Abstract
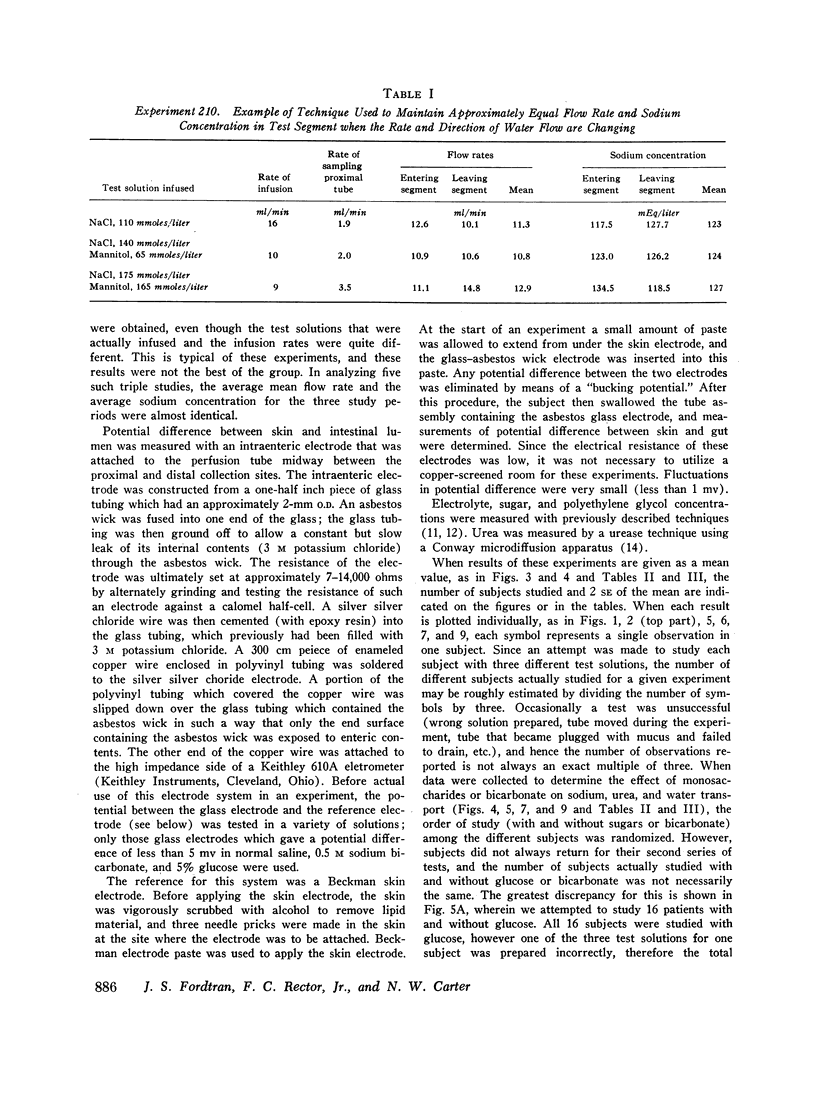
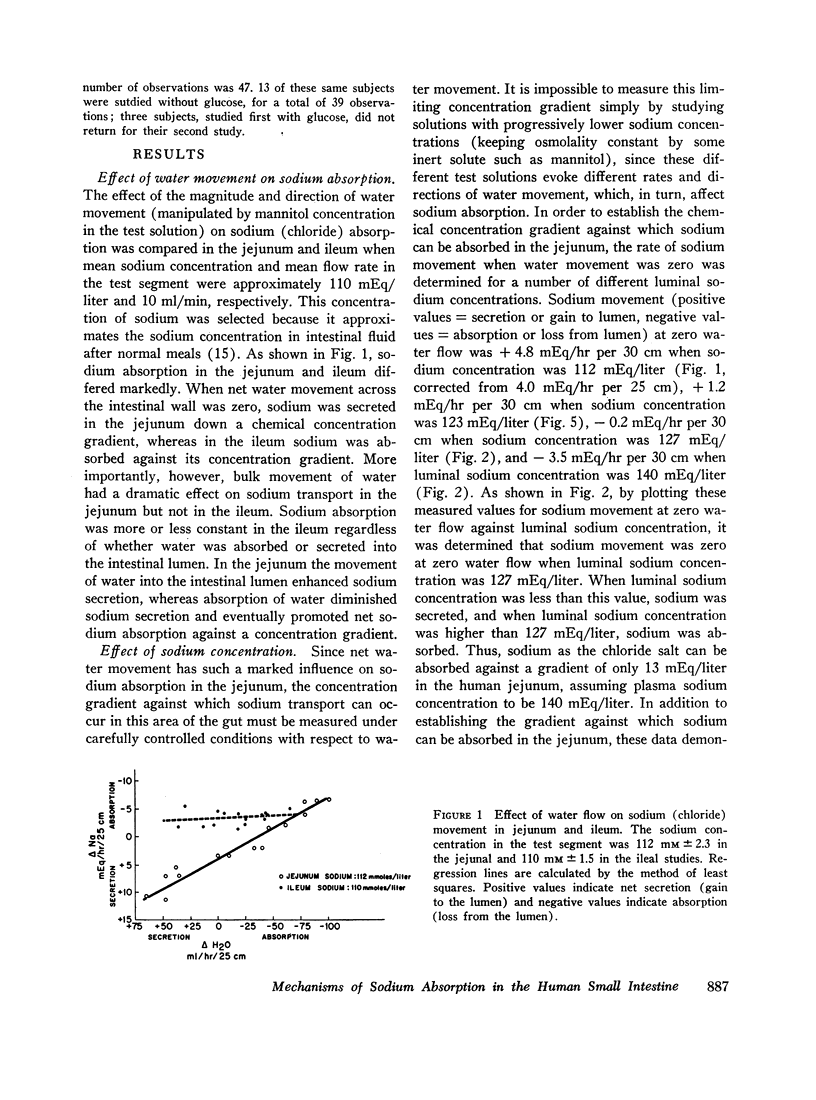
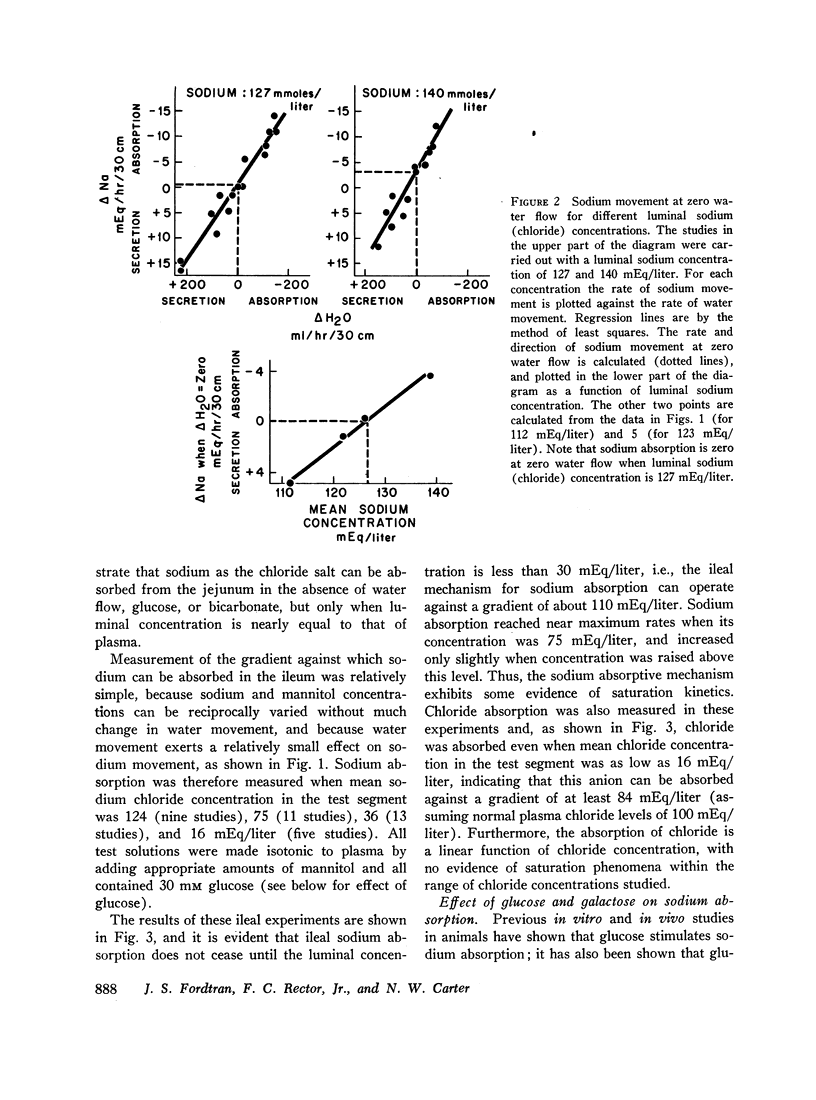
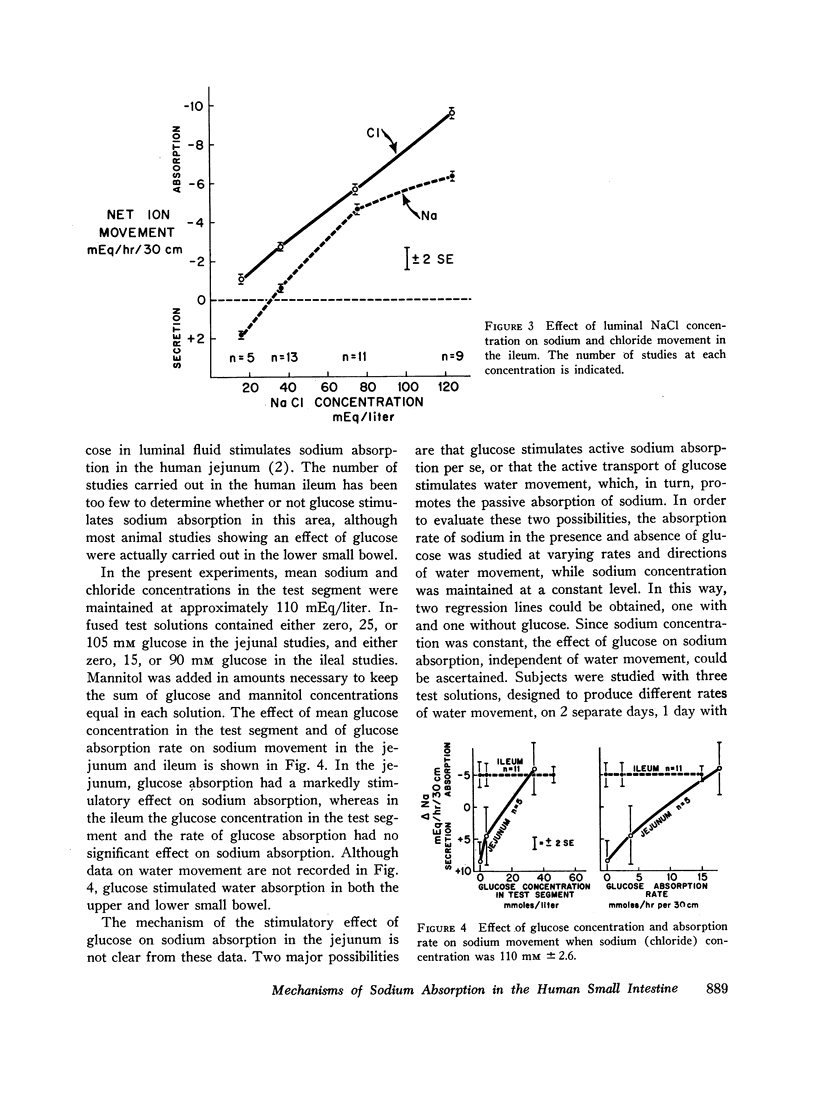
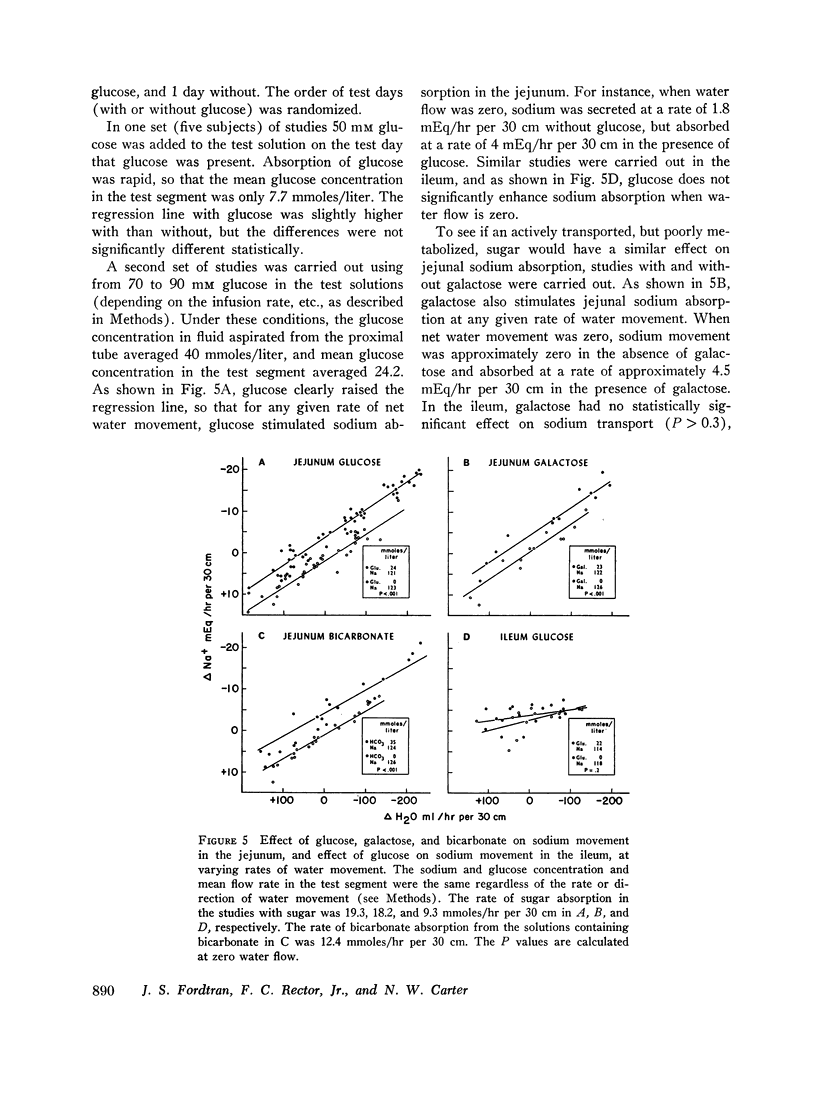
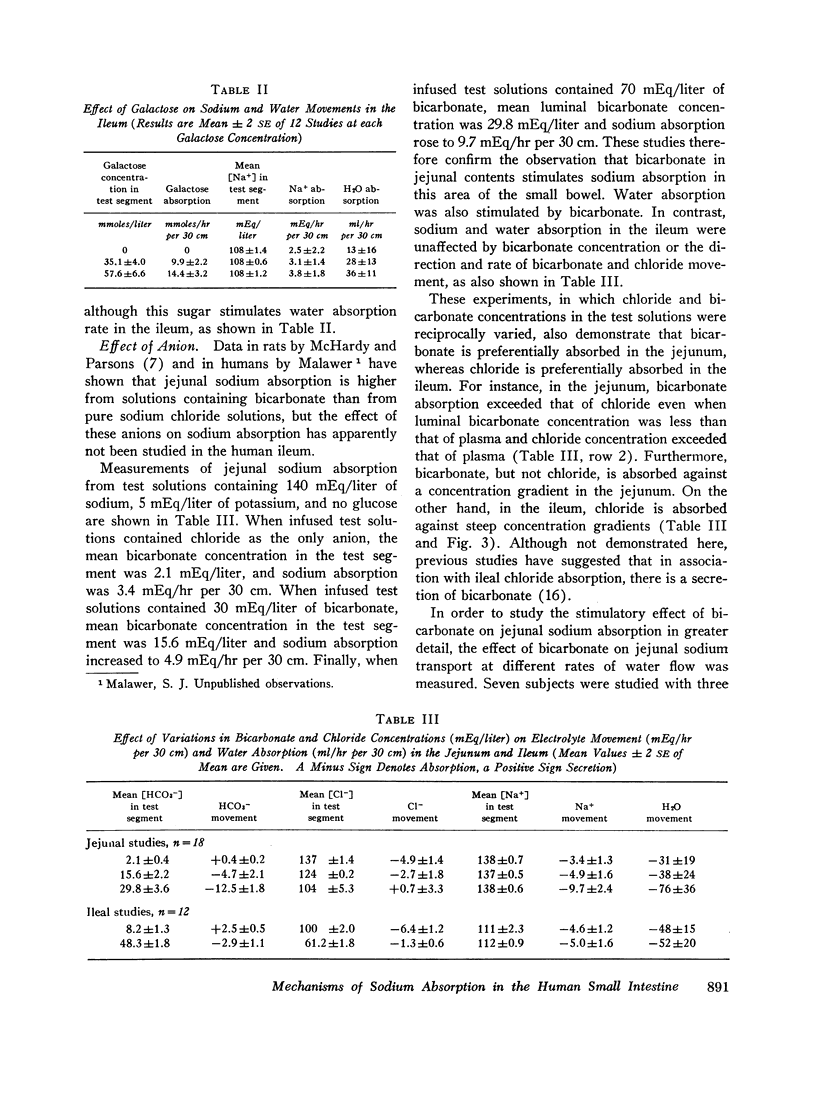
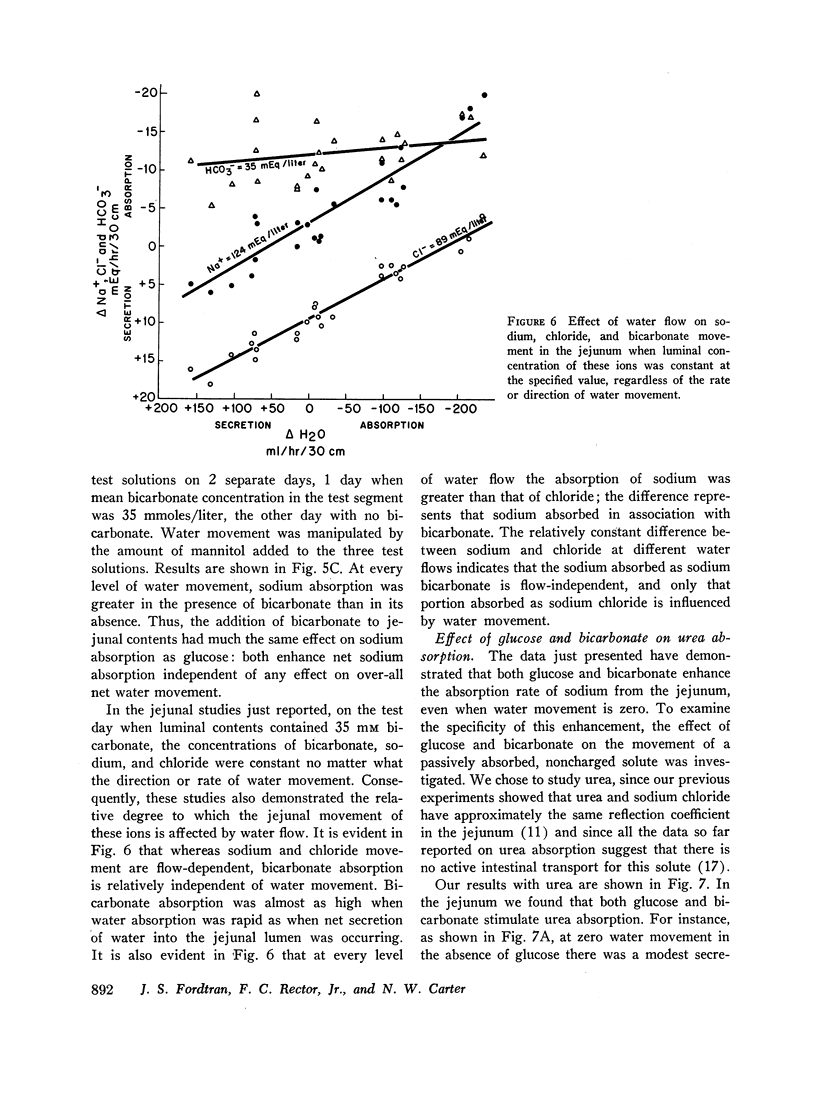
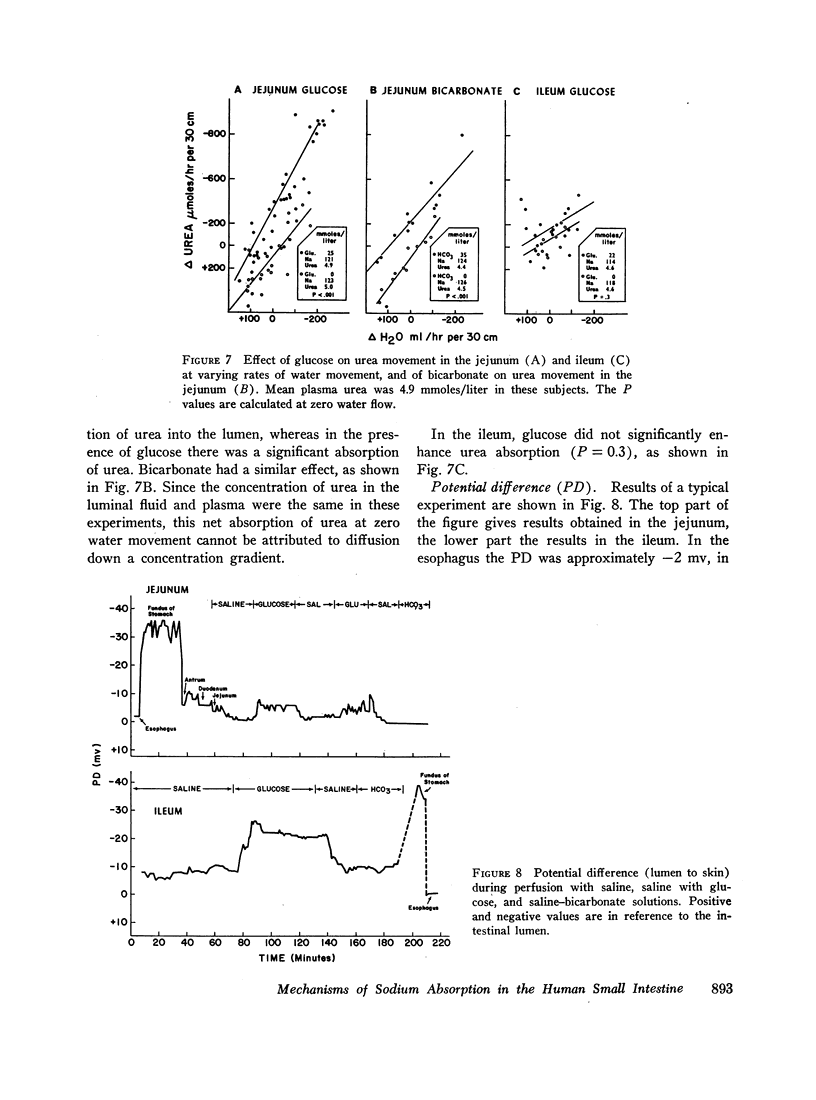
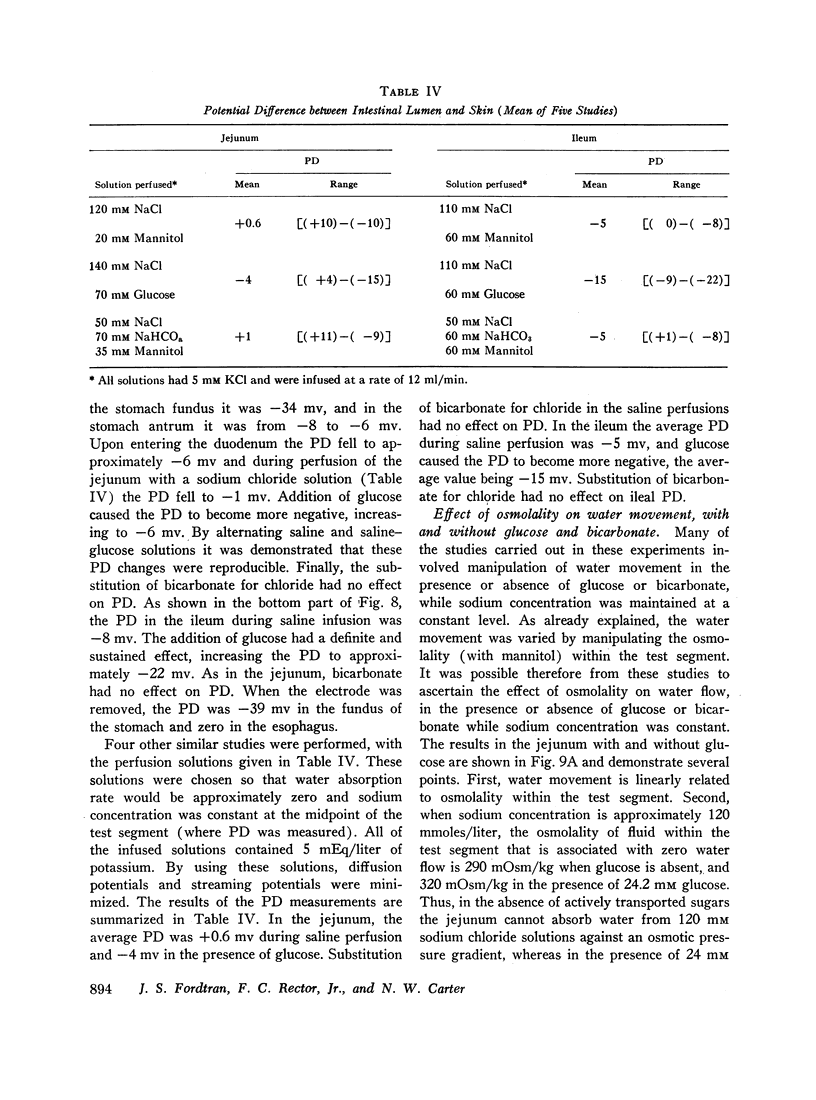
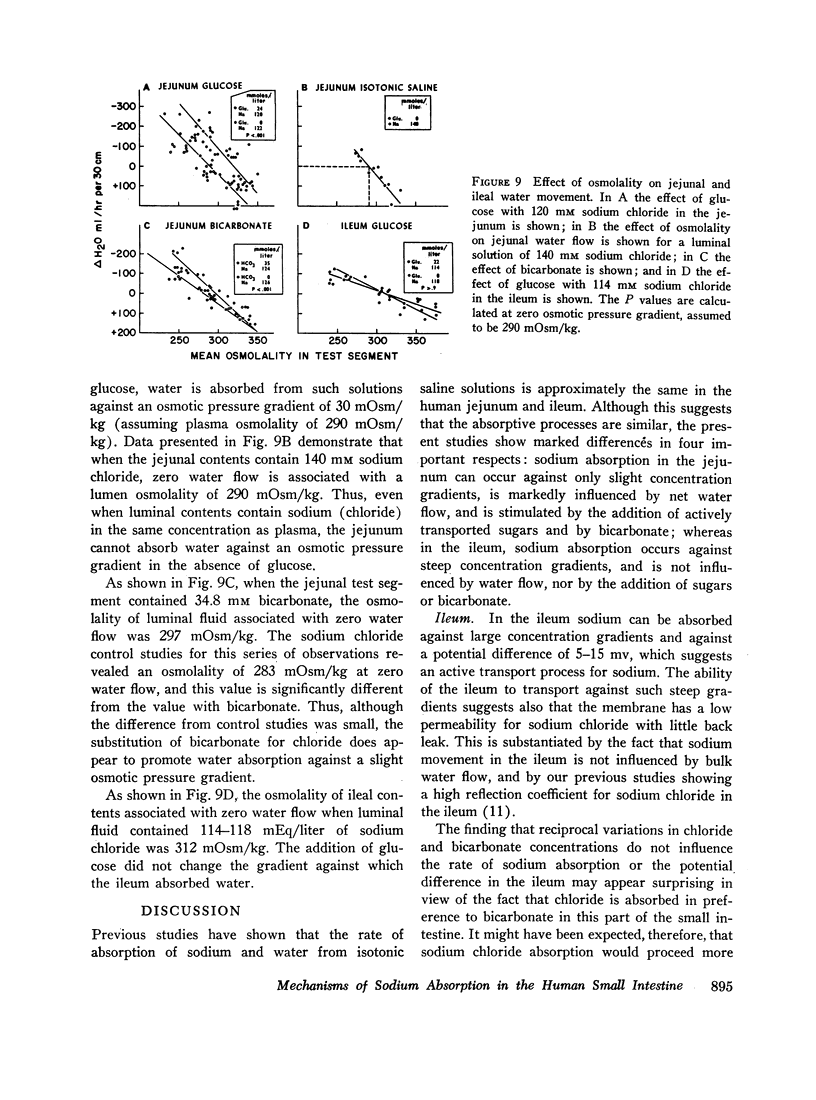
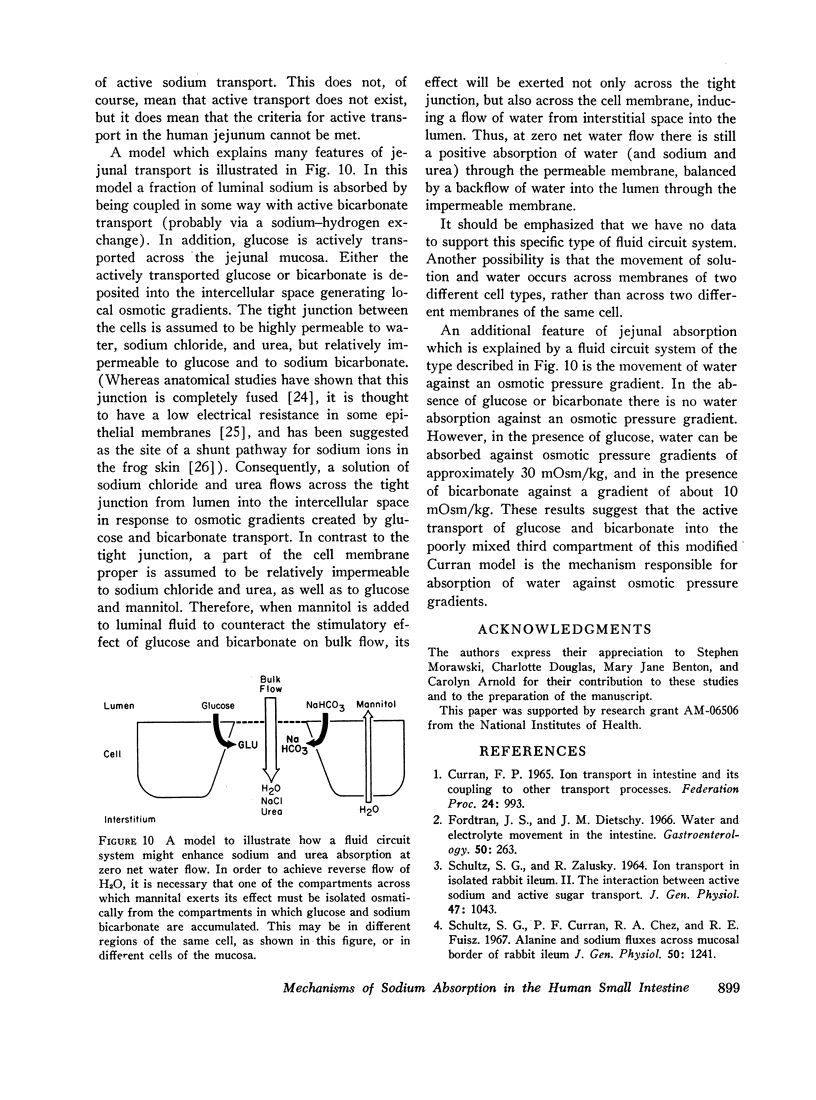
The present studies were designed to characterize sodium transport in the jejunum and ileum of humans with respect to the effects of water flow, sodium concentration, addition of glucose and galactose, and variations in aniomic composition of luminal fluid. In the ileum, sodium absorption occurred against very steep electrochemical gradients (110 mEq/liter, 5-15 mv), was unaffected by the rate or direction of water flow, and was not stimulated by addition of glucose, galactose, or bicarbonate. These findings led to the conclusion that there is an efficiently active sodium transport across a membrane that is relatively impermeable to sodium. In contrast, jejunal sodium (chloride) absorption can take place against only the modest concentration gradient of 13 mEq/liter, was dramatically influenced by water movement, and was stimulated by addition of glucose, galactose, and bicarbonate. The stimulatory effect of glucose and galactose was evident even when net water movement was inhibited to zero by mannitol. These observations led to the conclusion that a small fraction of jejunal sodium absorption was mediated by active transport coupled either to active absorption of bicarbonate or active secretion of hydrogen ions. The major part of sodium absorption, i.e. sodium chloride absorption, appeared to be mediated by a process of bulk flow of solution along osmotic pressure gradients. The stimulatory effect of glucose and galactose, even at zero water flow, was explained by a model in which the active transport of monosaccharide generates a local osmotic force for the absorption of solution (NaCl and water) from the jejunal lumen, which, in the presence of mannitol, is counterbalanced by a reverse flow of pure solvent (H2O) through a parallel set of channels which are impermeable to sodium. Support for the model was obtained by the demonstration that glucose and bicarbonate stimulated the absorption of the nonactively transported solute urea even when net water flow was maintained at zero by addition of mannitol to luminal contents.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curran P. F. Ion transport in intestine and its coupling to other transport processes. Fed Proc. 1965 Sep-Oct;24(5):993–999. [PubMed] [Google Scholar]
- Curran P. F., Schultz S. G., Chez R. A., Fuisz R. E. Kinetic relations of the Na-amino acid interaction at the mucosal border of intestine. J Gen Physiol. 1967 May;50(5):1261–1286. doi: 10.1085/jgp.50.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORDTRAN J. S., LEVITAN R., BIKERMAN V., BURROWS B. A., INGELFINGER F. J. The kinetics of water absorption in the human intestine. Trans Assoc Am Physicians. 1961;74:195–206. [PubMed] [Google Scholar]
- Fordtran J. S., Dietschy J. M. Water and electrolyte movement in the intestine. Gastroenterology. 1966 Feb;50(2):263–285. [PubMed] [Google Scholar]
- Fordtran J. S., Locklear T. W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966 Jul;11(7):503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- Fordtran J. S. Marker perfusion techniques for measuring intestinal absorption in man. Gastroenterology. 1966 Dec;51(6):1089–1093. [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Ewton M. F., Soter N., Kinney J. Permeability characteristics of the human small intestine. J Clin Invest. 1965 Dec;44(12):1935–1944. doi: 10.1172/JCI105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINDLE W., CODE C. F. Some differences between duodenal and ileal sorption. Am J Physiol. 1962 Aug;203:215–220. doi: 10.1152/ajplegacy.1962.203.2.215. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. A physical interpretation of the phenomenological coefficients of membrane permeability. J Gen Physiol. 1961 Sep;45:143–179. doi: 10.1085/jgp.45.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHARDY G. J., PARSONS D. S. The absorption of water and salt from the small intestine of the rat. Q J Exp Physiol Cogn Med Sci. 1957 Jan;42(1):33–48. doi: 10.1113/expphysiol.1957.sp001241. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Chez R. A., Fuisz R. E. Alanine and sodium fluxes across mucosal border of rabbit ileum. J Gen Physiol. 1967 May;50(5):1241–1260. doi: 10.1085/jgp.50.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Whalen G. E., Harris J. A., Geenen J. E., Soergel K. H. Sodium and water absorption from the human small intestine. The accuracy of the perfusion method. Gastroenterology. 1966 Dec;51(6):975–984. [PubMed] [Google Scholar]



