Abstract
Enterobacter species are the fourth most common cause of gram-negative bloodstream infection (BSI). We examined temporal changes and seasonal variation in the incidence rate of Enterobacter spp. BSI, estimated 28-day and 1-year mortality, and determined in vitro antimicrobial resistance rates of Enterobacter spp. bloodstream isolates in Olmsted County, Minnesota, from 1/1/1998 to 12/31/2007. Multivariable Poisson regression was used to examine temporal changes and seasonal variation in incidence rate and Kaplan-Meier method to estimate 28-day and 1-year mortality. The median age of patients with Enterobacter spp. BSI was 58 years and 53% were female. The overall age- and gender-adjusted incidence rate of Enterobacter spp. BSI was 3.3/100,000 person-years (95% confidence interval [CI]: 2.3-4.4). There was a linear trend of increasing incidence rate from 0.8 (95% CI: 0-1.9) to 6.2 (95% CI: 3.0-9.3) per 100,000 person-years between 1998 and 2007 (p=0.002). There was no significant difference in the incidence rate of Enterobacter spp. BSI during the warmest four months compared to the remainder of the year (incidence rate ratio 1.06 [95% CI: 0.47-2.01]). The overall 28-day and 1-year mortality rates of Enterobacter spp. BSI were 21% (95% CI: 8-34%) and 38% (95% CI: 22-53%), respectively. Up to 13% of Enterobacter spp. bloodstream isolates were resistant to third-generation cephalosporins. To our knowledge, this is the first population-based study to describe the epidemiology and outcome of Enterobacter spp. BSI. The increase in incidence rate of Enterobacter spp. BSI over the past decade, coupled with its associated antimicrobial resistance, dictate more investigation of this syndrome.
Keywords: bacteremia, epidemiology, mortality, seasonal variation, incidence, Enterobacter, antimicrobial resistance
Introduction
Enterobacter species are the fourth most common cause of gram-negative bloodstream infection (BSI) [1-3]. Population-based studies that specifically address the epidemiology, outcome, and in vitro antimicrobial resistance rates of Enterobacter spp. BSI are lacking and most surveys that have been published have been derived from referral tertiary care centers [4-10]. Therefore, we performed a population-based study to determine the incidence rate and examine temporal changes in the incidence rate of Enterobacter spp. BSI. In addition, we examined seasonal variation in incidence rate of Enterobacter spp. BSI because recent reports have demonstrated seasonal variation in both Escherichia coli [11] and Klebsiella pneumoniae BSI [12] and other syndromes of infections caused by gram-negative bacilli [13]. We also estimated the 28-day and 1-year mortality rates in patients with Enterobacter spp. BSI. Lastly, we examined the in vitro antimicrobial resistance rates of Enterobacter spp. bloodstream isolates in Olmsted County, Minnesota, from 1998 to 2007.
Methods
Setting
Olmsted County is located in southeastern Minnesota and has a population of 124,277 according to the 2000 census (US Census Bureau, Olmsted County QuickFacts [http://quickfacts.census.gov], accessed April 21, 2008). With the exception of a lower prevalence of injection drug use, a higher prevalence of middle-class individuals and a higher proportion being employed in the healthcare industry, the population characteristics of Olmsted County residents are similar to those of USA non-Hispanic whites [14,15]. The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Olmsted County, Minnesota. The microbiology laboratories at Mayo Medical Center and Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centers are geographically isolated from other urban centers as previously described [14,16,17]; therefore, local residents are able to obtain healthcare within the community, rather than seeking healthcare at a distant geographic location.
Case ascertainment
We used complete enumeration of Olmsted County, Minnesota, from 1 January 1998 to 31 December 2007. Using the microbiology databases at the Mayo Medical Center Rochester and Olmsted Medical Center, we identified 38 unique patients with first episodes of monomicrobial Enterobacter spp. BSI during the study period. Medical records were reviewed by the primary investigator (M.N.A.) to confirm the diagnosis, determine patient residency status, and obtain baseline clinical features and outcome.
Blood cultures were processed using standard microbiology techniques according to the Clinical and Laboratory Standards Institute (CLSI). Both laboratories are certified by the College of American Pathologists. CLSI methods were employed to evaluate in vitro antimicrobial susceptibility results of Enterobacter spp. bloodstream isolates. The study was approved by the institutional review boards of both institutions. The detailed case ascertainment and blood culture methods used were described elsewhere [17,18,19].
Case definition
Monomicrobial Enterobacter spp. BSI was defined as the growth of only Enterobacter spp. in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium spp., and Propionibacterium spp. Cases were classified according to the site of acquisition into nosocomial, healthcare-associated, and community-acquired [20]. The primary source of BSI was defined using the Centers for Disease Control and Prevention criteria [21].
Statistical analysis
Descriptive statistics were used to summarize the data: medians and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. The Fisher's exact test was used to evaluate associations between categorical variables and Wicoxon rank-sum test was used to test for differences in medians across continuous variables.
The incidence rate, expressed as the number of new cases of Enterobacter spp. BSI per 100,000 person-years, was calculated assuming that the entire population of Olmsted County was at risk of BSI. The 2000 Olmsted County census figures were used to compute the age-, gender- and calendar year-specific person-years denominator with a projected population growth rate after 2000 of 1.9% per year. The 10-year study period was divided into five two-year intervals (1998-1999, 2000-2001, 2002-2003, 2004-2005, and 2006-2007) and age was divided into five groups (0-18, 19-39, 40-59, 60-79, and ≥ 80 years). The incidence rate was directly adjusted to the USA 2000 white population. A 95% confidence interval (CI) for each incidence rate was estimated using a Poisson distribution.
To evaluate the association between seasonal variation and incidence rate of Enterobacter spp. BSI, the age- and gender-adjusted incidence rate was calculated for both the four warmest months (June through September) and the eight remaining months; the person-years denominator was multiplied by 1/3 and 2/3, respectively. The incidence rate ratio is the ratio of the incidence rate for the four warmest months relative to the incidence rate for the remaining eight months. A 95% CI for the incidence rate ratio was constructed using bootstrap resampling.
To create an additional measure of seasonal variation, the average monthly temperatures for Rochester, Minnesota, were obtained from historic city records (Weatherbase Historical Weather for Rochester, Minnesota, USA [http://www.weatherbase.com], accessed July 24, 2008). Incidence rates were calculated for each of the 12 months assuming a fixed population within a given year. To test for an association between average monthly temperature and the incidence rate of Enterobacter spp. BSI while adjusting for gender, age and calendar year, a multivariable Poisson regression model was used.
The Kaplan-Meier method was used to estimate the 28-day and 1-year all-cause mortality rates. Patients were followed from the date of first episode of Enterobacter spp. BSI until death or last healthcare encounter; long-term follow-up was available through the REP. Patients lost to follow-up were censored on the date of their last healthcare encounter. All analyses were carried out using the SAS statistical software package (version 8.2, SAS Institute, Cary, NC). The level of significance for statistical testing was defined as p<0.05 (2-sided).
Results
We identified 38 unique patients with Enterobacter spp. BSI during the study period; 26 had E. cloacae, 10 had E. aerogenes, and 2 had E. sakazakii BSI. The median age of patients with Enterobacter spp. BSI was 58 years (IQR: 45-75); and 20 (53%) were female. Most cases were healthcare-associated (58%) or nosocomial (21%); the remaining 21% of cases were community-acquired. The urinary tract was the most common identified primary source of infection (24%), followed by the gastrointestinal tract (18%), central venous catheter-related (11%), the respiratory tract (5%), skin and soft tissue (5%), bone and joint (3%), and central nervous system (3%). Twelve patients (32%) had primary BSI of unknown source.
The overall age- and gender-adjusted incidence rate of Enterobacter spp. BSI was 3.3 (95% CI: 2.3-4.4) per 100,000 person-years. The age-adjusted incidence rate of Enterobacter spp. BSI per 100,000 person-years was 3.9 (95% CI: 2.0-5.8) in males and 3.2 (95% CI: 1.8-4.6) in females. The incidence rate of Enterobacter spp. BSI increased linearly with age (p=0.007; Figure 1). After adjusting for gender and age, there was a linear increase in the incidence rate of Enterobacter spp. BSI from 1998 to 2007 (p=0.002; Figure 2).
Figure 1.
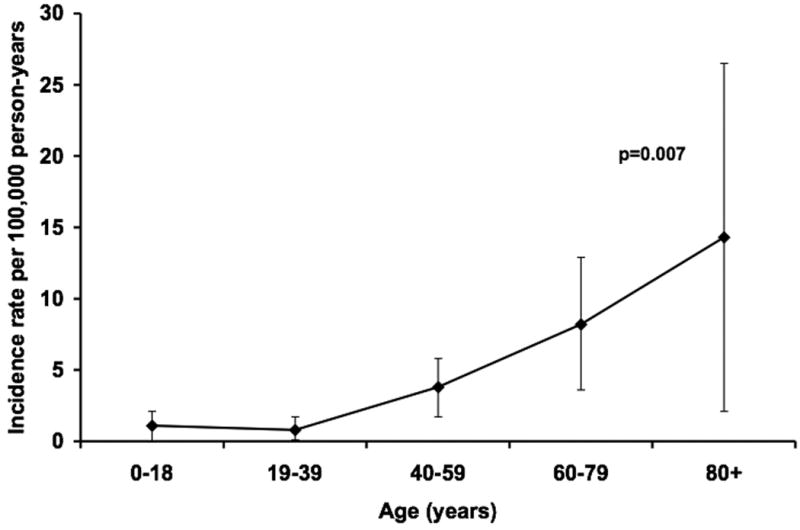
Gender-adjusted incidence rates of Enterobacter species bloodstream infection by age group, 1998-2007.
NOTE. Error bars indicate 95% confidence intervals. P-value denotes a linear change in incidence rate using Poisson regression.
Figure 2.
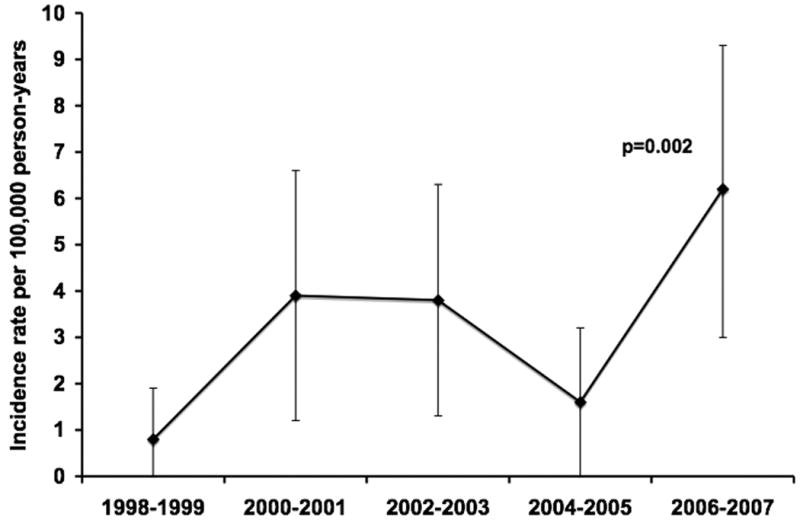
Age- and gender-adjusted incidence rates of Enterobacter species bloodstream infection by calendar year.
NOTE. Error bars indicate 95% confidence intervals. P-value denotes a linear change in incidence rate using Poisson regression.
The age- and gender-adjusted incidence rate of Enterobacter spp. BSI per 100,000 person-years was 3.5 (95% CI: 1.6–5.4) during the warmest four months of the year (June through September) compared to 3.3 (95% CI: 2.0–4.5) during the remainder of the year (incidence rate ratio 1.07 [95% CI: 0.47-2.01]). Additionally, there was no association between the incidence rate of Enterobacter spp. BSI and average temperature (p=0.83; Figure 3).
Figure 3.
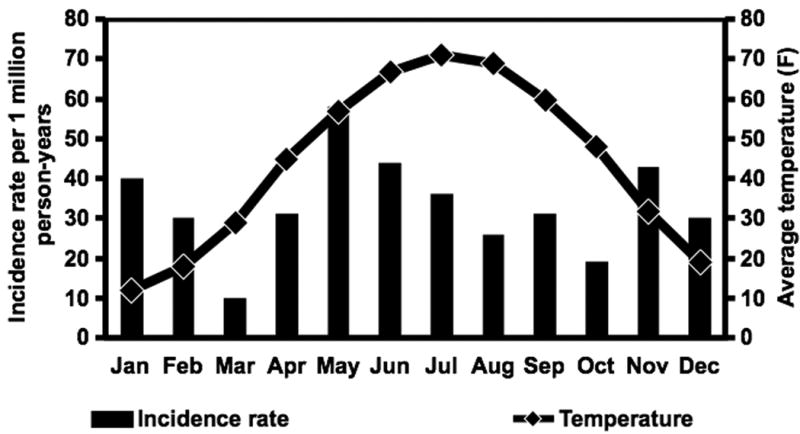
Monthly age- and gender-adjusted incidence rates of Enterobacter species bloodstream infection and average monthly temperatures, 1998-2007.
Patients with E. aerogenes BSI were older than those with E. cloacae BSI (median age 74 vs. 52 years, p=0.008; Table 1). Additionally, patients with E. aerogenes BSI were more likely than those with E. cloacae BSI to have a community-acquired site of infection acquisition (60% vs. 8%, p=0.002) and a urinary tract primary source of infection (50% vs. 15%, p=0.08).
Table 1.
Clinical characteristics of patients with Enterobacter species bloodstream infection.*
| Variable |
E. cloacae n=26 |
E. aerogenes n=10 |
|---|---|---|
| Age: median (IQR) | 52 (39-71) | 74 (61-82) |
| Female sex, n (%) | 15 (58) | 4 (40) |
| Site of acquisition, n (%) | ||
| Community-acquired | 2 (8) | 6 (60) |
| Healthcare-associated | 17 (65) | 3 (30) |
| Nosocomial | 7 (27) | 1 (10) |
| Primary source, n (%) | ||
| Urinary tract | 4 (15) | 5 (50) |
| Gastrointestinal tract | 5 (19) | 1 (10) |
| Central venous catheter-related | 4 (15) | 1 (10) |
| Other | 3 (12) | 1 (10) |
| Unknown | 10 (38) | 2 (20) |
IQR: interquartile range
A 51-year old male and a newborn female with healthcare-associated E. sakazakii bloodstream infection secondary to a gastrointestinal tract source and meningitis, respectively, are not shown.
The age- and gender-adjusted incidence rates of E. cloacae and E. aerogenes BSI per 100,000 person-years were 2.2 (95% CI: 1.4-3.1) and 0.9 (95% CI: 0.4-1.5), respectively. The age-adjusted incidence rates of E. cloacae BSI were comparable in males and females (2.2 [95% CI: 0.8-3.5] and 2.4 [95% CI: 1.2-3.7] per 100,000 person-years, respectively). The incidence rates of E. aerogenes BSI for males and females were 1.6 [95% CI: 0.3-2.8] and 0.6 [95% CI: 0-1.3] per 100,000 person-years, respectively.
Complete patient follow-up was obtained for most of the cohort; no patient was lost to follow-up within 28 days and only 3 (8%) were lost to follow-up within 1 year of Enterobacter spp. BSI. The overall 28-day and 1-year all-cause mortality rates of Enterobacter spp. BSI were 21% (95% CI: 8-34%) and 38% (95% CI: 22-53%), respectively. Although the overall 28-day all-cause mortality rate was relatively higher in patients with E. aerogenes as compared to those with E. cloacae BSI (30% [95% CI: 2-58%] vs. 15% [95% CI: 2-29%], Figure 4a), there was no apparent difference in the long-term survival (Figure 4b).
Figure 4.
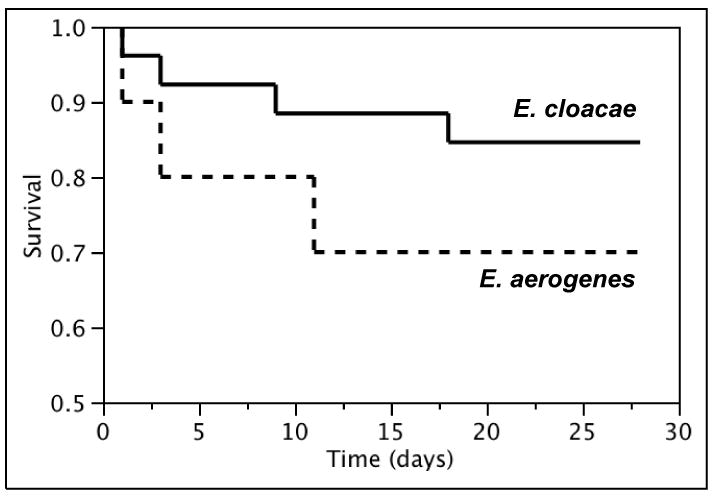
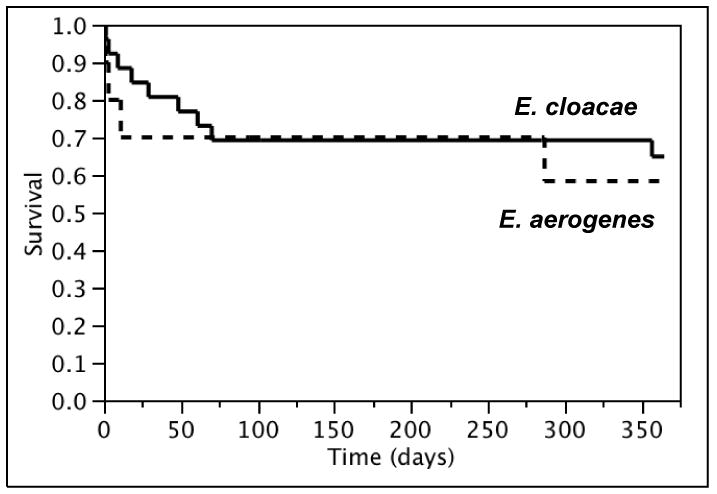
Kaplan-Meier 28-day (a) and 1-year (b) survival curves of patients with Enterobacter cloacae and Enterobacter aerogenes bloodstream infection, 1998-2007.
The in vitro antimicrobial susceptibility rates of Enterobacter spp. bloodstream isolates to all tested antimicrobial agents are shown in Table 2. Among all tested beta-lactams, ceftazidime and piperacillin-tazobactam had the lowest in vitro susceptibility rates (87% and 89%, respectively). No cefepime- or carbapenem-resistant Enterobacter spp. bloodstream isolates were detected in our population over the past decade.
Table 2.
In vitro antimicrobial susceptibility rates of Enterobacter species bloodstream isolates, 1998-2007.
| Antimicrobial | Number of susceptible isolates/number of isolates tested | Susceptibility % |
|---|---|---|
| Ceftazidime | 33/38 | 87 |
| Piperacillin-tazobactam | 33/37 | 89 |
| Ciprofloxacin | 36/38 | 95 |
| Levofloxacin | 35/37 | 95 |
| Trimethoprim-sulfamethoxazole | 37/38 | 97 |
| Gentamicin | 38/38 | 100 |
| Cefepime | 37/37 | 100 |
| Imipenem | 37/37 | 100 |
| Meropenem | 37/37 | 100 |
Discussion
To our knowledge, this is the first population-based study to describe the epidemiology, outcome, and in vitro antimicrobial resistance rates of Enterobacter spp. BSI. We demonstrated a linear trend of an increasing incidence rate of Enterobacter spp. BSI during the past decade. The age- and gender-adjusted incidence rate of Enterobacter spp. BSI increased from 0.8 (95% CI: 0-1.9) to 6.2 (95% CI: 3.0-9.3) per 100,000 person-years between 1998 and 2007. There was no seasonal variation in the incidence rate of Enterobacter spp. BSI.
Based on an age- and gender-adjusted incidence rate of 2.2 (95% CI: 1.4-3.1) per 100,000 person-years, E. cloacae was the fourth most common gram-negative bacillus to cause BSI in our population; Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa (incidence rates of 41.4 [95% CI: 37.6-45.3], 9.7 [95% CI: 7.8-11.6], and 4.7 [95% CI: 3.4-6.1] per 100 000 person-years, respectively) were the three most common causes of BSI due to gram-negative bacilli as previously described [11,17,22]. The incidence rate of BSI due to all four organisms increased with age. The median age of 52 years in patients with E. cloacae BSI was notably lower than that in patients with E. coli, K. pneumoniae, and P. aeruginosa BSI (69, 73 and 69 years, respectively). In contrast to E. coli BSI that was more common in females [11,23], and K. pneumoniae and P. aeruginosa BSIs that were more common in males [17,22,24,25], the incidence rate of E. cloacae BSI was not influenced by gender. Most cases of E. cloacae BSI were healthcare-associated or nosocomial and nearly one-third had no known primary source of infection, which is consistent with previous reports [5,9,10,26].
E. aerogenes is an uncommon cause of gram-negative BSI with an age- and gender-adjusted incidence rate of 0.9 (95% CI: 0.4-1.5) per 100,000 person-years. Although the incidence rate of E. aerogenes BSI in males was nearly three times that in females, the difference was not statistically significant due to the small number of patients with E. aerogenes BSI in our population. Patients with E. aerogenes were older and more likely to have a urinary tract primary source of infection than those with E. cloacae BSI, which is consistent with the results of a recent investigation [27].
Our finding of an increasing incidence rate of Enterobacter spp. BSI over the past decade is consistent with the results of a recent investigation that demonstrated increasing frequency of Enterobacter spp. as a cause of BSI in a tertiary care center in Spain from 1991 to 2006 [7]. It is also consistent with observations of earlier pediatric studies that suggested the emergence of Enterobacter spp. as an important cause of BSI in children [28,29]. The increase in incidence rate of Enterobacter spp. BSI in our population is unlikely due to changes in physicians' practices in obtaining blood cultures in febrile patients, because the incidence rates of BSI due to E. coli, Klebsiella spp., and other gram-negative bacilli have remained stable over the same time period [11,19,22]. Because most cases of Enterobacter spp. BSI were healthcare-associated or nosocomial, it is likely that changes within the local hospitals' environment have resulted in this increase in incidence rate. It is conceivable that an increasing number of patients are colonized with Enterobacter spp. following hospital admission or contact with the healthcare setting for an outpatient procedure such as urinary tract instrumentation, hemodialysis, or outpatient chemotherapy [30]. In addition, some studies have identified previous exposure to broad-spectrum antimicrobial agents, particularly third-generation cephalosporins, as a risk factor for Enterobacter spp. BSI [4,9,31]. It is possible that the increasing use of these antimicrobial agents in our local population is temporally associated with the increase in the incidence rate of Enterobacter spp. BSI, but these data were not collected to permit that determination.
Our observation of an increasing incidence rate of Enterobacter spp. BSI deserves additional study. Subsequent work should determine whether the increase in incidence rate of Enterobacter spp. BSI that was seen locally is also present in other locales. The development of a multi-national population-based study to examine temporal trends in Enterobacter spp. BSI with molecular testing of bloodstream isolates would provide vital clinical and microbiological information to advance this field.
Enterobacter spp. is more likely than other commonly isolated gram-negative bacilli, such as E. coli and Klebsiella spp., to be resistant to antimicrobial agents. For example, 13% of Enterobacter spp. bloodstream isolates in our local area were not susceptible to third-generation cephalosporins as compared to only 1% of both E. coli [32] and Klebsiella spp. bloodstream isolates [22]. The increased resistance is likely due to the inherent capability of Enterobacter spp. isolates to produce inducible chromosomal beta-lactamases, including Amp C. Even when the isolates demonstrate in vitro antimicrobial susceptibility, exposure to certain types of beta-lactam antibiotics, including cephalosporins and extended-spectrum penicillins, can cause a transient increase or induction of Amp C production, resulting in antimicrobial resistance and possibly a predisposition to treatment failures [31,33,34]. It has been demonstrated that 15-19% of Enterobacter spp. isolates develop resistance to third-generation cephalosporins during treatment [4,35]. Therefore, this increase in the incidence rate of Enterobacter spp. BSI makes the choice of an empiric antimicrobial regimen in patients with gram-negative BSI, while awaiting identification of the gram-negative bacillus, much more difficult, especially in patients with recent contact with the healthcare system and other identifiable risk factors for Enterobacter spp. BSI.
The lack of seasonal variation in the incidence rate of Enterobacter spp. BSI in this investigation is consistent with the results of a large study from four continents [12]. However, this is in contrast to our previous work that demonstrated a higher incidence rate of E. coli BSI during the warmest four months than in the reminder of the year [11]. The factors involved in the presence or absence of seasonal variation in BSI have yet to be identified.
Patients with E. cloacae BSI were younger than those with E. aerogenes BSI in our investigation and it is conceivable that the younger age accounted for the lower mortality rate in the former group. The relatively small sample size did not permit an examination of predictors of mortality in a multivariable model to confirm this notion. The 28-day all-cause mortality rate of 15% in patients with E. cloacae BSI was notably lower than the short-term mortality rates (21-69%) reported in investigations extending back to the 1980s [6,8-10,26,36,37]. In more recent investigations, mortality rates of 13-15% have been described and are consistent with our results [7,27]. We speculate that this decline in mortality could be due to advancements in critical care and antimicrobial management.
The unique availability of long-term patient follow-up through the REP resources in our population permitted an estimation of 1-year all-cause mortality rate following Enterobacter spp. BSI that has not been previously described. Over one-third of patients did not survive beyond one year following Enterobacter spp. BSI, and was most likely due to multiple comorbid conditions that characterized these patients. This observation was similar to what we previously observed in patients with Klebsiella spp. BSI [22].
The in vitro antimicrobial resistance rates of Enterobacter spp. bloodstream isolates in our population-based study were notably lower than those reported previously from tertiary care centers. Resistance rate to third-generation cephalosporins was 13% in our study as compared to 17-51% in hospital-based investigations [2,27,38-40]. Similarly, the 5% resistance rate to fluoroquinolones was also lower than previously reported rates of 8-14% from tertiary care centers [2,27,38-41]. Carbapenem-resistance rates among Enterobacter spp. isolates were under 1% in both population- and hospital-based studies [27,38,40]. The lower antimicrobial resistance rates reported from population-based studies as compared to investigations from tertiary care centers were likely due to referral bias that can adversely affect isolate susceptibility data in tertiary care centers [17]. It is conceivable that referral patients underwent more procedures, antimicrobial exposure and complications that prompted transfer to tertiary care centers and predisposed to colonization or infection with bacteria that harbored antimicrobial resistance.
The strength of this study is the population-based design and, therefore, lack of referral bias. Contrary to previous hospital-based studies that have estimated the incidence rate of Enterobacter spp. BSI per the number of admissions to a particular hospital, we determined the incidence rate by 100,000 person-years in a well-defined population.
Our study has limitations. First, our data is derived from one geographic area. Studies from multiple geographic locations may provide a more comprehensive view. Second, we did not perform pulse-field gel electrophoresis to investigate whether the increasing incidence rate of Enterobacter spp. BSI was a result of clonal spread of a single strain in the medical facilities in our region or due to patient-unique strains. For example, the peak in incidence rate of Enterobacter spp. BSI that was observed in the 2006-2007 interval might have been related to a nosocomial outbreak rather than a reflection of a true increase in incidence rate of Enterobacter spp. BSI among the general population. Third, the lack of seasonal variation in Enterobacter spp. BSI might have been due to lack of power due to the relatively small sample size. Finally, the population of Olmsted County consists mainly of middle class whites; therefore, our study results may be generalized only to communities with similar population characteristics.
In summary, Enterobacter spp. has emerged as an important cause of gram-negative BSI. The increase in incidence rate of Enterobacter spp. BSI over the past 10 years should prompt physicians to have a higher suspicion for Enterobacter spp. in patients with suspect or proven BSI. Empiric antimicrobial coverage should include coverage for these microorganisms, particularly in patients with frequent contact with the healthcare setting. For as yet unexplained reasons, the more recent mortality rate in patients with Enterobacter spp. BSI has declined.
Acknowledgments
The authors thank Emily Vetter and Mary Ann Butler for providing us with vital data from the microbiology laboratory databases at the Mayo Clinic, Rochester and Olmsted Medical Center.
The authors thank Susan Schrage, Susan Stotz, RN, and all the staff at the Rochester Epidemiology Project for their administrative help and support.
The study received funding from the Small Grants Program and the Baddour Family Fund at the Mayo Clinic, Rochester, MN. The funding source had no role in study design. This work was made possible by research grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, U.S. Public Health Service).
Footnotes
Transparency Declaration:
MNA and BDL have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential conflicts of interest. MNA, BDL, JEE, and LMB: No conflict.
References
- 1.Diekema DJ, Pfaller MA, Jones RN, et al. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29(3):595–607. doi: 10.1086/598640. [DOI] [PubMed] [Google Scholar]
- 2.Decousser JW, Pina P, Picot F, et al. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J Antimicrob Chemother. 2003;51(5):1213–22. doi: 10.1093/jac/dkg201. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hasan MN, Razonable RR, Eckel-Passow JE, Baddour LM. Incidence rate and outcome of Gram-negative bloodstream infection in solid organ transplant recipients. Am J Transplant. 2009;9(4):835–43. doi: 10.1111/j.1600-6143.2009.02559.x. [DOI] [PubMed] [Google Scholar]
- 4.Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585–90. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 5.Bouza E, Garcia de la Torre M, Erice A, Loza E, Diaz-Borrego JM, Buzon L. Enterobacter bacteremia An analysis of 50 episodes. Arch Intern Med. 1985;145(6):1024–7. doi: 10.1001/archinte.145.6.1024. [DOI] [PubMed] [Google Scholar]
- 6.Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin Infect Dis. 2004;39(6):812–8. doi: 10.1086/423382. [DOI] [PubMed] [Google Scholar]
- 7.Marcos M, Inurrieta A, Soriano A, et al. Effect of antimicrobial therapy on mortality in 377 episodes of Enterobacter spp bacteraemia. J Antimicrob Chemother. 2008;62(2):397–403. doi: 10.1093/jac/dkn155. [DOI] [PubMed] [Google Scholar]
- 8.Lin YC, Chen TL, Ju HL, et al. Clinical characteristics and risk factors for attributable mortality in Enterobacter cloacae bacteremia. J Microbiol Immunol Infect. 2006;39(1):67–72. [PubMed] [Google Scholar]
- 9.Ye Y, Li JB, Ye DQ, Jiang ZJ. Enterobacter bacteremia: Clinical features, risk factors for multiresistance and mortality in a Chinese University Hospital. Infection. 2006;34(5):252–7. doi: 10.1007/s15010-006-5038-3. [DOI] [PubMed] [Google Scholar]
- 10.Haddy RI, Cecil ML, Norris LL, Markert RJ. Enterobacter bacteremia in the community hospital. J Fam Pract. 1991;32(6):601–6. [PubMed] [Google Scholar]
- 11.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Seasonal variation in Echerichia coli bloodstream infection: a population-based study. Clin Microbiol Infect. 2009;15(10):951–3. doi: 10.1111/j.1469-0691.2009.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson DJ, Richet H, Chen LF, et al. Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J Infect Dis. 2008;197(5):752–6. doi: 10.1086/527486. [DOI] [PubMed] [Google Scholar]
- 13.Perencevich EN, McGregor JC, Shardell M, et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(12):1124–31. doi: 10.1086/592698. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 15.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88(6):582–8. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 16.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293(24):3022–8. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008;121(8):702–8. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167(8):834–9. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Recurrent gram-negative bloodstream infection: a 10-year population-based cohort study. J Infect. 2010 doi: 10.1016/j.jinf.2010.03.028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 21.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect control. 1988;16(3):128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Epidemiology and outcome of Klebsiella species bloodstream infection: a population-based study. Mayo Clin Proc. 2010;85(2):139–44. doi: 10.4065/mcp.2009.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laupland KB, Gregson DB, Church DL, Ross T, Pitout JD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14(11):1041–7. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 24.Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122(9):866–73. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection. 2010;38(1):25–32. doi: 10.1007/s15010-009-9145-9. [DOI] [PubMed] [Google Scholar]
- 26.Watanakunakorn C, Weber J. Enterobacter bacteremia: a review of 58 episodes. Scand J Infect Dis. 1989;21(1):1–8. doi: 10.3109/00365548909035673. [DOI] [PubMed] [Google Scholar]
- 27.Song EH, Park KH, Jang EY, et al. Comparison of the clinical and microbiologic characteristics of patients with Enterobacter cloacae and Enterobacter aerogenes bacteremia: a prospective observation study. Diagn Microbiol Infect Dis. 2010;66(4):436–40. doi: 10.1016/j.diagmicrobio.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Andresen J, Asmar BI, Dajani AS. Increasing Enterobacter bacteremia in pediatric patients. Pediatr Infect Dis J. 1994;13(9):787–92. doi: 10.1097/00006454-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Trad O, Jumaa PA, Afify Z. The changing pattern of bloodstream infection in pediatric oncology patients in the United Arab Emirates. Pediatr Hematol Oncol. 2003;20(4):281–9. [PubMed] [Google Scholar]
- 30.Chow JW, Yu VL, Shlaes DM. Epidemiologic perspectives on Enterobacter for the infection control professional. Am J Infect control. 1994;22(4):195–201. doi: 10.1016/0196-6553(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 31.Kaye KS, Engemann JJ, Fraimow HS, Abrutyn E. Pathogens resistant to antimicrobial agents: epidemiology, molecular mechanisms, and clinical management. Infect Dis Clin North Am. 2004;18(3):467–511. viii. doi: 10.1016/j.idc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: a population-based study, 1998-2007. J Antimicrob Chemother. 2009;64(1):169–74. doi: 10.1093/jac/dkp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161–82. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller MA, Jones RN, Marshall SA, et al. Inducible amp C beta-lactamase producing gram-negative bacilli from blood stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE) Diagn Microbiol Infect Dis. 1997;28(4):211–9. doi: 10.1016/s0732-8893(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 35.Kaye KS, Cosgrove S, Harris A, Eliopoulos GM, Carmeli Y. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob Agents Chemother. 2001;45(9):2628–30. doi: 10.1128/AAC.45.9.2628-2630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen G, Schonheyder HC, Sorensen HT. Antibiotic therapy and outcome of monomicrobial gram-negative bacteraemia: a 3-year population-based study. Scand J Infect Dis. 1997;29(6):601–6. doi: 10.3109/00365549709035903. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher PG. Enterobacter bacteremia in pediatric patients. Rev Infect Dis. 1990;12(5):808–12. doi: 10.1093/clinids/12.5.808. [DOI] [PubMed] [Google Scholar]
- 38.Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–9. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 40.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 41.Kang CI, Kim SH, Park WB, et al. Clinical epidemiology of ciprofloxacin resistance and its relationship to broad-spectrum cephalosporin resistance in bloodstream infections caused by Enterobacter species. Infect Control Hosp Epidemiol. 2005;26(1):88–92. doi: 10.1086/502492. [DOI] [PubMed] [Google Scholar]


