Abstract
BTBR T+tf/J (BTBR) is an inbred mouse strain that displays social deficits and repetitive behaviors analogous to the first and third diagnostic symptoms of autism. We previously reported an unusual pattern of ultrasonic vocalizations in BTBR pups that may represent a behavioral homologue to the second diagnostic symptom of autism, impaired communication. The present study investigated the social and vocal repertoire in adult BTBR mice, to evaluate the role of ultrasonic vocalizations in multiple social situations at the adult stage of development. Three different social contexts were considered: male-female, male-male (resident-intruder) and female-female interactions. Behavioral responses and ultrasonic vocalizations were recorded for BTBR and for the highly social control strain C57BL/6J (B6). No episodes of overt fighting or mating were observed during the short durations of the three different experimental encounters.
BTBR displayed lower levels of vocalizations and social investigation in all three social contexts as compared to B6. In addition, the correlation analyses between social investigation and USVs emission rate revealed that in B6 mice the two variables were positively correlated in all the three different social settings, whereas in BTBR mice the positive correlation was significant only in the male-female interactions. These findings strongly support the value of simultaneously recording two aspects of the mouse social repertoire, social motivation and bioacoustic communication. Moreover, our findings in adults are consistent with previous results in pups, showing an unusual vocal repertoire in BTBR as compared to B6.
Keywords: ultrasonic vocalizations, social approach, social motivation, communication, Autism
Introduction
Ultrasonic vocalizations (USV) are emitted by mice not only during infancy (Noirot & Pye 1969; Scattoni et al. 2008a; Winslow 2009), but in different social contexts throughout their lifespan (Nyby & Whitney 1978; Nyby 2001; Portfors 2007; Scattoni et al. 2009). The USV emission of adult mice has been primarily reported in reproductive contexts (Nyby 2001), with males being responsible for most of the calls (Maggio et al. 1983; Nyby & Whitney 1978; Wang et al. 2008; Whitney & Nyby 1979). At variance with rats, gerbils and wood mice, adult mouse USV were not detected during male-male agonistic encounters in laboratory settings (Nyby & Whitney 1978; Nyby 2001; Sales 1972a). During female–female encounters (D’Amato & Moles 2001; D’Udine et al. 1982; Maggio & Whitney 1985; Moles et al. 2007), mice emit a large number of USV, at absolute rates comparable to those of male–female interactions. Recently, mouse USV were reported in adolescent C57BL/6J (B6) and BALB/cJ mice of both sexes during social interactions after weaning (Panksepp et al. 2007).
Ultrasonic emission is a consistent and robust phenomenon in rodents during adult social interactions both in males and females, and can be considered an index of social interest and motivation (Moles et al. 2007; Nyby 2001; Scattoni et al. 2009). In fact, adult mouse USV have been positively correlated with social investigation, such as anogenital sniffing, both in the sexual and female-female social interactions (Moles et al. 2007; Nyby 1983; Sales 1972b). Behavioral and playback studies attributed an affiliative function to male and female USV, facilitating approach behavior and proximity (Hammerschmidt et al. 2009; Moles et al. 2007; Pomerantz et al. 1983). Male USV emitted during courtship are thought to be important to maintain female proximity and to reduce female aggression, thus facilitating copulation (Pomerantz et al. 1983). Female USV facilitate proximity between the resident and the intruder, which may help the resident to acquire relevant social information about the intruder (Moles et al. 2007).
The study of social behavior is essential for the understanding of biochemical, genetic and environmental factors underlying many psychiatric disorders which are characterized by aberrant social interactions, such as autism and schizophrenia. BTBR T+tf/J (BTBR) is an inbred strain of mice that displays several behavioral traits relevant to autism, including reduced social approach in adults and an unusual pattern of ultrasonic vocalizations in pups (Bolivar et al. 2007; McFarlane et al. 2008; Moy & Nadler 2008; Moy et al. 2007; Scattoni et al. 2008a; Silverman et al. 2010; Yang et al. 2009; Yang et al. 2007a; Yang et al. 2007b), resembling the atypical vocalizations seen in some autistic infants (Frith & Happe 1994; Johnson 2008; Kanner 1971). Comprehensive analyses of procedural abilities revealed that general health, motor functions, and olfactory abilities were normal in BTBR, with no evidence for high anxiety-like traits, supporting an interpretation of highly specific social deficits (Benno et al. 2009; McFarlane et al. 2008; Moy et al. 2007; Yang et al. 2009). In addition, auditory-evoked brainstem response data suggested that BTBR hearing abilities are comparable to those of other mouse strains (Scattoni et al., unpublished data). Cross-fostering studies and unpublished observations of maternal behavior determined that BTBR pups reared with either B6 or BTBR mothers displayed low sociability as adults, excluding the explanation that early maternal care factors determined sociability in the BTBR inbred strain (Yang et al. 2007b).
The goal of the present study was to investigate vocalizations in adult BTBR versus B6 mice engaged in various types of reciprocal social interactions. Autistic people often display a lack of joint attention and spontaneous seeking to share interests with other people, and low social reciprocity, while the ability to recognize different individuals is intact. To model this selective social deficit, we evaluated the social approach towards a freely moving partner, by both social investigation and social communication in multiple social situations.
Three different social encounters were employed to investigate adult USV: 1) Courtship by a male towards a sexually receptive female; 2) Male resident-intruder interactions; 3) Female-female interactions. Simultaneous recording of social interactions and USV during the test session enabled correlations between two aspects of the mouse social repertoire. The present experimental design addresses mouse endophenotypes relevant to two of the three diagnostic symptoms of autism, abnormal reciprocal interaction and impaired communication.
Materials and methods
Animals and housing
Subject mice were 2-month-old C57BL/6J (B6, N= 36 males and 36 females) and BTBR T+tf/J (BTBR, N= 36 males and 36 females) mice. Breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at the National Institute of Mental Health in Bethesda, MD, USA. About two weeks after pairing for breeding, the females were individually housed and subsequently inspected daily for pregnancy and delivery. After weaning on postnatal day 21, mice were socially housed in groups of 2–4 with same-sex partners. All mice were housed in polycarbonate Makrolon cages (369 × 156 × 132 mm, 435 cm2; 1145T; Tecniplast, Milan, Italy) in a temperature and humidity controlled vivarium, with food and water available ad libitum. In addition to standard bedding, paper strips, a nestlet square and a cardboard tube were provided in each cage. The colony room was maintained on a 12:12 light/dark cycle with lights on at 06:00 h. Behavioral testing was conducted between 14:00 and 17:00h, towards the end of the light phase of the circadian cycle. All procedures were conducted in strict compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals, and approved by the National Institute of Mental Health Animal Care and Use Committee.
Behavioral observations
Two-month-old mice, separate groups of 12 B6 and 12 BTBR for each test, were evaluated in three different social interactions: 1) male-female; 2) male-male and 3) female-female encounters. Behavioral tests were conducted in the standard Tecniplast cage of the resident mouse under red light, videotaped using a Panasonic monochrome CCD camera and subsequently analyzed with Noldus Observer 8.0 software (Noldus Information Technology, Leesburg, VA, USA). All the tests were conducted in a sound-attenuating chamber (model ENV-018V, Med Associates Inc, St. Albans, VT).
Adult Social Interactions
An unfamiliar stimulus mouse was placed into the home cage of an isolated test mouse who had resided in the cage for the previous 5 days, for a 5 minute session of male-female interactions, or for a 3 minute session for male-male and female-female interactions. In addition to the resident mouse, the cage contained litter (1.5 cm deep) and the lid was removed. The videocamera was mounted facing the side of the cage, to record the session for subsequent scoring of social investigation parameters. The ultrasonic microphone was mounted 20 cm above the cage, to record the session for subsequent scoring of USV parameters.
Stimulus mice were matched to the subject mice by age, body weight, and strain. Stimulus mice were bred in the NIMH colony as described above, and maintained in social groups of four per home cage. On the day of male-female testing, the vaginal estrous condition of each female was assessed as previously described (Rugh 1990). Only females in estrous were selected for the test. A total of 72 stimulus mice (N=36 each strain) were employed. Each was used only once.
The ultrasonic microphone (Avisoft UltraSoundGate condenser microphone capsule CM16, Avisoft Bioacoustics, Berlin, Germany), suspended 20 cm above the cage, was sensitive to frequencies between 10 to 180 kHz. Vocalizations were recorded using Avisoft Recorder software version 3.2. Settings included sampling rate at 250 kHz; format 16 bit. For acoustical analysis, recordings were transferred to Avisoft SASLab Pro (Version 4.40) and a fast Fourier transformation (FFT) was conducted as previously described (Scattoni et al. 2008a). Spectrograms were generated with an FFT-length of 512 points and a time window overlap of 75% (100% Frame, Hamming window). The spectrogram was produced at a frequency resolution of 488 Hz and a time resolution of 1 ms. A lower cut-off frequency of 20 kHz was used to reduce background noise outside the relevant frequency band to 0 dB. Parameters analyzed for each test included number of calls, mean duration of calls, qualitative and quantitative analyses of sound frequencies measured in terms of frequency and amplitude at the maximum of the spectrum. Total calling time was computed by summing duration of each call emitted by the subject. This parameter provides an indication of the portion of time spent vocalizing in each min of testing. Start times for the video and audio files were synchronized. However, because behavioral events occurred in a time frame of seconds, and vocalizations occurred in a time frame of milliseconds, it was not possible to synchronize scoring of behaviors with calls using the currently available recording technology. The software used for the behavioral (Noldus, Observer 3.0) and spectrographic (Avisoft Bioacoustics, Avisoft SASLabPro version 4.40) analyses cannot be combined on the same screen because they proceed with different speeds.
Waveform patterns of calls were examined in depth in the sonograms collected from every mouse tested. Using the entire 3 or 5 minute session recordings, we classified 839 BTBR calls (N=9 subjects) and 4543 B6 calls (N=11 subjects) in the male-female encounter, 395 BTBR calls (n=12) and 2026 B6 calls (n=7) in the male-male encounter, 55 BTBR calls (n=6) and 1805 B6 calls (n=9) in the female-female encounter. The rest of the subjects (total N=12 each strain) were not analyzed because they did not emit vocalizations. Each call was identified as one of 10 distinct categories, based on internal pitch changes, lengths and shapes, using previously published categorizations (Branchi et al. 1998; Brudzynski et al. 1999; Panksepp et al. 2007; Scattoni & Branchi 2009; Scattoni et al. 2008a). Quantitative analysis was performed by visual inspection using the interactively (section labels) function of the Avisoft software. For some recordings, the automatic threshold-based element separation was ineffective, either because of strong ambient noise or poor structured vocalizations. For this reason, the element borders have been defined manually by inserting section labels.
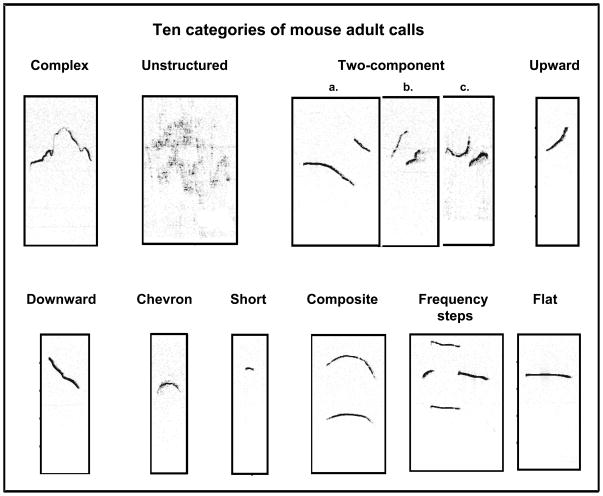
Classification of USV focused on the ten waveform patterns described below, and illustrated visually in Figure 1.
Figure 1.
Typical sonograms of ultrasonic vocalizations, classified into ten distinct categories of calls emitted by adult mice.
Complex calls displayed one sound component containing two or more directional changes in frequency with varying direction and rate of changes, each ≥6.25 kHz.
Unstructured calls did not contain a main sound component and their shape cannot be assimilated to any of the other categories (see figure S1 for details).
Two-component calls consisted of two components: a main call (with a flat or downward frequency change) with an additional short component of higher frequency.
Upward-modulated calls exhibited a continuous increase in frequency that was ≥12.5 kHz, with a terminal dominant frequency at least 6.25 kHz more than the frequency at the beginning of the vocalization.
Downward-modulated calls exhibited a continuous decrease in frequency that was ≥12.5 kHz, with a terminal dominant frequency at least 6.25 kHz less than the initial frequency of the vocalization.
Chevron calls resembled an ‘inverted-U shape’, which was identified by a continuous increase in frequency ≥12.5 kHz followed by a decrease that was ≥6.25 kHz.
Short calls were punctuated and shorter than 5 ms.
Composite calls were formed by two parallel lines consisting of a continuous sound with a minor frequency modulation and a single higher amplified harmonic component.
Frequency steps calls consisted of three components: a main call resembling the composite calls flanked by two discontinuous “step” on the sonographic display, but with no gaps on the time scale.
Flat calls presented a constant frequency, including the initial and terminal frequency with changes ≤3 kHz.
Inter-rater reliability in scoring the call categories was 98% between the two investigators who scored the calls. Scoring was conducted blind to the mouse strain. Call category data were subjected to two different analyses: 1) strain-dependent effects on the probability of producing calls (proportion of calls in each category for each subject) from each of the ten categories of USV, as described below under Statistical analysis, within and across social encounters; 2) a descriptive analysis which included strain-dependent effects on the percentage of calls emitted by each subject in each of the ten categories of USV; and strain-dependent effects on quantitative parameters such as mean duration, peak frequencies, and amplitude of calls, emitted by each subject in each of the ten categories of USV (Table 1).
Table 1.
Quantitative Analysis of Ultrasonic Vocalizations of B6 and BTBR Adults in Three Social Encounters
| Male Female | Cx | Un | Tc | Up | Dw | Ch | Sh | Cp | Fs | Fl | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B6 | Duration (ms) | 53,5 ± 20,6 | - | 37,0 ± 16,4 | 19,7 ± 3,7 | 33,0 ± 6,9 | 33,9 ± 9,7 | 9,4 ± 2,2 | 23,5 ± 5,7 | 70,9 ± 22,6 | 26,2 ± 5,3 |
| Peak Freq (kHz) | 74,6 ± 3,8 | - | 75,6 ± 3,9 | 78,6 ± 4,1 | 78,0 ± 7,0 | 84,9 ± 6,3 | 81,2 ± 5,8 | 87,6 ± 21,6 | 72,5 ± 2,6 | 74,7 ± 3,5 | |
| Peak Amp (dB) | 63,8 ± 2,8 | - | 62,6 ± 3,2 | 65,9 ± 2,4 | 63,7 ± 2,7 | 64,6 ± 3,7 | 60,8 ± 2,6 | 65,3 ± 2,8 | 64,6 ± 3,4 | 59,5 ± 1,9 | |
| BTBR | Duration (ms) | 47,4 ± 14,1 | 39,7 ± 22,7 | 27,7 ± 3,5 | 20,2 ± 9,1 | 29,4 ± 13,7 | 31,0 ± 15,0 | 8,4 ± 2,7 | 32,2 ± 1,9 | 31,7 ± 13,3 | 23,9 ± 1,2 |
| Peak Freq (kHz) | 72,8 ± 9,7 | 72,2 ± 18,7 | 75,3 ± 7,1 | 74,5 ± 5,9 | 77,2 ± 5,3 | 77,6 ± 4,9 | 85,8 ± 11,7 | 68,8 ± 17,7 | 64,9 ± 4,4 | 67,9 ± 7,7 | |
| Peak Amp (dB) | 66,5 ±2,2 | 65,3 ± 1,6 | 63,3 ± 1,5 | 63,9 ± 3,5 | 65,0 ± 0,4 | 60,1 ± 1,3 | 62,4 ± 4,0 | 63,2 ± 0,3 | 67,3 ± 2,1 | 61,9 ± 4,2 | |
| Male Male | Cx | Un | Tc | Up | Dw | Ch | Sh | Cp | Fs | Fl | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B6 | Duration (ms) | 64,3 ± 20,4 | - | 44,2 ± 26,5 | 17,6 ± 3,2 | 40,0 ± 16,4 | 33,8 ± 20,4 | 6,6 ± 0,5 | 86,3 ± 16,9 | 88,5 ± 25,6 | 22,7 ± 11,9 |
| Peak Freq (kHz) | 72,3 ± 5,1 | - | 75,4 ± 4,9 | 77,5 ± 4,8 | 81,3 ± 11,5 | 77,8 ± 10,8 | 77,5 ± 5,7 | 57,7 ± 1,2 | 73,0 ± 11,3 | 71,3 ± 6,9 | |
| Peak Amp (dB) | 55,9 ± 5,3 | - | 56,2 ± 7,3 | 57,6 ± 6,4 | 55,3 ± 8,4 | 56,5 ± 9,1 | 54,6 ± 7,6 | 49,0 ± 0,1 | 56,6 ± 6,6 | 54,8 ± 7,5 | |
| BTBR | Duration (ms) | 30,8 ± 10,0 | 18,7 ± 6,1 | 29,8 ± 4,9 | 22,4 ± 4,9 | 14,2 ± 4,2 | 13,5 ± 1,8 | 7,7 ± 2,1 | 32,8 ± 7,1 | 37,8 ± 10,1 | 19,9 ± 5,7 |
| Peak Freq (kHz) | 65,8 ± 9,8 | 71,0 ± 10,6 | 81,2 ± 9,6 | 65,1 ± 5,8 | 68,7 ± 16,5 | 75,4 ± 5,8 | 75,5 ± 17,0 | 61,3 ± 10,7 | 62,0 ± 3,4 | 65,1 ± 6,6 | |
| Peak Amp (dB) | 64,3 ± 3,5 | 64,4 ± 2,2 | 62,1 ± 7,3 | 67,9 ± 2,6 | 65,2 ± 4,3 | 66,0 ± 4,6 | 63,4 ± 1,5 | 68,8 ± 2,7 | 75,3 ± 7,7 | 65,1 ± 3,6 | |
| Female Female | Cx | Un | Tc | Up | Dw | Ch | Sh | Cp | Fs | Fl | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B6 | Duration (ms) | 67,3 ± 23,6 | 44,2 ± 29,4 | 39,8 ± 10,9 | 17,5 ± 3,4 | 30,8 ± 10,6 | 30,8 ± 10,7 | 7,5 ± 1,3 | 76,2 ± 15,5 | 72,8 ± 9,2 | 27,9 ± 3,7 |
| Peak Freq (kHz) | 77,2 ± 11,3 | 69,2 ± 3,1 | 72,5 ± 6,2 | 87,1 ± 9,1 | 83,0 ± 12,8 | 85,3 ± 6,3 | 79,4 ± 7,6 | 65,5 ± 7,6 | 72,0 ± 4,1 | 73,5 ± 6,9 | |
| Peak Amp (dB) | 64,9 ± 3,7 | 59,4 ± 8,4 | 62,9 ± 4,3 | 65,9 ± 2,6 | 65,4 ± 5,2 | 65,0 ± 4,1 | 62,7 ± 3,1 | 65,5 ± 3,5 | 64,3 ± 2,2 | 59,1 ± 8,6 | |
| BTBR | Duration (ms) | 29,1 ± 12,1 | 11,2 ± 2,2 | 28,3 ± 9,1 | 20,8 ± 6,1 | 16,2 ± 10,7 | - | 5,6 ± 1,6 | 14,3 ± 1,5 | 35,3 ± 1,7 | 22,5 ± 3,2 |
| Peak Freq (kHz) | 88,0 ± 10,4 | 80,5 ± 7,9 | 63,3 ± 2,9 | 100,5 ± 18,5 | 72,4 ± 17,9 | - | 81,0 ± 10,3 | 91,7 ± 7,9 | 79,1 ± 6,5 | 90,3 ± 14,3 | |
| Peak Amp (dB) | 69,1 ± 3,8 | 58,2 ± 4,5 | 65,7 ± 6,5 | 68,9 ± 1,9 | 66,5 ± 8,1 | - | 61,7 ± 3,2 | 64,7 ± 4,9 | 65,4 ± 2,1 | 66,5 ± 2,3 | |
Data are mean values ± standard deviations.
Social interactions were scored from the videotapes for the frequencies and durations of the following behavioral responses performed by the subject mouse: anogenital sniffing (direct contact with the anogenital area), body sniffing (sniffing or snout contact with the flank area), nose to nose sniffing (sniffing or snout contact with the head/neck/mouth area), locomotor activity by line crossings, rearing up against the wall of the home cage, digging in the bedding, and grooming (self-cleaning, licking any part of its own body). No observations of mounting, fighting, tail rattling and wrestling behaviors were observed during any of the social interactions considered in the study. Scoring was conducted by four investigators uninformed of the strain. Inter-rater reliability was 97%.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to analyze social interaction parameters and USV quantitative parameters in each of the three social contexts. A mixed-model ANOVA with repeated measures was used to analyze sniffing behaviors, number of USV and probability of vocalizations with strain as between-subject factor and i. sniffing of different body areas (anogenital, body or nose to nose); ii. USV test minutes (3 or 5min); and iii. ten call categories as within-subject factors. Probability of vocalizations within each strain was calculated as number of calls in each category for each subject/total number of calls analyzed in each subject and standardized by angular transformation. To better analyze strain differences within call categories, a nonparametric analysis (Mann Whitney test) was performed for each category. Post-hoc comparisons were performed using Tukey’s HSD test only when a significant F-value was determined. Pearson’s product moment statistics were used to run correlation analyses between social investigation and numbers of ultrasonic vocalizations. For all comparisons, significance was set at p < 0.05.
Results
Male-female social interaction test
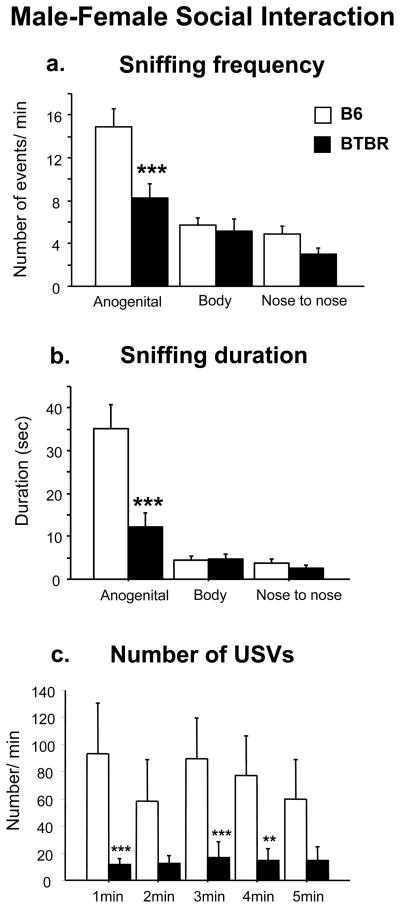
Analysis of the social sniffing response in adult male mice interacting with an estrus female revealed a main effect of strain [frequency, F1,22 = 6.29, p=0.02; duration, F1,22 = 9.65, p<0.01], body areas [frequency, F2,44 = 52.67, p <0.0001; duration, F2,44 = 42.39, p<0.0001], and their interaction [frequency, F2,44 = 8.26, p <0.001; duration, F2,44 = 13.36, p<0.0001] (Figure 2a and b). As expected, B6 male mice sniffed B6 female partner more frequently and for longer time in the anogenital area than other parts of the body [Tukey’s HSD test: p <0.0001 for anogenital sniffing frequency and duration versus body and nose to nose sniffing]. In fact, in the first phases of the social encounter, the area of interest is typically the anogenital region of the stimulus mouse (Gheusi et al. 1994; Winslow 2003). By contrast, BTBR male mice sniffed the anogenital area of the estrus BTBR female partner less as compared to B6 [Tukey’s HSD test: p <0.001 for anogenital sniffing frequency and duration of B6 versus BTBR].
Figure 2.
Male-female social interaction: A five minute session measured parameters of direct interaction of a male with a sexually receptive female of the same strain. a) Sniffing frequency, b) sniffing duration, c) number of ultrasonic vocalizations. Data are expressed as mean + SEM throughout Figures 2–4 and in Table 1 and 2. N=12 each strain. ***p<0.001 and **p<0.01 for BTBR compared to B6.
A main effect of strain was detected for the total number of vocalizations across the 5 minute test session with the estrus female [F1,22 = 4.79, p =0.03; Figure 2c]. Analysis of total calling time showed that adult B6 male mice tended to spend more time calling than adult BTBR male mice [F1,18 = 3.81, p =0.06] in response to the female presence. In general, BTBR male mice emitted a lower number of ultrasonic vocalizations than B6 male mice. The strain difference was significant on the first, third and fourth minute of the social encounter [Tukey’s HSD test for B6 versus BTBR: 1st and 3rd min, p <0.001, 4th min, p <0.01]. No significant strain differences were detected on mean duration of the calls [F1,18 = 0.05, p =0.82], or on peak of frequencies [F1,18 = 0.37, p =0.55]. BTBR males emitted calls with lower amplitude than B6 males [F1,18 = 8.24, p =0.01]. Analysis of total calling time showed that adult B6 male mice tended to spend more time calling than adult BTBR male mice [F1,18 = 3.81, p =0.06] in response to the female presence.
BTBR spent more time exploring the environment than B6. A significant effect of strain was found on behavioral measurements commonly used to evaluate general exploratory activity, including rearing [duration, F1,22 = 5.65, p < 0.05] and digging [F1,22 = 5.29, p < 0.05] (Table 2). In contrast, there was no significant effect of strain on locomotor activity [F1,22 = 1.94, p = 0.18], grooming frequency [F1,22 = 2.88, p = 0.11] or duration [F1,22 = 0.06, p = 0.99] (data not shown).
Table 2.
Exploration of the cage environment
| Male-Female | B6 | BTBR |
|---|---|---|
| Crossings | 87.4±6.5 | 99.6±5.8 |
| Rearing (s) | 42.9±3.6 | 55.9±4.1* |
| Digging (s) | 20.7±3.7 | 41.4±8.2* |
| Male-Male | ||
| Crossings | 45.5±2.8 | 45.2±3.1 |
| Rearing (s) | 22.4±2.3 | 33.5±3.5* |
| Digging (s) | 14.1±5.2 | 6.4±2.9 |
| Female-Female | ||
| Crossings | 37.2±4.8 | 37.5±3.8 |
| Rearing (s) | 25.2±1.9 | 30.4±5.2 |
| Digging (s) | 5.9±2.1 | 2.5±0.7 |
Data are mean values ± SEM. Crossing data are number of events/3–5min; Rearing and Digging data are durations.
Indicates significant strain differences p< 0.05
Male-male social interaction test
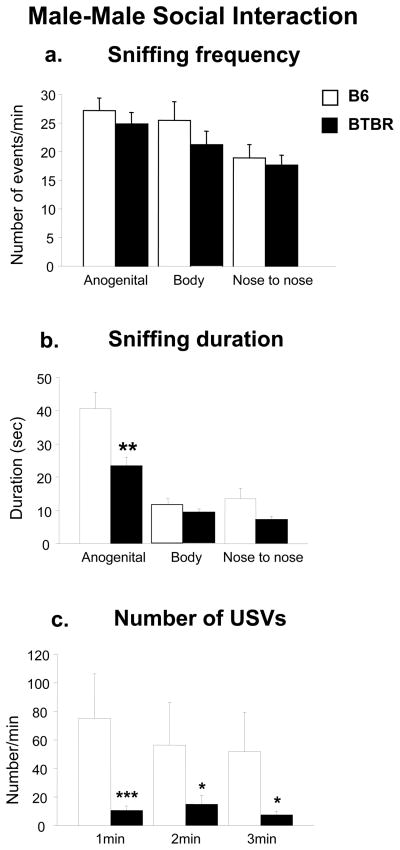
Analysis of the social sniffing response in adult male mice interacting with another male revealed a significant main effect of strain on time spent sniffing [duration, F1,22 = 7.60, p =0.01], body areas [frequency, F2,44 = 24.58, p <0.0001; duration, F2,44 = 69.03, p <0.0001], and strain × body areas interaction [duration, F2,44 = 6.52, p<0.01](Figure 3a and b). Post hoc comparisons revealed that both strains sniffed the anogenital area more than other body areas [p<0.0001 for B6; p<0.05 for BTBR]. BTBR males showed a lower amount of anogenital sniffing to a male partner in comparison to B6 males [Tukey’s HSD test: p <0.0001 for B6 versus BTBR].
Figure 3.
Male-male social interaction: A three minute session measured parameters of interaction of a resident male with an unfamiliar male of the same strain. a) Sniffing frequency, b) sniffing duration, c) number of ultrasonic vocalizations. N=12 each strain. ***p<0.001; **p<0.01 and *p<0.05 for BTBR compared to B6 pups.
Adult BTBR male mice emitted significantly fewer USV when compared to adult B6 male mice [F1,22 = 4.31, p =0.04], during each minute of the male-male social interaction session [Tukey’s HSD test for B6 versus BTBR: 1st min, p <0.001; 2nd and 3rd min, p<0.05], (Figure 3c). As shown in Table 1, mean USV durations and peak frequencies showed no strain differences [duration, F1,17 = 3.12, p =0.10; peak frequency, F1,17 = 0.44, p =0.51]. USV peak amplitudes analysis revealed that BTBR males emitted calls with lower amplitude than B6 males [F1,17 = 11.91, p <0.01]. Analysis of total calling time showed that BTBR males spent less time vocalizing than B6 males [F1,17 = 6.72, p =0.01].
No significant strain differences were detected on locomotor activity [F1,22 = 0.01, p = 0.95], time spent digging [F1,22 = 1.59, p =0.22] or grooming [frequency, F1,22 = 0.40, p =0.53; duration, F1,22 = 0.27, p =0.61] (data not shown). In agreement with male-female interaction data, BTBR male mice spent more time rearing than B6 male mice [F1,22 = 6.76, p <0.05] (Table 2).
Female-female social interaction test
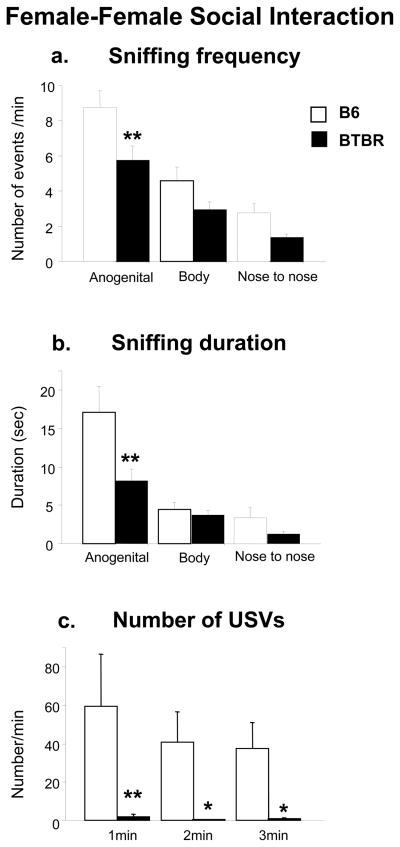
Analysis of the social sniffing response in adult female mice interacting with another female revealed significant differences between strains on frequency [F1,22 = 9.64, p< 0.005] (Figure 4a), duration [F1,22 = 6.83, p = 0.01](Figure 4b), and body areas [frequency, F2,44 = 37.05, p < 0.0001; duration, F2,44 = 24.27, p < 0.0001]. In general, BTBR mice spent less time investigating the female partner than B6 females. The two-way interaction strain × body areas for duration was significant [F2,44 = 3.78, p = 0.03]. Post hoc analysis revealed that B6 females spent more time sniffing the anogenital area of the stimulus than BTBR females [Tukey’s HSD test: p <0.01 for B6 versus BTBR].
Figure 4.
Female-female social interaction: A three minutes session measured parameters of interaction of a resident female with an unfamiliar female of the same strain. a) Sniffing frequency, b) sniffing duration, c) number of ultrasonic vocalizations. N=12 each strain. **p<0.01 and *p<0.05 for BTBR compared to B6 pups.
Adult female BTBR emitted significantly fewer USV during the female-female interaction test when compared with adult female B6 [F1,22 = 8.91, p< 0.01], (Figure 4c). Strain differences were significant during each minute of the interaction [Tukey’s HSD test for B6 versus BTBR: 1st min, p <0.01; 2nd min, p <0.05, 3rd min, p<0.05], (Figure 4c). Analysis of USV durations revealed that BTBR females emitted calls with shorter duration than B6 females [F1,13 = 4.68, p< 0.05]. USV peak frequencies and peak amplitudes showed no strain differences during the female-female social interaction test [peak frequency, F1,13= 0.43, p = 0.52; peak amplitude, F1,13= 0.26, p = 0.62]. Analysis of total calling time showed that BTBR females spent less time vocalizing than B6 females [F1,13 = 6.45, p =0.02].
No significant strain differences were detected in females on behavioral responses associated with locomotor activity and exploration of the environment [crossing, F1,22 = 0.01, p = 0.97; rearing, F1,22 = 0.86, p = 0.36; digging, F1,22 = 0.38, p = 0.54; grooming frequency, F1,22 = 0.06, p = 0.81; grooming duration, F1,22 = 0.38, p = 0.54] (Table 2).
Correlation between social investigation and USV emission rates
As shown in Table 3, in the male-female social interaction, number of ultrasonic vocalizations emitted by B6 and BTBR males were positively correlated with time spent socially investigating the same-strain female [B6, r = 0.982, p <0.0001; BTBR, r = 0.978, p <0.0001; see Figure S2 for details]. In the male-male social interactions, B6 call emission was significantly correlated with social investigation [r = 0.655, p = 0.02;], while in BTBR mice there was no significant correlation [r = 0.218, p = 0.49]. Similarly, in female-female social interactions, B6 call emission was significantly correlated with social investigation [r = 0.874, p < 0.001], but BTBR call emission was not correlated with social investigation [r = 0.044, p = 0.89].
Table 3.
Correlations (r value and * significance) between number of Ultrasonic Vocalizations and Social Investigation
| Social Encounters | B6 | BTBR |
|---|---|---|
| Male-Female | 0,98** | 0,97** |
| Male-Male | 0,65* | 0.21 |
| Female-Female | 0,87** | 0.13 |
Pattern of sonographic structure among strains and within each social encounters
Male-female social interaction test
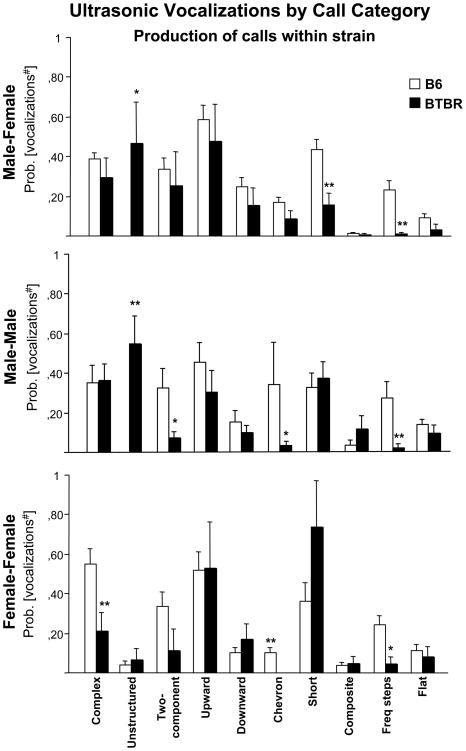
Proportions of calls within each category are shown in Figures 5 and 6. The pattern of sonographic structure differed across strains [F1,18 = 15.9, p < 0.001] and call category [F9,162 = 6.74, p < 0.0001]. A significant strain × category interaction was detected [F9,162 = 2.86, p < 0.01]. Analysis of each USV category separately revealed a strain-dependent effect on the proportion of calls in three of the ten call categories. Specifically, BTBR emitted fewer short calls [Mann-Whitney, p<0.005], fewer frequency steps calls [Mann-Whitney, p<0.005] and more unstructured calls [Mann-Whitney, p<0.05] than B6 (Figure 5).
Figure 5.
Production of calls within each of 10 qualitative vocalization categories in adult B6 and BTBR for each type of social encounter. Probability of producing calls from each of the ten categories of USV (proportion of each call category within the total calls for the subject). Category initials correspond to the descriptions in the Methods section: Cx = complex, Un = unstructured, Tc = two-component, Up = upward, Dw = downward, Ch = chevron, Sh = short, Cp = composite, Fs = frequency steps, Fl = flat. Number of total calls analyzed: 839 BTBR calls (n=9), 4543 B6 calls (n=11) in the male-female encounter, 395 BTBR calls (n=12), 2026 B6 calls (n=7) in the male-male encounter, 55 BTBR calls (n=6), 1805 B6 calls (n=9) in the female-female encounter. **p<0.01 and *p<0.05 for the comparisons between strains.
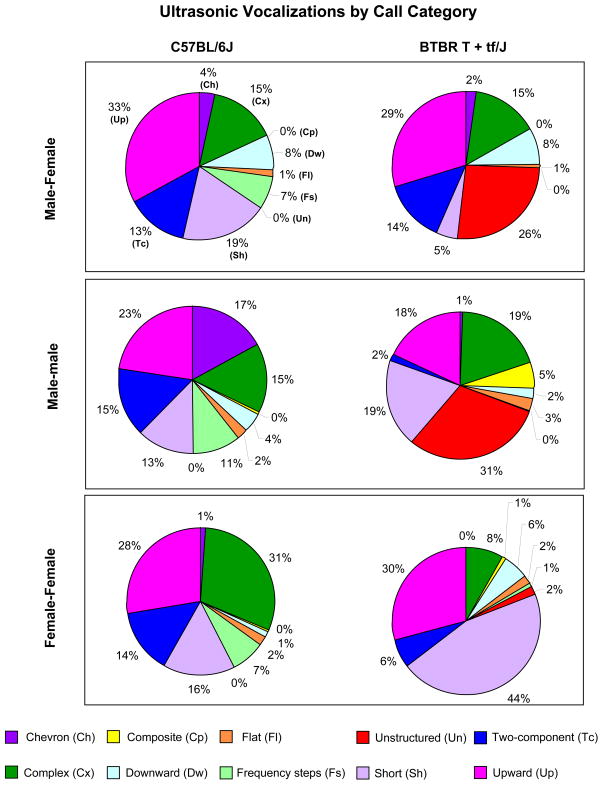
Figure 6.
Distributions of call categories in adult B6 and BTBR during each type of social encounter. Pie graphs display the percentages of the different call categories within each strain, for male-female, male-male, and female-female social interactions. Percentages were calculated in each strain as number of calls in each category for each subject/total number of calls analyzed in each subject.
Male-male social interaction test
As shown in Figures 5, a detailed qualitative analysis of the adult male vocalizations revealed a significant difference on the pattern of call categories between BTBR and B6 male mice [strain, F1,17 = 4.69, p <0.05; category, F9,153 = 3.12, p < 0.001; strain × category interaction, F9,153 = 3.72, p < 0.001]. When analyzing each USV category separately, a strain-dependent effect was found on the proportion of calls in four of the ten call categories. Specifically, adult male BTBR mice emitted fewer frequency steps [Mann-Whitney, p=0.01], two-components [Mann-Whitney, p<0.05], chevron-like [Mann-Whitney, p<0.05], and more unstructured [Mann-Whitney, p=0.01] calls as compared to adult B6 male mice during an encounter with another male.
Female-female social interaction test
A detailed examination of the sonograms generated for each subject revealed strain-dependent differences in USV categories for interacting females [category, F9,117 = 9.59, p < 0.0001; strain × category interaction, F9,117 = 2.81, p = 0.01]. A strain effect was found on the proportion of calls in three of the ten call categories. BTBR females emitted fewer complex calls [Mann-Whitney, p=0.01], fewer frequency steps calls [Mann-Whitney, p<0.05] and fewer chevron calls [Mann-Whitney, p=0.01] than B6 females (Figure 5).
Pattern of sonographic structure across social encounters
Proportions of calls within each category were analyzed across three social encounters. The pattern of sonographic structure differed across strains [F1,48 = 18.2, p < 0.0001], but did not differ acroos social encounters [social encounter, F2,48 = 0.02, p < 0.98; social encounter × strain, F2,48 = 0.25, p < 0.77].
Descriptive analysis
Strain differences in call categories are shown in Figure 6 and Table 1. Adult B6 mice emitted a wide repertoire of calls and similar waveform patterns in each of the three types of social encounters. BTBR emitted a narrower repertoire of calls. The most striking difference was the high levels of unstructured calls in the male-female (BTBR 26% versus B6 0%) and male-male encounters (BTBR 31% versus B6 0%). These unstructured calls did not contain a main sound component and their shape could not be classified into any of the other categories (see example in Figure 1). BTBR emitted minimal numbers of frequency steps (<1%) in all the social contexts. In addition, BTBR males emitted fewer shorts than B6 males (BTBR 5% versus B6 19%) during courtship. By contrast, when BTBR resident males interacted with an intruder male, emitted more shorts (BTBR 19% versus B6 13%), composite (BTBR 5% versus B6 0%) and fewer two-components (BTBR 2% versus B6 15%) and chevron (BTBR 1% versus B6 17%) calls than B6 males. In the female-female interactions, BTBR resident females emitted a higher percentage of shorts (BTBR 44% versus B6 16%) and downwards (BTBR 6% versus B6 1%) but fewer complex calls (BTBR 8% versus B6 31%) and two-components (BTBR 6% versus B6 14%) than B6 females.
Discussion
BTBR T+ tf/J is an inbred mouse strain that displays social abnormalities and repetitive behaviors analogous to the first and third diagnostic symptoms of autism (Bolivar et al. 2007; McFarlane et al. 2008; Moy & Nadler 2008; Moy et al. 2007; Yang et al. 2009; Yang et al. 2007a; Yang et al. 2007b). We previously reported unusual repertoire of vocalizations in BTBR pups separated from their mothers and siblings (Scattoni et al. 2008a) indicative of a potential communication deficit during early stages of ontogeny. We now report that adult BTBR emit unusual properties of vocalizations as compared to B6, in three different social contexts.
During male-female interactions, the social test which elicited the highest numbers of USV in B6, adult BTBR male mice showed less social investigation of the female than B6, as detected by a reduction of anogenital sniffing (see Video 1 and Video 2 provided in the Supplementary Materials), and emitted fewer calls than B6. Concordance between these independent parameters supports the growing literature that vocalizations are a component of social interactions in adult mice (D’Amato 1991; Holy & Guo 2005; Moles et al. 2007; Portfors 2007; Wang et al. 2008; White et al. 1998). Importantly, the pattern of vocalizations differed between the two strains. Specifically, two call categories, “short” and “frequency steps,” were frequent among B6 male calls but considerably less frequent among BTBR calls, whereas unstructured calls were present only in the BTBR male vocal repertoire.
During male-male interactions, BTBR displayed less anogenital sniffing than B6, although comparable levels were obtained on other sniffing parameters. Adult BTBR males emitted fewer vocalizations than adult B6 males during male-male encounters. Both BTBR and B6 male vocal repertoire was limited to complex, upward and short calls. However, BTBR males emitted fewer “two-components”, “chevron” and “frequency steps” and again a higher number of “unstructured” calls in comparison to B6. In a mouse resident-intruder paradigm, no ultrasonic vocalizations have been previously reported (Nyby 2001; Sales 1972a; Whitney & Nyby 1983) The resident-intruder test usually involves a dominant-subordinate relationship that leads to overt agonistic behavior. In our testing sessions, however, no episodes of fighting were observed in B6 or BTBR dyads, probably due to the short time of interaction, 3 minutes. It is likely that a clear social hierarchy between resident and intruder was not established, and dyads were more involved in affiliative/social behaviors with concomitant emission of ultrasonic vocalizations. To our knowledge, the current data represent the first evidence of male ultrasonic vocalizations during male-male interactions.
During female-female interactions, BTBR engaged in less social investigation than B6, and displayed a nearly complete absence of ultrasonic emissions. B6 females engaged in high levels of social interaction and vocalizations as previously reported (Maggio & Whitney 1985; Scattoni et al. 2008b). The adult female B6 vocal repertoire mainly consisted of five out of ten call categories, namely “complex”, “two-components”, “upward”, “shorts” and “frequency steps”. By contrast, calls detected during BTBR female-female interactions were primarily the “upward” and “short” call categories only. It is worth noting that the female-female social context is the only one eliciting emission of unstructured calls both in B6 and BTBR mice (Figure 5). USV emitted by the resident female facilitates proximity of the intruded female, and may help the resident to acquire relevant social information about the intruder (Moles et al. 2007). In agreement with male BTBR data, BTBR female dyads showed less social interactions and emitted fewer vocalizations as compared to B6 female dyads.
One of the most frequent BTBR call categories, “Unstructured”, was never reported by other investigators in other mouse strains (Branchi et al. 1998; Gourbal et al. 2004; Holy & Guo 2005; Panksepp et al. 2007) but a similar pattern (defined as complex sonogram not resembling any of the other USV shapes) is described in a pivotal paper about call categorizations of neonatal Sprague-Dawley rat USV (see Figure 5 in (Brudzynski et al. 1999)). Our data provide evidence that “unstructured” calls are also present in the B6 females. Therefore, it appears unlikely that their production is due to motor control deficits or larynx changes of the BTBR strain.
We recognize that the unstructured calls represent a peculiar, unique sonographic pattern, characterized by the lack of a well-defined wave shape (Figure 1 and Figure S1 in the Supplementary Materials). In Figure S1 we illustrate examples of the unstructured calls, audible mouse emissions, and cage noises, to clarify that the unstructured calls are not an artifact and do not resemble either audible or sonic signals recorded during adult social interactions. In fact, their frequency and energy ranges, shown in Table 1, were comparable to those of the other USV categories. Notably, the BTBR unstructured call frequency range (72.2 ± 18 kHz and 71.0 ± 10.6 kHz) fell within the usual range of the adult mouse ultrasonic vocalizations (50–90 kHz) (Holy & Guo 2005; Liu et al. 2003; Nyby 2001). Like other ultrasonic calls, unstructured calls were perceived as whistle-like sounds (audiofiles Audio1 and Audio2). Interestingly, we noticed that unstructured calls by BTBR males were primarily emitted by those individuals that vocalized less (<64 calls). If unstructured calls are a distinctive feature of low vocalizers, this could explain why unstructured calls were not been previously described in strains with higher vocalization rates.
It is important to note that the short session durations, 3 to 5 minutes, have been selected to mainly focus our analysis on social approach and motivation rather than on sexual or aggressive behaviors. In fact, no episodes of overt fighting or mating were observed during the three different experimental encounters. BTBR displayed high repetitive self-grooming during longer experimental sessions (10–20 minutes), e.g. within a social test apparatus when another stranger animal is present or when the subject is alone in a bare cage (McFarlane et al. 2008; Yang et al. 2009; Yang et al. 2007a; Yang et al. 2007b). In contrast, during the short test sessions used in the present study, BTBR did not display high repetitive self-grooming.
Olfactory investigation allows the mouse to gather biologically meaningful information on the identity of a conspecific, such as social status and sex (Brennan 2004; Brown 1975; Hurst et al. 2001; Keverne 2005). Moreover, the USV emitted during social interactions seem to have an affiliative function in facilitating proximity, a pre-requisite for the acquisition of relevant information about the intruder by the resident mouse. In all the social contexts considered, adult BTBR mice showed decreased social investigation associated with lower levels of vocalizations indicative of impaired social competencies. Both in male–female and female–female interaction, the resident animal is the vocalizing and investigating subject, whereas the intruder usually explores the new environment and tend to avoid the prolonged and persistent social investigation performed by the resident (D’Amato & Moles 2001; Moles et al. 2007; Wang et al. 2008; Whitney et al. 1973). When facing a female or a male intruder, BTBR resident male mice preferred to explore their home cage environment, as indicated by high levels of rearing, rather than to focus on social cues.
In addition, the correlation analyses between social investigation and USVs emission rate revealed that in B6 mice the two variables were positively correlated in all the three different social settings, whereas in BTBR mice such positive correlation was significant only in the male-female interaction dyads, a social context with high biological significance, because of higher levels of male social responsiveness triggered by a sexually receptive female. There is compelling evidence that ultrasonic vocalizations in this context serve not only to establish or to maintain social contact (Hammerschmidt et al. 2009; Pomerantz et al. 1983) but are predictors of mating opportunities and are associated with reward expectations. Male-female interactions have been extensively used to investigate mouse vocalizations, in the context in which male mice are exposed to female pheromonal cues contained in fresh urine (Holy & Guo 2005; Malkesman et al.; Wohr et al.). In agreement with our findings, adult male BTBR mice displayed lower scent marking and minimal ultrasonic vocalization responses to female urine obtained from both B6 and BTBR females (Wohr et al.)). In assessing male social motivation in response to a female stimulus, a positive correlation was reported between ultrasonic vocalizations and the total number of scent marks deposited by both B6 and BTBR male subjects in the presence of same strain female urine (Wohr et al.)). In BTBR male mice, both female presence and female olfactory cues thus elicit a lower level of social investigation, scent marks and ultrasonic vocalizations, but still maintain a positive correlation between the social behavioral responses and vocalization rates. While still correlated, social responses and vocal performances are dramatically reduced in magnitude in BTBR, thus confirming in the male-female setting the same decreased social motivation reported for BTBR mice in male-male and female-female settings.
Positive correlations between USV emission rate and social investigation were previously reported for several other mouse strains, including DBA/2J, C57BL/6J, A/J, BALB/cJ, and outbred albino NMRI (Moles et al. 2007; Nyby 1983; Panksepp et al. 2007). In comparison, BTBR appears to be an outlier, contributing further evidence for an unusual BTBR social profile relevant to an autism-like phenotype.
Significant strain differences emerged when comparing the vocal repertoire, i.e. the relative frequencies of the 10 different call categories, across the three different social encounters. to identify similarities or differences in the vocal patterns elicited by different social partners. It appears that the use of the different call categories is strain-specific. General characteristics of calls were similar in B6 across their different types of social interactions, and similar in BTBR across their different types of social interactions. Taken together, these data indicate that BTBR mice respond to an unfamiliar partner with a behavioral and vocal repertoire that is reduced and unusual for the mouse social repertoire, at least as compared to the social B6 inbred strain.
Our results are consistent with previous studies on animal models of autism that reported deficits in communication during courtship (Jamain et al. 2008) and female-female social interactions (Scattoni et al. 2008b). Mice with a loss-of-function mutation in the murine NLGN4 ortholog Nlgn4, which encodes the synaptic cell adhesion protein neuroligin-4, were found to exhibit deficits in reciprocal social interactions and communication (Jamain et al. 2008). Latency to start calling, upon contact with a female mouse in estrous, was 3.2 times longer in Nlgn4 null mutants as compared to wildtype littermate controls, and the total number of calls per session was reduced by 48% in Nlgn4 mutants as compared to wildtype controls (Jamain et al. 2008). Null mutation of the Avpr1b gene resulted in reduced USV emission by adult females during the resident–intruder test, while social sniffing levels were unaltered (Scattoni et al. 2008b).
Our findings of an unusual pattern of vocalization categories in adult BTBR confirm our previous results in BTBR pups separated from their mothers and nest (Scattoni et al. 2008a). Both developmental ages displayed fewer call categories than their B6 controls. By contrast, BTBR pups emitted more and louder USV than B6, while BTBR adults emitted fewer USV than B6, suggesting that emission rate and wideness of the vocal repertoire are two totally unrelated call features. Since the well-replicated social deficits in BTBR have led to the proposal of this strain as a mouse model of autism (Bolivar et al. 2007; McFarlane et al. 2008; Moy & Nadler 2008; Moy et al. 2007; Yang et al. 2009; Yang et al. 2007a; Yang et al. 2007b), it is interesting to speculate that the more frequent and louder USV of BTBR pups are analogous to the unusual cries, guttural grunts and squeals reported for some infants later diagnosed with autism (Johnson 2008; Sheinkopf et al. 2000; Zwaigenbaum et al. 2005). Fewer USV in adult BTBR during social encounters may be analogous to language impairments in autistic children and adults (Happe 1993; Lord et al. 2000; Siegal & Blades 2003; Tager-Flusberg & Caronna 2007). Corroborative results on social sniffing and vocalizations, in each of the adult social encounters, support the value of simultaneously scoring these two aspects of the mouse social repertoire, which may reflect components of social motivation and bioacoustic communication. Future studies will be devoted to correlate specific sequences of call categories within a bout of ultrasonic vocalizations to specific behavioral responses. Taken together, our results encourage the evaluation of social investigation and ultrasonic calls as fundamental endophenotypes of the adult mouse social repertoire, which may be relevant to the first and second diagnostic symptoms of autism.
Supplementary Material
Audio 1 and Audio 2. Two audioclips representing examples of unstructured calls emitted by BTBR males during male-female and male-male social encounters. Recordings were collected and converted from mouse’s ultrasonic range to the human hearing range by Avisoft software. In order to appreciate the waveforms in greater detail, the wav file was converted from the sample rate of 250 kHz in the original wav file to 11.025 kHz, resulting in a slower speed for human listening.
Examples of “unstructured” mouse calls, audible calls, and non-mouse noises. Spectrograms illustrate that the unstructured calls resemble some properties of other mouse vocalizations but do not resemble the sonic and noise signals recorded in the adult social interactions sonograms. a) Pup wriggling calls; b) Female audible calls during male approach; c) cage noises produced during male fighting; d) cages noises produced during contact of the body of the mouse with the arena wall.
During male-female social interaction, the number of ultrasonic vocalizations emitted by B6 and BTBR males was positively correlated with time spent socially investigating the same-strain female. Ten out of 12 BTBR males emit less than 25 calls in five minutes. Thus the relationship between the two variables is primarily due to the presence of the 2 males emitting between 50 and 100 USVs. By contrast, B6 males emitted variable amounts of calls and engaged in variable amounts of social investigation, producing values for both parameters that were more homogeneously scattered along the regression line.
Video 1 and Video 2. During male-female encounters, adult BTBR male mice showed less social investigation of the female than B6 male mice. Video 1: B6 male socially investigating a B6 sexually receptive female. Video 2: BTBR male socially investigating a BTBR sexually receptive female.
Acknowledgments
We are grateful for the excellent technical contributions of our students Maria Adelaide Marconi, Ludovica De Benedetti, and Gloria Matte Bon, ISS, and Mark Harris, NIMH. Special thanks go to Professor Stefan Brudzynsky, Brock University, Dr Markus Wöhr, University of Marburg, and Raimund Specht, Avisoft Bioacoustics, for their expert advice concerning the unstructured call categorization. We thank Adam Katz, NIMH, for the editing of the supplementary movies. This work was supported by the National Institute of Mental Health Intramural Research Program and Istituto Superiore di Sanità 530F/52 ‘Neurobehavioral phenotyping of genetically modified mouse models of mental retardation’.
References
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33:249–256. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Brennan PA. The nose knows who’s who: chemosensory individuality and mate recognition in mice. Horm Behav. 2004;46:231–240. doi: 10.1016/j.yhbeh.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Brown RE. Effects of social isolation in adulthood on odor preferences and urine-marking in male rats. Behav Neural Biol. 1975;44:139–143. doi: 10.1016/s0163-1047(85)91301-9. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34:195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- D’Amato FR. Courtship ultrasonic vocalizations and social status in mice. Anim Behav. 1991;41:875–885. [Google Scholar]
- D’Amato FR, Moles A. Ultrasonic vocalizations as an index of social memory in female mice. Behav Neurosci. 2001;115:834–840. doi: 10.1037//0735-7044.115.4.834. [DOI] [PubMed] [Google Scholar]
- D’Udine B, Robinson DJ, Oliverio A. An analysis of single-gene effects on audible and ultrasonic vocalizations in the mouse. Behav Neural Biol. 1982;36:197–203. doi: 10.1016/s0163-1047(82)90189-3. [DOI] [PubMed] [Google Scholar]
- Frith U, Happe F. Language and communication in autistic disorders. Philos Trans R Soc Lond B Biol Sci. 1994;346:97–104. doi: 10.1098/rstb.1994.0133. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthe RM, Goodall G, Dantzer R. Ethological study of the effects of tetrahydroaminoacridine (THA) on social recognition in rats. Psychopharmacology (Berl) 1994;114:644–650. doi: 10.1007/BF02244996. [DOI] [PubMed] [Google Scholar]
- Gourbal BE, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe FG. Communicative competence and theory of mind in autism: a test of relevance theory. Cognition. 1993;48:101–119. doi: 10.1016/0010-0277(93)90026-r. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP. Recognition of autism before age 2 years. Pediatr Rev. 2008;29:86–96. doi: 10.1542/pir.29-3-86. [DOI] [PubMed] [Google Scholar]
- Kanner L. Follow-up study of eleven autistic children originally reported in 1943. J Autism Child Schizophr. 1971;1:119–145. doi: 10.1007/BF01537953. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Odor here, odor there: chemosensation and reproductive function. Nat Neurosci. 2005;8:1637–1638. doi: 10.1038/nn1205-1637. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Maggio JC, Maggio JH, Whitney G. Experience-based vocalization of male mice to female chemosignals. Physiol Behav. 1983;31:269–272. doi: 10.1016/0031-9384(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry. 67:864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot E, Pye D. Sound analysis of ultrasonic distress calls of mouse pups as a function of their age. Anim Behav. 1969:340–349. [Google Scholar]
- Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- Nyby J, Whitney G. Ultrasonic communication of adult myomorph rodents. Neuroscience & Biobehavioral Reviews. 1978;2:1–14. [Google Scholar]
- Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton, Florida: 2001. pp. 3–18. [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Rugh R. The Mouse: Its reproduction and development. Oxford University Press; Oxford: 1990. [Google Scholar]
- Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972a;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. Journal of Zoology. 1972b;168:149–164. [Google Scholar]
- Scattoni ML, Branchi I. Vocal repertoire in mouse pups: strain differences. In: Brudzinsky SM, editor. Handbook of Mammalian Vocalization. Academic Press; Oxford: 2009. pp. 88–96. [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008a;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008b;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Mundy P, Oller DK, Steffens M. Vocal atypicalities of preverbal autistic children. J Autism Dev Disord. 2000;30:345–354. doi: 10.1023/a:1005531501155. [DOI] [PubMed] [Google Scholar]
- Siegal M, Blades M. Language and auditory processing in autism. Trends Cogn Sci. 2003;7:378–380. doi: 10.1016/s1364-6613(03)00194-3. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: autism and other pervasive developmental disorders. Pediatr Clin North Am. 2007;54:469–481. vi. doi: 10.1016/j.pcl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Whitney G, Coble JR, Stockton MD, Tilson EF. Ultrasonic emissions: do they facilitate courtship of mice. J Comp Physiol Psychol. 1973;84:445–452. doi: 10.1037/h0034899. [DOI] [PubMed] [Google Scholar]
- Whitney G, Nyby J. Cues that elicit ultrasounds from adult male mice. American Zoologist. 1979;19:457–463. [Google Scholar]
- Whitney G, Nyby J. Sound communication among adults. In: Willot JF, editor. The auditory psychology of the mouse. Charles C Thomas; Springfield: 1983. pp. 98–129. [Google Scholar]
- Winslow JT. Curr Protoc Neurosci. Unit 8. John Wiley and Sons, Inc; 2003. Mouse social recognition and preference; pp. 1–16. [DOI] [PubMed] [Google Scholar]
- Winslow JT. Ultrasonic vocalizations by infant mice: an ethological expression of separation anxiety. In: Gould TD, editor. Mood and anxiety related phenotypes in mice. 42. Chapter 5. Humana Press; New York City: 2009. pp. 67–84. [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Audio 1 and Audio 2. Two audioclips representing examples of unstructured calls emitted by BTBR males during male-female and male-male social encounters. Recordings were collected and converted from mouse’s ultrasonic range to the human hearing range by Avisoft software. In order to appreciate the waveforms in greater detail, the wav file was converted from the sample rate of 250 kHz in the original wav file to 11.025 kHz, resulting in a slower speed for human listening.
Examples of “unstructured” mouse calls, audible calls, and non-mouse noises. Spectrograms illustrate that the unstructured calls resemble some properties of other mouse vocalizations but do not resemble the sonic and noise signals recorded in the adult social interactions sonograms. a) Pup wriggling calls; b) Female audible calls during male approach; c) cage noises produced during male fighting; d) cages noises produced during contact of the body of the mouse with the arena wall.
During male-female social interaction, the number of ultrasonic vocalizations emitted by B6 and BTBR males was positively correlated with time spent socially investigating the same-strain female. Ten out of 12 BTBR males emit less than 25 calls in five minutes. Thus the relationship between the two variables is primarily due to the presence of the 2 males emitting between 50 and 100 USVs. By contrast, B6 males emitted variable amounts of calls and engaged in variable amounts of social investigation, producing values for both parameters that were more homogeneously scattered along the regression line.
Video 1 and Video 2. During male-female encounters, adult BTBR male mice showed less social investigation of the female than B6 male mice. Video 1: B6 male socially investigating a B6 sexually receptive female. Video 2: BTBR male socially investigating a BTBR sexually receptive female.