Abstract
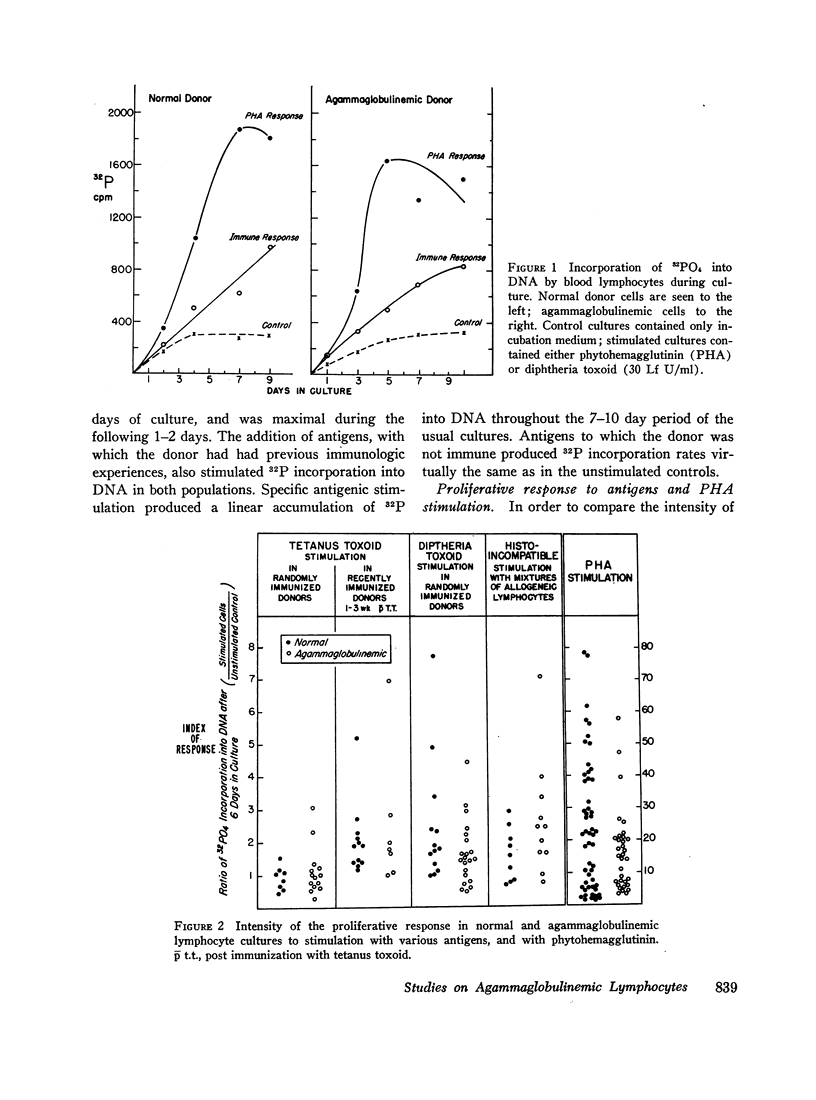
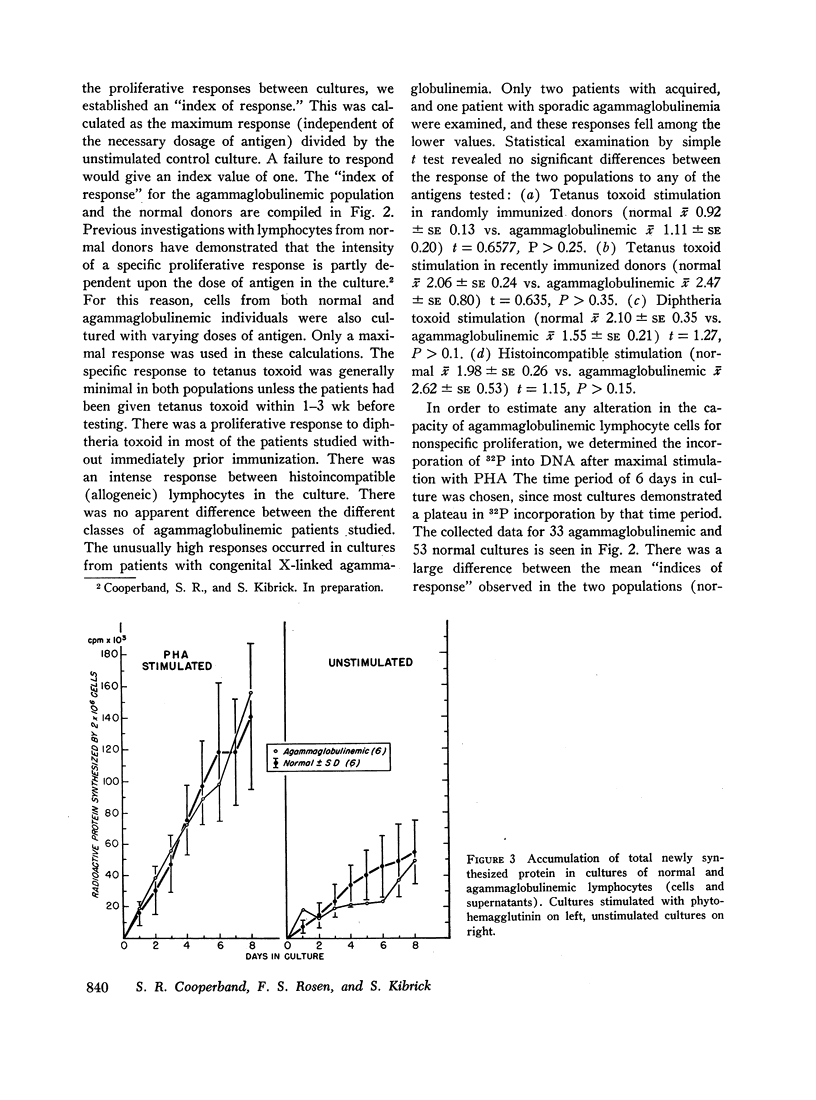
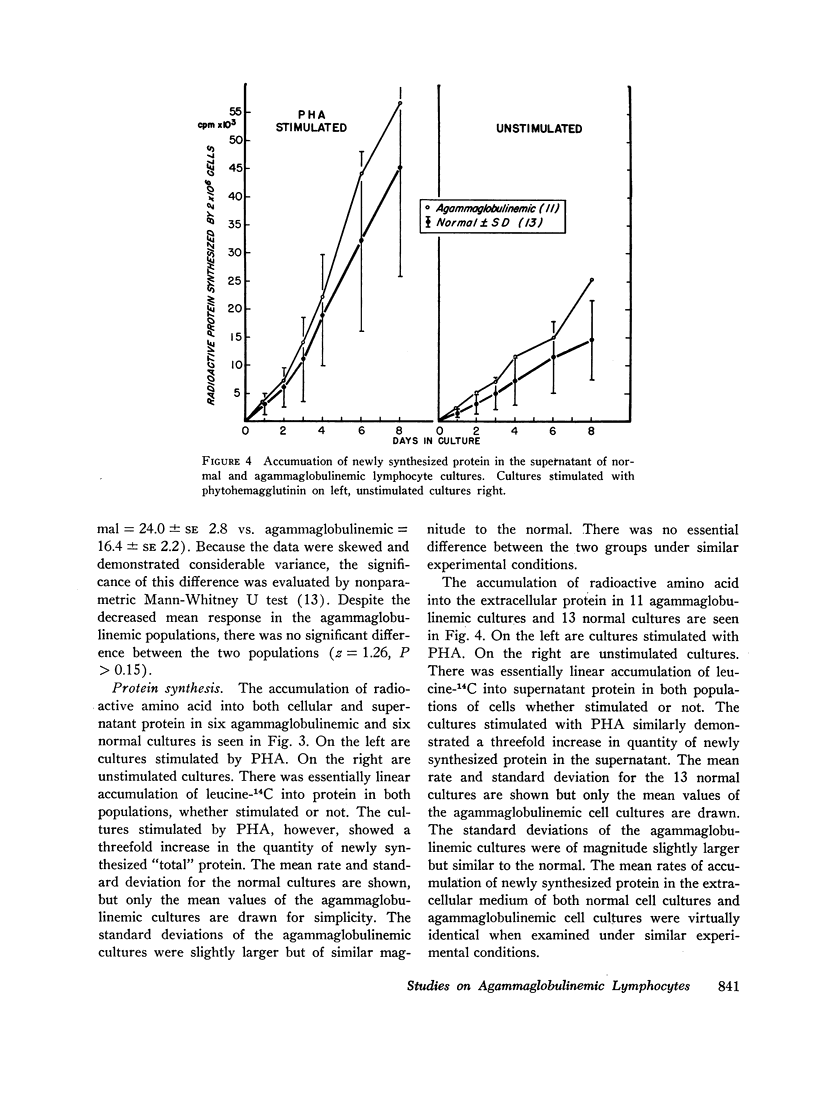
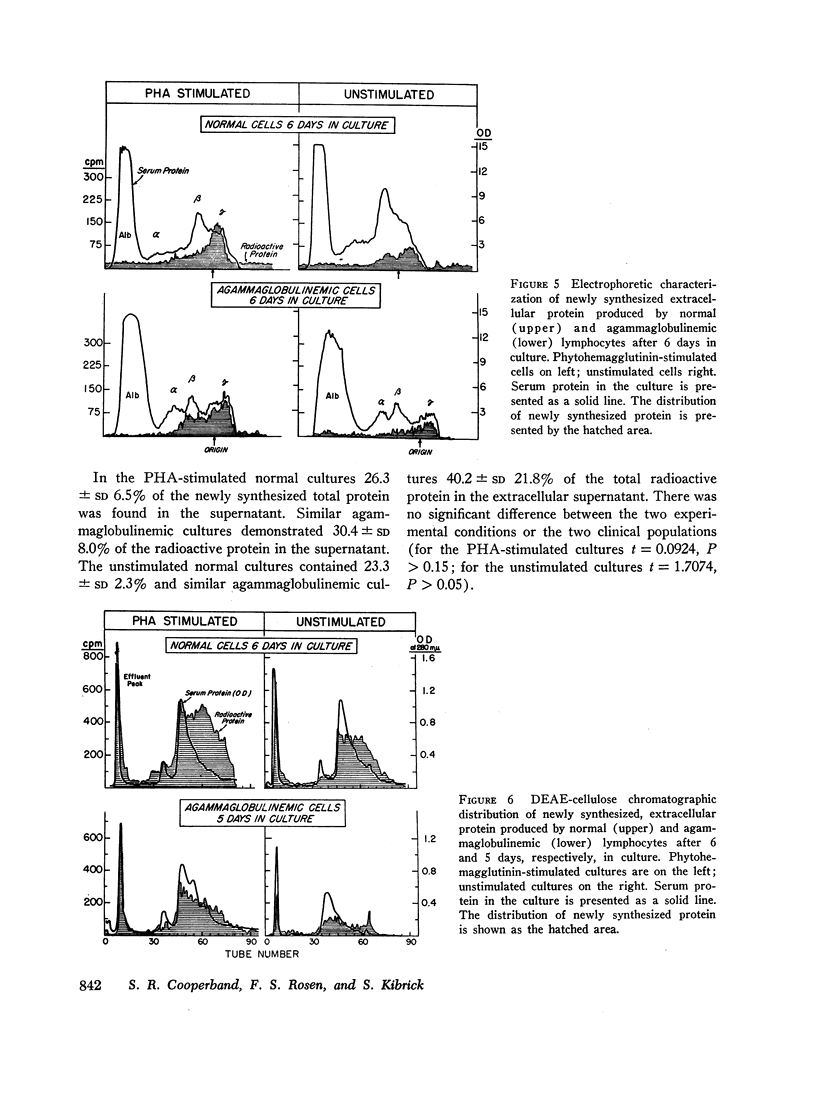
Circulating lymphocytes from patients with congenital X-linked agammaglobulinemia, sporadic congenital agammaglobulinemia, and acquired agammaglobulinemia have been cultured in vitro. They have been shown to proliferate in a normal manner under stimulus of phytohemagglutinin and antigens to which the patient was sensitized. Agammaglobulinemic cells have been shown to synthesize protein at a rate similar to that of normal cells, and the character of the extracellular protein produced is also similar. Agammaglobulinemic lymphocytes have been found to produce a small quantity of immunoglobulin G, similar to that found in normal cell cultures. The quantity of immunoglobulin produced may be increased by exposure of the cells to phytohemagglutinin. From these data, it appears that the basic lesion responsible for agammaglobulinemia is not a deficiency in lymphocyte-mediated antigen recognition or cellular proliferation. It would also appear that the basic deficiency in these disorders does not involve the structural or regulatory genes necessary for the synthesis of immunoglobulins. By exclusion, the pathogenesis of the deficiency would appear to involve cells other than circulating lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH F., HIRSCHHORN K. GAMMA-GLOBULIN PRODUCTION BY HUMAN LYMPHOCYTES IN VITRO. Exp Cell Res. 1963 Dec;32:592–595. doi: 10.1016/0014-4827(63)90197-6. [DOI] [PubMed] [Google Scholar]
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Fudenberg H. H. Defective RNA Synthesis in Lymphocytes from Patients with Primary Agammaglobulinemia. Science. 1965 Dec 3;150(3701):1311–1312. doi: 10.1126/science.150.3701.1311. [DOI] [PubMed] [Google Scholar]
- Cooperband S. R., Green J. A., Kennedy M. A., Grant M. M. Dissociation and inhibition of the stimulatory effect of phytohaemagglutinin on protein and DNA synthesis in human lymphocyte cultures. Nature. 1967 Jun 17;214(5094):1240–1241. doi: 10.1038/2141240a0. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., KUNKEL H. G., FRANKLIN E. C. Interaction of the rheumatoid factor with antigen-antibody complexes and aggregated gamma globulin. J Exp Med. 1958 Jul 1;108(1):105–120. doi: 10.1084/jem.108.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELVES M. W., ROATH S., ISRAELS M. C. FAILURE OF LYMPHOCYTES FROM HYPOGAMMAGLOBULINAEMIC SUBJECTS TO TRANSFORM IN CULTURE. Br Med J. 1964 Oct 24;2(5416):1051–1052. doi: 10.1136/bmj.2.5416.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY F. L., LAWRENCE M. E. QUANTITATIVE DETERMINATION OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND GAMMA-1-MACROGLOBULINS IN HUMAN SERUM. J Immunol. 1963 Nov;91:597–603. [PubMed] [Google Scholar]
- FAHEY J. L., McCOY P. F., GOULIAN M. Chromatography of serum proteins in normal and pathologic sera: the distribution of protein-bound carbohydrate and cholesterol, siderophilin, thyroxin-binding protein, B12-binding protein, alkaline and acid phosphatases, radio-iodinated albumin and myeloma proteins. J Clin Invest. 1958 Feb;37(2):272–284. doi: 10.1172/JCI103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUDENBERG H. H., HIRSCHHORN K. AGAMMAGLOBULINEMIA: THE FUNDAMENTAL DEFECT. Science. 1964 Aug 7;145(3632):611–612. doi: 10.1126/science.145.3632.611. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., ZAK S. J. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956 Jul;18(1):109–149. [PubMed] [Google Scholar]
- KAHAN F. M. Purification and measurement of microgram amounts of radioactive nucleic acids and proteins from animal cells in tissue culture. Anal Biochem. 1960 Sep;1:107–126. doi: 10.1016/0003-2697(60)90003-8. [DOI] [PubMed] [Google Scholar]
- KULNEFF N., PEDERSEN K. O., WALDENSTROM J. Drei Fälle von Agammaglobulinämie; ein klinischer, genetischer und physikalisch-chemischer Beitrag zur Kenntnis des Proteinstoffwechsels. Schweiz Med Wochenschr. 1955 Apr 16;85(16):363–368. [PubMed] [Google Scholar]
- Latham W. C., Jenness C. P., Timperi R. J., Michelsen C. B., Zipilivan E. M., Edsall G., Ley H. L., Jr Purification and characterization of tetanus toxoid and toxin. I. Fractionation of tetanus toxoid by gel filtration. J Immunol. 1965 Sep;95(3):487–493. [PubMed] [Google Scholar]
- PEARMAIN G., LYCETTE R. R., FITZGERALD P. H. Tuberculin-induced mitosis in peripheral blood leucocytes. Lancet. 1963 Mar 23;1(7282):637–638. doi: 10.1016/s0140-6736(63)91275-3. [DOI] [PubMed] [Google Scholar]
- PORTER H. M. Immunologic studies in congenital agammaglobulinemia with emphasis on delayed hypersensitivity. Pediatrics. 1957 Dec;20(6):958–965. [PubMed] [Google Scholar]
- SCHREK R. Cell transformations and mitoses produced in vitro by tuberculin purified protein derivative in human blood cells. Am Rev Respir Dis. 1963 May;87:734–738. doi: 10.1164/arrd.1963.87.5.734. [DOI] [PubMed] [Google Scholar]
- SCHUBERT W. K., FOWLER R., Jr, MARTIN L. W., WEST C. D. Homograft rejection in children with congenital immunological defects: agammaglobulinemia and Aldrich syndrome. Transplant Bull. 1960 Jul;26:125–128. doi: 10.1097/00006534-196007000-00044. [DOI] [PubMed] [Google Scholar]
- Turner K. J., Forbes I. J. Synthesis of proteins by human leukocytes in vitro. II. Chemical characterization. J Immunol. 1966 Jun;96(6):926–935. [PubMed] [Google Scholar]



