towards constriction by impaired vasodilation due to alteration in β-adrenergic receptor signaling. The important role of β-adrenergic receptor mediated vasodilatory effects is critical for blood pressure maintenance and the resensitization of these receptors is a potential therapeutic path. Development of a GRK2 inhibitor, comprised of the last 194 amino acids of the C-terminal end of GRK2, has given us a tool to selectively block desensitization of GRK2-sensitive GPCRs. This molecule has been termed βARKct or GRK2ct (GRK2 was originally named β-adrenergic receptor kinase – βARK1) and it has been shown to have diverse effects. Koch et al. have found that cardiac-specific overexpression of the βARKct increased cardiac contractility through enhanced β-adrenergic signaling and prevented the progression of heart failure in animal models of the disease [13, 98]. Viral vector-mediated gene delivery of this peptide to myocardium led to marked improvement of cardiac function in a model of ischemic heart disease [99]. Given the recent push for novel gene therapy development through successful clinical application for instance in ophthalmology and cancer therapy, and first clinical application of genes therapeutic in heart failure [100], we expect this field to develop rapidly and look forward to applications relevant in hypertension.
Conclusion
The etiology of hypertension remains elusive. It is a complex and multifactorial disease and as such is unlikely to be successfully treated with a singular approach. Current standard therapy includes inhibition of angiotensin II AT1 receptors as well as enhancement of nitric oxide-mediated relaxation of VSM. Further, β-adrenergic receptors are also blocked and the mechanisms of action is thought to be through cardiac effects. It appears that the majority of GPCRs are regulated by GRKs suggesting that, in multifactorial diseases such as hypertension, GRKs might represent valuable targets for novel therapeutic approaches since they act in a more generalized fashion on the intracellular mediators of GPCRs rather than just blocking one selective agonist from exerting its action. To overcome the imbalance of arterial relaxation and contraction we will have to outsmart the intricate mechanisms within vascular tissues. GPCR regulation offers a broad and potent base for further study and development of therapeutic options to overcome a devastating disease.
Table 1.
Summary of studies elucidating the implications of GPCR-mediated blood pressure regulation being altered by GRKs and RGSs.
| Regulator | Function in blood pressure regulation | Reference |
|---|---|---|
| GRK2 | GRK2 is increased in lymphocytes in Hypertension | Gros, 1997; Gros, 2000 |
| Increased GRK2 in VSM causes elevated blood pressure and lead to increased vascular wall thickness | Eckhart, 2002 | |
| GRK2 reduces NO production in endothelial cells | Liu, 2005 | |
| GRK2 regulates Endothelin-1-mediated vasoconstriction | Morris, 2009 | |
| Increased GRK2 levels correlate with high blood pressure | Cohn, 2009 | |
| GRK5 | GRK5 upregulation in response to elevated blood pressure | Ishizaka, 1997 |
| VSM-overexpression of GRK5 leads to gender specific high blood pressure and vascular hypercontractility | Keys, 2005 | |
| RGS2 | Lack of RGS2 produces a spontaneous hypertension and are more sensitive to induced hypertension |
Heximer, 2003 Hercule, 2007 |
| RGS2 phosphorylation by PKGIα diminishes Gq activity | Tang, 2003 | |
| RGS2 polymorphisms are associated with hypertension and obesity | Riddle, 2006, Kohara, 2008 Sartori, 2008 | |
| RGS5 | RGS5 knockout leads to hypotension and lean habitus |
Cho, 2008 Nisancioglu, 2008 |
| Tumours lacking RGS5 are more susceptible to therapy | Hamzah, 2008 | |
| Decreased RGS5 in VSM of artherosclerotic lesions | Li, 2004 |
Acknowledgments
This work was funded by the NHLBI (National Heart, Lung and Blood Institute), the WW Smith Charitable Trust (A.D.E.), and a Swiss National Science Foundation Grant (H.L.B.) as well as under a grant with the Pennsylvania Department of Health, which specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 2.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 3.Asmar R, Rudnichi A, Blacher J, London GM, Safar ME. Pulse pressure and aortic pulse wave are markers of cardiovascular risk in hypertensive populations. Am J Hypertens. 2001;14:91–97. doi: 10.1016/s0895-7061(00)01232-2. [DOI] [PubMed] [Google Scholar]
- 4.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 5.Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. Faseb J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 6.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 7.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bregeon J, Loirand G, Pacaud P, Rolli-Derkinderen M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2009;297:C1062–1070. doi: 10.1152/ajpcell.00174.2009. [DOI] [PubMed] [Google Scholar]
- 9.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 10.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 11.Weiss ER, Raman D, Shirakawa S, Ducceschi MH, Bertram PT, Wong F, Kraft TW, Osawa S. The cloning of GRK7, a candidate cone opsin kinase, from cone- and rod-dominant mammalian retinas. Mol Vis. 1998;4:27. [PubMed] [Google Scholar]
- 12.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 13.Martini JS, Raake P, Vinge LE, DeGeorge BR, Jr, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–1042. doi: 10.1161/01.hyp.33.4.1036. [DOI] [PubMed] [Google Scholar]
- 15.Ping P, Anzai T, Gao M, Hammond HK. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol. 1997;273:H707–717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 16.Vinge LE, Oie E, Andersson Y, Grogaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and beta-arrestin isoforms in congestive heart failure in rats. Am J Physiol Heart Circ Physiol. 2001;281:H2490–2499. doi: 10.1152/ajpheart.2001.281.6.H2490. [DOI] [PubMed] [Google Scholar]
- 17.Kamikawa Y, Cline WH, Jr, Su C. Diminished purinergic modulation of the vascular adrenergic neurotransmission in spontaneously hypertensive rats. Eur J Pharmacol. 1980;66:347–353. doi: 10.1016/0014-2999(80)90467-7. [DOI] [PubMed] [Google Scholar]
- 18.Anand-Srivastava MB, Picard S, Thibault C. Altered expression of inhibitory guanine nucleotide regulatory proteins (Gi alpha) in spontaneously hypertensive rats. Am J Hypertens. 1991;4:840–843. doi: 10.1093/ajh/4.10.840. [DOI] [PubMed] [Google Scholar]
- 19.Feldman RD, Lawton WJ, McArdle WL. Low sodium diet corrects the defect in lymphocyte beta-adrenergic responsiveness in hypertensive subjects. J Clin Invest. 1987;79:290–294. doi: 10.1172/JCI112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman RD, Gros R. Impaired vasodilator function in hypertension: the role of alterations in receptor-G protein coupling. Trends Cardiovasc Med. 1998;8:297–305. doi: 10.1016/s1050-1738(98)00022-x. [DOI] [PubMed] [Google Scholar]
- 21.Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8:321–335. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
- 22.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–489. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- 23.Siehler S. Regulation of RhoGEF proteins by G(12/13)-coupled receptors. Br J Pharmacol. 2009;158:41–49. doi: 10.1111/j.1476-5381.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, Wieland T. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280:11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 25.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 26.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 27.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang T, Wu L, Wang R. Altered expression of BK channel beta1 subunit in vascular tissues from spontaneously hypertensive rats. Am J Hypertens. 2006;19:678–685. doi: 10.1016/j.amjhyper.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 30.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 31.Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. Pathophysiology of voltage-gated K(+) channels in vascular smooth muscle cells: Modulation by protein kinases. Prog Biophys Mol Biol. 2009 doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 33.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Ruiz R, Penela P, Penn RB, Mayor F., Jr Analysis of the human G protein-coupled receptor kinase 2 (GRK2) gene promoter: regulation by signal transduction systems in aortic smooth muscle cells. Circulation. 2000;101:2083–2089. doi: 10.1161/01.cir.101.17.2083. [DOI] [PubMed] [Google Scholar]
- 35.Theilade J, Lerche Hansen J, Haunso S, Sheikh SP. Extracellular signal-regulated kinases control expression of G protein-coupled receptor kinase 2 (GRK2) FEBS Lett. 2002;518:195–199. doi: 10.1016/s0014-5793(02)02701-1. [DOI] [PubMed] [Google Scholar]
- 36.Theilade J, Hansen JL, Haunso S, Sheikh SP. MAP kinase protects G protein-coupled receptor kinase 2 from proteasomal degradation. Biochem Biophys Res Commun. 2005;330:685–689. doi: 10.1016/j.bbrc.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 37.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 38.Luo J, Benovic JL. G protein-coupled receptor kinase interaction with Hsp90 mediates kinase maturation. J Biol Chem. 2003;278:50908–50914. doi: 10.1074/jbc.M307637200. [DOI] [PubMed] [Google Scholar]
- 39.Lombardi MS, Kavelaars A, Penela P, Scholtens EJ, Roccio M, Schmidt RE, Schedlowski M, Mayor F, Jr, Heijnen CJ. Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. Mol Pharmacol. 2002;62:379–388. doi: 10.1124/mol.62.2.379. [DOI] [PubMed] [Google Scholar]
- 40.Gros R, Benovic JL, Tan CM, Feldman RD. G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest. 1997;99:2087–2093. doi: 10.1172/JCI119381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gros R, Chorazyczewski J, Meek MD, Benovic JL, Ferguson SS, Feldman RD. G-Protein-coupled receptor kinase activity in hypertension : increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension. 2000;35:38–42. doi: 10.1161/01.hyp.35.1.38. [DOI] [PubMed] [Google Scholar]
- 42.Gros R, Tan CM, Chorazyczewski J, Kelvin DJ, Benovic JL, Feldman RD. G-protein-coupled receptor kinase expression in hypertension. Clin Pharmacol Ther. 1999;65:545–551. doi: 10.1016/S0009-9236(99)70074-3. [DOI] [PubMed] [Google Scholar]
- 43.Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, Eckhart AD. G protein-coupled receptor kinase 2 expression and activity are associated with blood pressure in black Americans. Hypertension. 2009;54:71–76. doi: 10.1161/HYPERTENSIONAHA.108.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ergul A. Hypertension in black patients: an emerging role of the endothelin system in salt-sensitive hypertension. Hypertension. 2000;36:62–67. doi: 10.1161/01.hyp.36.1.62. [DOI] [PubMed] [Google Scholar]
- 45.Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- 46.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sterne-Marr R, Dhami GK, Tesmer JJ, Ferguson SS. Characterization of GRK2 RH domain-dependent regulation of GPCR coupling to heterotrimeric G proteins. Methods Enzymol. 2004;390:310–336. doi: 10.1016/S0076-6879(04)90020-1. [DOI] [PubMed] [Google Scholar]
- 48.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 49.Huang J, Zhou H, Mahavadi S, Sriwai W, Murthy KS. Inhibition of Galphaq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am J Physiol Cell Physiol. 2007;292:C200–208. doi: 10.1152/ajpcell.00103.2006. [DOI] [PubMed] [Google Scholar]
- 50.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–758. doi: 10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- 51.Feldman RD. Deactivation of vasodilator responses by GRK2 overexpression: a mechanism or the mechanism for hypertension? Mol Pharmacol. 2002;61:707–709. doi: 10.1124/mol.61.4.707. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 53.Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD. Vascular smooth muscle G(q) signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3072–3079. doi: 10.1152/ajpheart.00880.2007. [DOI] [PubMed] [Google Scholar]
- 54.Peppel K, Jacobson A, Huang X, Murray JP, Oppermann M, Freedman NJ. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells attenuates mitogenic signaling via G protein-coupled and platelet-derived growth factor receptors. Circulation. 2000;102:793–799. doi: 10.1161/01.cir.102.7.793. [DOI] [PubMed] [Google Scholar]
- 55.Peppel K, Zhang L, Huynh TT, Huang X, Jacobson A, Brian L, Exum ST, Hagen PO, Freedman NJ. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells reduces neointimal hyperplasia. J Mol Cell Cardiol. 2002;34:1399–1409. doi: 10.1006/jmcc.2002.2092. [DOI] [PubMed] [Google Scholar]
- 56.Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res. 2000;86:43–50. doi: 10.1161/01.res.86.1.43. [DOI] [PubMed] [Google Scholar]
- 57.Vinge LE, Andressen KW, Attramadal T, Andersen GO, Ahmed MS, Peppel K, Koch WJ, Freedman NJ, Levy FO, Skomedal T, Osnes JB, Attramadal H. Substrate specificities of g protein-coupled receptor kinase-2 and -3 at cardiac myocyte receptors provide basis for distinct roles in regulation of myocardial function. Mol Pharmacol. 2007;72:582–591. doi: 10.1124/mol.107.035766. [DOI] [PubMed] [Google Scholar]
- 58.Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115:2971–2974. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishizaka N, Alexander RW, Laursen JB, Kai H, Fukui T, Oppermann M, Lefkowitz RJ, Lyons PR, Griendling KK. G protein-coupled receptor kinase 5 in cultured vascular smooth muscle cells and rat aorta. Regulation by angiotensin II and hypertension. J Biol Chem. 1997;272:32482–32488. doi: 10.1074/jbc.272.51.32482. [DOI] [PubMed] [Google Scholar]
- 64.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation. 2005;112:1145–1153. doi: 10.1161/CIRCULATIONAHA.104.531657. [DOI] [PubMed] [Google Scholar]
- 65.Yi XP, Gerdes AM, Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002;39:1058–1063. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- 66.Wieland T, Mittmann C. Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol Ther. 2003;97:95–115. doi: 10.1016/s0163-7258(02)00326-1. [DOI] [PubMed] [Google Scholar]
- 67.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 68.Gu S, Cifelli C, Wang S, Heximer SP. RGS proteins: identifying new GAPs in the understanding of blood pressure regulation and cardiovascular function. Clin Sci (Lond) 2009;116:391–399. doi: 10.1042/CS20080272. [DOI] [PubMed] [Google Scholar]
- 69.Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest. 2003;111:1259. doi: 10.1172/JCI200315598A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci U S A. 1997;94:14389–14393. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shankaranarayanan A, Thal DM, Tesmer VM, Roman DL, Neubig RR, Kozasa T, Tesmer JJ. Assembly of high order G alpha q-effector complexes with RGS proteins. J Biol Chem. 2008;283:34923–34934. doi: 10.1074/jbc.M805860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Hashim S, Anand-Srivastava MB. Angiotensin II-evoked enhanced expression of RGS2 attenuates Gi-mediated adenylyl cyclase signaling in A10 cells. Cardiovasc Res. 2005;66:503–511. doi: 10.1016/j.cardiores.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Romero DG, Plonczynski MW, Gomez-Sanchez EP, Yanes LL, Gomez-Sanchez CE. RGS2 is regulated by angiotensin II and functions as a negative feedback of aldosterone production in H295R human adrenocortical cells. Endocrinology. 2006;147:3889–3897. doi: 10.1210/en.2005-1532. [DOI] [PubMed] [Google Scholar]
- 74.Hercule HC, Tank J, Plehm R, Wellner M, da Costa Goncalves AC, Gollasch M, Diedrich A, Jordan J, Luft FC, Gross V. Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp Physiol. 2007;92:1014–1022. doi: 10.1113/expphysiol.2007.038240. [DOI] [PubMed] [Google Scholar]
- 75.Sun X, Kaltenbronn KM, Steinberg TH, Blumer KJ. RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol Pharmacol. 2005;67:631–639. doi: 10.1124/mol.104.007724. [DOI] [PubMed] [Google Scholar]
- 76.Obst M, Tank J, Plehm R, Blumer KJ, Diedrich A, Jordan J, Luft FC, Gross V. NO-dependent blood pressure regulation in RGS2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1012–1019. doi: 10.1152/ajpregu.00288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Mendelsohn ME. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. 2003;9:1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 78.Chatterjee TK, Eapen AK, Fisher RA. A truncated form of RGS3 negatively regulates G protein-coupled receptor stimulation of adenylyl cyclase and phosphoinositide phospholipase C. J Biol Chem. 1997;272:15481–15487. doi: 10.1074/jbc.272.24.15481. [DOI] [PubMed] [Google Scholar]
- 79.Shi CS, Lee SB, Sinnarajah S, Dessauer CW, Rhee SG, Kehrl JH. Regulator of G-protein signaling 3 (RGS3) inhibits Gbeta1gamma 2-induced inositol phosphate production, mitogen-activated protein kinase activation, and Akt activation. J Biol Chem. 2001;276:24293–24300. doi: 10.1074/jbc.M100089200. [DOI] [PubMed] [Google Scholar]
- 80.Vogt A, Lutz S, Rumenapp U, Han L, Jakobs KH, Schmidt M, Wieland T. Regulator of G-protein signalling 3 redirects prototypical Gi-coupled receptors from Rac1 to RhoA activation. Cell Signal. 2007;19:1229–1237. doi: 10.1016/j.cellsig.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Chakir K, Daya SK, Aiba T, Tunin RS, Dimaano VL, Abraham TP, Jaques-Robinson KM, Lai EW, Pacak K, Zhu WZ, Xiao RP, Tomaselli GF, Kass DA. Mechanisms of enhanced beta-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009;119:1231–1240. doi: 10.1161/CIRCULATIONAHA.108.774752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- 83.Xiang Y, Kobilka B. The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc Natl Acad Sci U S A. 2003;100:10776–10781. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu LA, Chen W, Premont RT, Cong M, Lefkowitz RJ. G protein-coupled receptor kinase 5 regulates beta 1-adrenergic receptor association with PSD-95. J Biol Chem. 2002;277:1607–1613. doi: 10.1074/jbc.M107297200. [DOI] [PubMed] [Google Scholar]
- 85.Mittmann C, Chung CH, Hoppner G, Michalek C, Nose M, Schuler C, Schuh A, Eschenhagen T, Weil J, Pieske B, Hirt S, Wieland T. Expression of ten RGS proteins in human myocardium: functional characterization of an upregulation of RGS4 in heart failure. Cardiovasc Res. 2002;55:778–786. doi: 10.1016/s0008-6363(02)00459-5. [DOI] [PubMed] [Google Scholar]
- 86.Cho H, Park C, Hwang IY, Han SB, Schimel D, Despres D, Kehrl JH. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol. 2008;28:2590–2597. doi: 10.1128/MCB.01889-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamirisa P, Blumer KJ, Muslin AJ. RGS4 inhibits G-protein signaling in cardiomyocytes. Circulation. 1999;99:441–447. doi: 10.1161/01.cir.99.3.441. [DOI] [PubMed] [Google Scholar]
- 88.Rogers JH, Tamirisa P, Kovacs A, Weinheimer C, Courtois M, Blumer KJ, Kelly DP, Muslin AJ. RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload. J Clin Invest. 1999;104:567–576. doi: 10.1172/JCI6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaen C, Doupnik CA. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J Biol Chem. 2006;281:34549–34560. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- 90.Adams LD, Geary RL, McManus B, Schwartz SM. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–631. doi: 10.1161/01.res.87.7.623. [DOI] [PubMed] [Google Scholar]
- 91.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. Faseb J. 2003;17:440–442. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- 92.Seki N, Sugano S, Suzuki Y, Nakagawara A, Ohira M, Muramatsu M, Saito T, Hori T. Isolation, tissue expression, and chromosomal assignment of human RGS5, a novel G-protein signaling regulator gene. J Hum Genet. 1998;43:202–205. doi: 10.1007/s100380050071. [DOI] [PubMed] [Google Scholar]
- 93.Zhou J, Moroi K, Nishiyama M, Usui H, Seki N, Ishida J, Fukamizu A, Kimura S. Characterization of RGS5 in regulation of G protein-coupled receptor signaling. Life Sci. 2001;68:1457–1469. doi: 10.1016/s0024-3205(01)00939-0. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR. Receptor-selective effects of endogenous RGS3 and RGS5 to regulate mitogen-activated protein kinase activation in rat vascular smooth muscle cells. J Biol Chem. 2002;277:24949–24958. doi: 10.1074/jbc.M203802200. [DOI] [PubMed] [Google Scholar]
- 95.Nisancioglu MH, Mahoney WM, Jr, Kimmel DD, Schwartz SM, Betsholtz C, Genove G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28:2324–2331. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 97.Anderson KM, Eckhart AD, Willette RN, Koch WJ. The myocardial beta-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension. 1999;33:402–407. doi: 10.1161/01.hyp.33.1.402. [DOI] [PubMed] [Google Scholar]
- 98.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 99.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]