Abstract
Little is known about patients prescribed high doses of opioids to treat chronic non-cancer pain, though these patients may be at higher risk for medication-related complications. We describe the prevalence of high-dose opioid use and associated demographic and clinical characteristics among veterans treated in a VA regional healthcare network. Veterans with chronic non-cancer pain prescribed high doses of opioids (>=180 mg/day morphine equivalent; n=478) for 90+ consecutive days were compared to two groups with chronic pain: Traditional-dose (5–179 mg/day; n=500) or no opioid (n=500). High-dose opioid use occurred in 2.4% of all chronic pain patients and in 3.4% of all chronic pain patients prescribed opioids long-term. The average dose in the high-dose group was 324.9 (SD=285.1) mg/day. The only significant demographic difference among groups was race (p=0.03) with black veterans less likely to receive high doses. High-dose patients were more likely to have four or more pain diagnoses and the highest rates of medical, psychiatric, and substance use disorders. After controlling for demographic factors and VA facility, neuropathy, low back pain, and nicotine dependence diagnoses were associated with increased likelihood of high-dose prescriptions. High-dose patients frequently did not receive care consistent with treatment guidelines: there was frequent use of short-acting opioids, urine drug screens were administered to only 40.8% of patients in the prior year, and 32.0% received concurrent benzodiazepine prescriptions, which may increase risk for overdose and death. Further study is needed to identify better predictors of high-dose usage, as well as the efficacy and safety of such dosing.
Keywords: Chronic pain, Opioids, Epidemiology, Quality of life, Pain/drug therapy
Introduction
The use of opioids for chronic non-cancer pain has increased in recent years [10,31] despite uncertainty regarding the efficacy of opioids in reducing pain or improving function [24,32]. A summary of the literature regarding opioid medications to treat chronic non-cancer pain indicates strong evidence of initial effectiveness in pain reduction, but less evidence to support long-term effectiveness [6,13].
Doses of opioid medications often remain stable [47] and the vast majority of pain patients are on opioid doses less than 100 mg morphine equivalents per day [40]. However, a small proportion of patients are prescribed high doses (defined here as greater than 180 mg morphine equivalent per day [5]). Limited empirical data are available regarding the prevalence of high-dose opioid use, clinical or demographic factors associated with high-dose opioid use, or outcomes associated with long-term high-dose opioid therapy [12]. Of the few randomized clinical trials that included high doses of opioids, time on active treatment was limited to one to eight weeks [5]. A planned three year registry study of patients taking controlled-release oxycodone for non-cancer pain found that, of 233 patients enrolled, 5% were taking 100 mg or more of oxycodone per day [40]. However, treatment discontinuation was high, as 133 (57%) participants voluntarily terminated the study, and the data on long-term benefit are not available. In a study of 1,226 patients with disabling musculoskeletal disorders, higher baseline opioid doses (greater than 61 mg morphine equivalent per day) were associated with poorer outcomes from an interdisciplinary functional restoration program [28].
There are numerous risks associated with opioids, including side effects, misuse, diversion, and other adverse health effects [27,34,35,44], in addition to concerns about effectiveness. These concerns are heightened for patients on high doses of opioids. There may also be additional side effects associated with high-dose opioid use that are not present at standard dose, such as arrhythmia, thyroid disease, hypogonadism with sexual dysfunction, or hyperalgesia [4,16,18,30]. Recent data from a retrospective cohort study examined patient factors and risks associated with opioid use. Analyzing data from 9,940 patients who were prescribed long-term opioid therapy, those patients who were prescribed doses greater than 100 mg morphine equivalent per day were at the greatest risk for overdose, relative to patients taking lower doses [21]. Additionally, risk of fractures for chronic pain patients older than 60 were highest among patients prescribed 50 mg per day morphine equivalent or more of opioids [45].
Few studies have examined the progression of opioids from traditional to high doses. Among patients with painful spine conditions, smoking status and nonsurgical treatment predicted long-term opioid use [29]. Other studies have examined factors associated with opioid prescribing and found that history of comorbid psychiatric disorders and substance use disorders were significantly linked to opioid prescriptions [8,49,50]. It is unclear whether these variables are also associated with high doses of opioids. The purpose of the present study was to describe the prevalence, demographic characteristics, and clinical features of chronic non-cancer pain patients who are prescribed high doses of opioid medications.
Methods
Data were collected from veterans receiving treatment at any VA facility in the Pacific Northwest (Washington, Oregon, Idaho, and Alaska) as part of routine care. Data were extracted from the Veterans Integrated Service Network (VISN)-20 Data Warehouse [51]. The Data Warehouse contains data from the main clinical software packages of regional VA healthcare facilities and two national VA databases. The Data Warehouse is updated monthly and reliability checks are performed regularly. This study was approved by the Institutional Review Board at the Portland VA Medical Center.
Inclusion/Exclusion Criteria
Patients who received any medical care in VISN-20 at any time during calendar year 2008 were eligible for study inclusion. To identify patients with chronic pain, we reviewed electronic medical record pain Numeric Rating Scores (NRS; 26) during calendar year 2008. Patients with pain intensity scores ≥ 4 on the NRS, a scale of 0 – 10 (with 0 being low and 10 being high), recorded in at least three different months during 2008 were considered to have chronic pain. This is consistent with common definitions of chronic pain that require at least three to six month duration of moderate to severe pain [14,25]. Although consensus on the optimal NRS for moderate pain is lacking, most studies use either 4 or 5; we used 4 as the cutoff in this study due to its consistency with VA clinical practice and policy [15]. For those patients whose first pain scores occurred in November or December, the first two months of 2009 were also considered. Included subjects were classified into one of three groups: chronic non-cancer pain patients prescribed high doses of opioid medications (defined as ≥ 180 mg morphine equivalent per day) for at least 90 consecutive days, chronic non-cancer pain patients prescribed traditional doses of opioid medications (defined as 5 – 179 mg morphine equivalent per day) for at least 90 consecutive days, or chronic non-cancer pain patients not prescribed any opioid medications in the prior year. Previous studies have used 90 days of opioid use to indicate chronic use [22,53]. To calculate a morphine equivalent, each prescription was categorized into an opioid category and multiplied by a conversion factor to determine the same milligram amount per day of morphine (Table 1). The conversion factors were based on previously published recommendations [37,52,53].
Table 1.
Morphine Equivalent Conversion Factor.
| Type of Opioid | Morphine equivalent conversion factor/mg of opioid |
|---|---|
| Codeine | 0.17 |
| Fentanyl Transdermal | 3.6 |
| Hydrocodone | 1.0 |
| Hydromorphone | 4.0 |
| Methadone | 2.5 |
| Morphine | 1.0 |
| Oxycodone | 2.0 |
| Propoxyphene | 0.15 |
Note. The conversion factor for Transdermal Fentanyl assumes that one patch provides the dispensed micrograms per hour and remains affixed for three days.
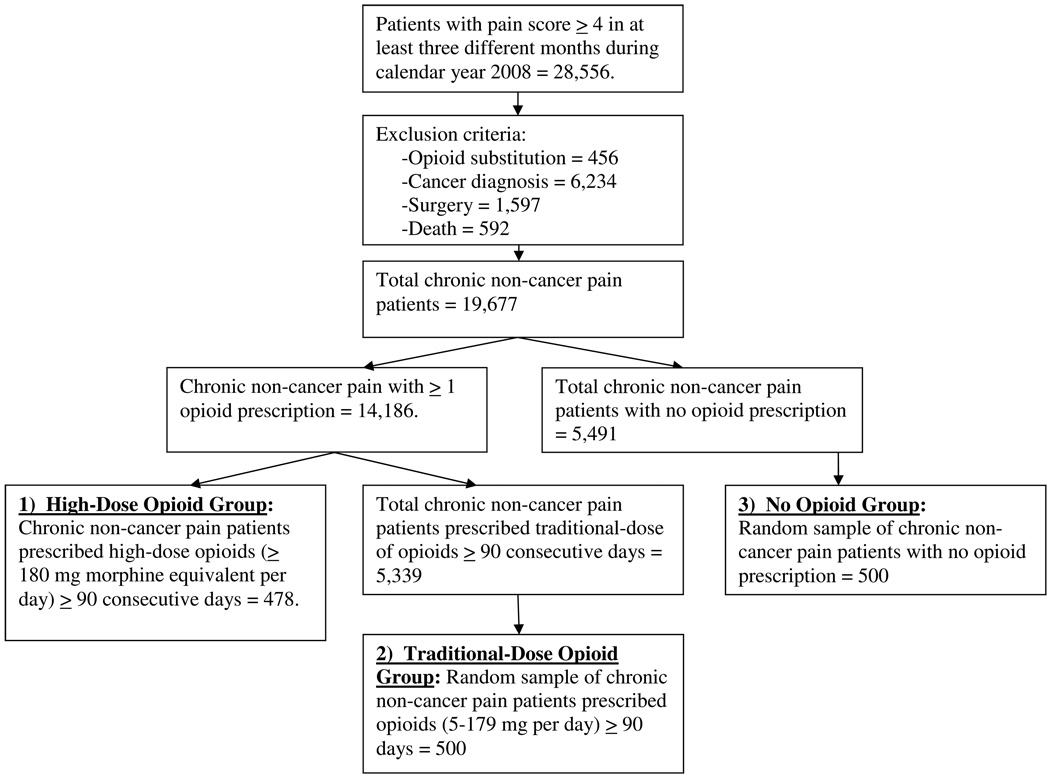
Patients were excluded from the study if they had any visits to a VA opioid substitution (methadone maintenance) program in the region during 2008, had any cancer diagnosis in the six years prior to their index date, or any surgery within the past six months. In order to exclude patients who might have a terminal illness during the study period, we also excluded any patient who subsequently died in 2008 (Figure 1).
Figure 1.
Data Flow for Patients with Chronic Noncancer Pain on High Doses of Opioid Medications.
Defining Study Samples
Four hundred seventy-eight patients were identified as being in the high-dose opioid chronic pain group for at least 90 consecutive days. We also identified 5,339 patients who met chronic non-cancer pain criteria and who received traditional opioid use (opioid dose range from 5 – 179 mg morphine equivalent per day) for at least 90 consecutive days; 500 of these patients were selected as one comparison group. Among the patients meeting our chronic non-cancer pain inclusion criteria, there were 5,491 who had no opioid use in 2008. We randomly selected 500 of these patients to serve as an additional comparison group. In order to identify the two comparison groups, we used a random number generator to assign a random number to each subject in the group, and we then selected the first 500 subjects. In both the high-dose and traditional-dose groups we created an index date, to be used as a reference point for collecting medical diagnoses and pharmacy data. The index date was calculated as the earliest date in 2008 of a 90 consecutive day episode of opioid use. Clinical data for patients were retrieved for five prior years from the index date. The index date for the no opioid group was the date of their earliest pain score ≥ 4 in calendar year 2008.
Collection of Demographic Data, Medical Diagnoses, and Pharmacy Data
Demographic data including age, gender, race, marital status, and VA service-connected disability status were collected for all patients. Body mass index was derived from weight and height measurements at outpatient visits within six months of the index date, and was calculated by dividing weight in kilograms by the square of height in meters. Inpatient and outpatient diagnoses were based on International Classification of Diseases, Clinical Modification – 9th Revision (ICD-9-CM). If a diagnostic code for a disorder was made any time in the five years prior to the index date, the individual was coded as having the disorder. Past-year inpatient hospitalization data and outpatient diagnoses were used to calculate a Charlson Comorbidity score [11], a measure which uses ICD-9-CM diagnostic codes for 17 health conditions to document the burden of medical comorbidity [42,43]; higher scores indicate more severe illness. Pharmacy data were reviewed to extract information on prescriptions of current opioids and non-opioid analgesics (data were available only from VA pharmacies).
Statistical Analyses
Demographic and diagnostic data were analyzed using chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables. Post-hoc tests were evaluated with Scheffe. A forward stepwise logistic regression model was used to evaluate characteristics associated with prescriptions of high doses of opioid medications. The dependent variable was prescription for high-dose (versus traditional-dose) of opioids. For this analysis, participants who were not prescribed any opioid medications were excluded, as the goal was to examine differences between patients prescribed traditional doses of opioids versus those prescribed high doses of opioids. Age, gender, and VA facility (one of eight medical centers in Washington, Oregon, Idaho, or Alaska) were entered into the model in Step 1. Age and gender were included because these variables have been associated with pain and opioid use in prior studies. The variable for VA facility was included to help control for practice variation, as regional deviations in opioid prescribing practices could impact the results. Other independent variables were eligible to be entered in Step 2 if they significantly differed (p ≤ 0.05) between groups in bivariate testing. We also included variables that summed the total number of pain diagnoses, and total number of psychiatric diagnoses with which a participant was diagnosed; these variables were included to provide additional measures of comorbidity. In the regression analyses, only participants for whom all variables were available were included.
Results
Demographic Characteristics
High-dose opioids were prescribed for 2.4% of all participants with chronic non-cancer pain, 3.4% of all chronic non-cancer pain patients who received any prescription for opioid therapy, and 8.2% of chronic non-cancer pain patients who are prescribed opioids long-term (Figure 1). Demographic characteristics are reported in Table 2. The average participant was male (90.7%), 55.1 (SD=12.6) years old, married (49.3%) or divorced (32.5%), white (70.8%), overweight (mean BMI=31.1, SD=7.1), and had a VA service-connected disability (64.3%). The average pain numeric rating score did not differ between the two opioid groups, though both were higher than in the non-opioid group. Race significantly differed amongst groups (χ2 (6) = 20.2, p = 0.03), as white veterans were over-represented in the high-dose group and black veterans were more likely to be included in the traditional-dose group or no opioid group (race data were missing for 14.8% of participants). No significant differences were detected among the three groups with respect to age, sex, marital status, BMI, or whether they had a VA service-connected disability.
Table 2.
Comparison of Demographic Characteristics by Study Group.
| High-Dose Opioid Group (n=478) |
Traditional- Dose Opioid Group (n=500) |
No opioid Group (n=500) |
Test (df) | p-value | |
|---|---|---|---|---|---|
| Average Daily Dose Morphine | 324.9 (285.1) | 32.4 (20.1) | 0 | F (1, 977) = 523.4 | < 0.001 |
| Equivalent (SD) | |||||
| Range, Median | 180 – 5496, 273 | 5 – 128, 27.5 | 0 | ||
| Age | 54.9 (10.7) | 54.7 (12.5) | 55.8 (14.2) | F (2, 1477) = .97 | 0.38 |
| Body Mass Index | 31.2 (7.7) | 31.2 (6.9) | 30.9 (6.8) | F (2, 1143) = .13 | 0.88 |
| Male Gender | 435 (91.0%) | 461 (92.2%) | 445 (89.0%) | χ2 (2) = 3.1 | 0.21 |
| Marital Status | χ2 (8) = 13.9 | 0.09 | |||
| Single/Never Married | 39 (8.2%) | 62 (12.4%) | 59 (11.8%) | ||
| Married | 264 (55.2%) | 236 (47.2%) | 229 (45.8%) | ||
| Separated/Divorced | 142 (29.7%) | 166 (33.2%) | 173 (34.6%) | ||
| Widow | 17 (3.6%) | 23 (4.6%) | 26 (5.2%) | ||
| Unknown | 16 (3.3%) | 13 (2.6%) | 13 (2.6%) | ||
| Race | χ2 (6) = 20.2 | 0.03 | |||
| Caucasian | 365 (76.4%) | 341 (68.2%) | 346 (69.2%) | ||
| Black | 14 (2.9%) | 43 (8.6%) | 39 (7.8%) | ||
| Other | 13 (2.7%) | 11 (2.2%) | 19 (3.8%) | ||
| Unknown or Declined to answer | 86 (18.0%) | 105 (21.0%) | 96 (19.2%) | ||
| VA Service-Connected | 311 (65.1%) | 327 (65.4%) | 312 (62.4%) | χ2 (2) = 1.2 | 0.56 |
| Average Pain Score | 6.7 (1.3)a | 6.6 (1.3)a | 6.2 (1.3)b | F (2, 1477) = 17.8 | < 0.001 |
Note. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing.
Pain and other Medical Diagnoses
The high-dose group had a greater number of documented diagnoses for pain conditions compared with the traditional-dose group and the no opioid group (Table 3). The traditional-dose group also had higher rates than the no opioid group for most pain diagnoses. The proportion of patients in each group who were diagnosed with four or more pain diagnoses significantly differed; 35.6% of the high-dose group, 26.0% of the traditional-dose group, and 14.0% of the no opioid group had four or more pain diagnoses (χ2 (2) = 60.9, p < 0.001). Patients in the no opioid group were most likely to have only one pain diagnosis, χ2 (2) = 15.7, p < 0.001 (19.0% of the no opioid group, 11.3% of the high-dose group, and 11.6% of the traditional-dose group).
Table 3.
Pain Diagnoses in the Past Five Years.
| High-Dose Opioid Group (n=478) |
Traditional- Dose Opioid Group (n=500) |
No opioid Group (n=500) |
Test (df) | p-value | |
|---|---|---|---|---|---|
| Fibromyalgia | 79 (16.5%) | 55 (11.0%) | 31 (6.2%) | χ2 (2) = 26.3 | < 0.001 |
| Inflammatory | 35 (7.3%) | 22 (4.4%) | 14 (2.8%) | χ2 (2) = 11.2 | 0.004 |
| Bowel Disease | |||||
| Low Back Pain | 399 (83.5%) | 371 (74.2%) | 234 (46.8%) | χ2 (2) = 164.5 | < 0.001 |
| Migraine Headache | 102 (21.3%) | 112 (22.4%) | 72 (14.4%) | χ2 (2) = 12.0 | 0.002 |
| Neck or Joint Pain | 392 (82.0%) | 401 (80.2%) | 315 (63.0%) | χ2 (2) = 58.1 | < 0.001 |
| Neuropathy | 86 (18.0%) | 54 (10.8%) | 56 (11.2%) | χ2 (2) = 13.8 | 0.001 |
| Rheumatism/ Arthritis |
343 (71.8%) | 321 (64.2%) | 236 (47.2%) | χ2 (2) = 65.4 | < 0.001 |
Regarding other medical diagnoses (Table 4), there were statistically significant differences among the three groups in rates of hypertension, congestive heart failure, chronic pulmonary disease and paraplegia/hemiplegia, with the high-dose group having the highest rates of diagnoses for all conditions except hypertension, which was more common among patients in the traditional-dose group. There were also significant differences in rates of several psychiatric diagnoses, with patients in the high-dose group often having the highest frequencies. Patients in the high-dose group had significantly higher scores than the no opioid group on the Charlson Comorbidity Index (F (2, 1477) = 5.2, p = 0.006), indicating more severe illness; scores from the traditional-dose group and no opioid group did not significantly differ.
Table 4.
Medical and Psychiatric Diagnoses in the Past Five Years.
| High-Dose Opioid Group (n=478) |
Traditional- Dose Opioid Group (n=500) |
No Opioid Group (n=500) |
Test (df) | p-value | |
|---|---|---|---|---|---|
| Medical Diagnoses | |||||
|
| |||||
| Hypertension | 298 (62.3%) | 346 (69.2%) | 292 (58.4%) | χ2 (2) = 12.9 | 0.002 |
| Congestive Heart | 59 (12.3%) | 43 (8.6%) | 32 (6.4%) | χ2 (2) = 10.7 | 0.005 |
| Failure | |||||
| Myocardial Infarction | 34 (7.1%) | 32 (6.4%) | 21 (4.2%) | χ2 (2) = 4.1 | 0.13 |
| Diabetes | 151 (31.6%) | 129 (25.8%) | 133 (26.6%) | χ2 (2) = 4.8 | 0.09 |
| Sleep Apnea | 97 (20.3%) | 96 (19.2%) | 82 (16.4%) | χ2 (2) = 2.6 | 0.27 |
| Cerebrovascular | 57 (11.9%) | 51 (10.2%) | 51 (10.2%) | χ2 (2) = 1.0 | 0.61 |
| Disease | |||||
| Peripheral Vascular | 55 (11.5%) | 45 (9.0%) | 38 (7.6%) | χ2 (2) = 4.5 | 0.11 |
| Disease | |||||
| Chronic Pulmonary | 176 (36.8%) | 175 (35.0%) | 146 (29.2%) | χ2 (2) = 7.0 | 0.03 |
| Disease | |||||
| Paraplegia and/or | 26 (5.4%) | 16 (3.2%) | 11 (2.2%) | χ2 (2) = 7.7 | 0.02 |
| Hemiplegia | |||||
|
| |||||
| Psychiatric Diagnoses | |||||
|
| |||||
| Major Depressive | 320 (66.9%) | 303 (60.6%) | 200 (40.0%) | χ2 (2) = 79.3 | < 0.001 |
| Disorder | |||||
| Dysthymic Disorder | 87 (18.2%) | 62 (12.4%) | 50 (10.0%) | χ2 (2) = 14.8 | 0.001 |
| Bipolar Disorder | 28 (5.9%) | 45 (9.0%) | 42 (8.4%) | χ2 (2) = 3.8 | 0.15 |
| Panic Disorder | 33 (6.9%) | 35 (7.0%) | 15 (3.0%) | χ2 (2) = 9.8 | 0.008 |
| Posttraumatic Stress | 179 (37.4%) | 169 (33.8%) | 134 (26.8%) | χ2 (2) = 13.1 | 0.001 |
| Disorder | |||||
| Other Anxiety Disorder | 122 (25.5%) | 122 (24.4%) | 79 (15.8%) | χ2 (2) = 16.4 | < 0.001 |
| Schizophrenia | 13 (2.7%) | 24 (4.8%) | 18 (3.6%) | χ2 (2) = 3.0 | 0.23 |
| Any Sleep Disorder | 103 (21.5%) | 102 (20.4%) | 64 (12.8%) | χ2 (2) = 15.2 | 0.001 |
| Any Alcohol or | 175 (36.6%) | 165 (33.0%) | 114 (22.8%) | χ2 (2) = 23.8 | < 0.001 |
| Substance Use Disorder | |||||
| Nicotine Disorder | 172 (36.0%) | 157 (31.4%) | 99 (19.8%) | χ2 (2) = 33.3 | < 0.001 |
|
| |||||
| Past-Year Charlson Comorbidity Score, Mean (SD) |
1.2 (1.6)a | 1.0 (1.5)a,b | 0.9 (1.4)b |
F (2, 1477) = 5.2 |
0.006 |
Note. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing.
Medications
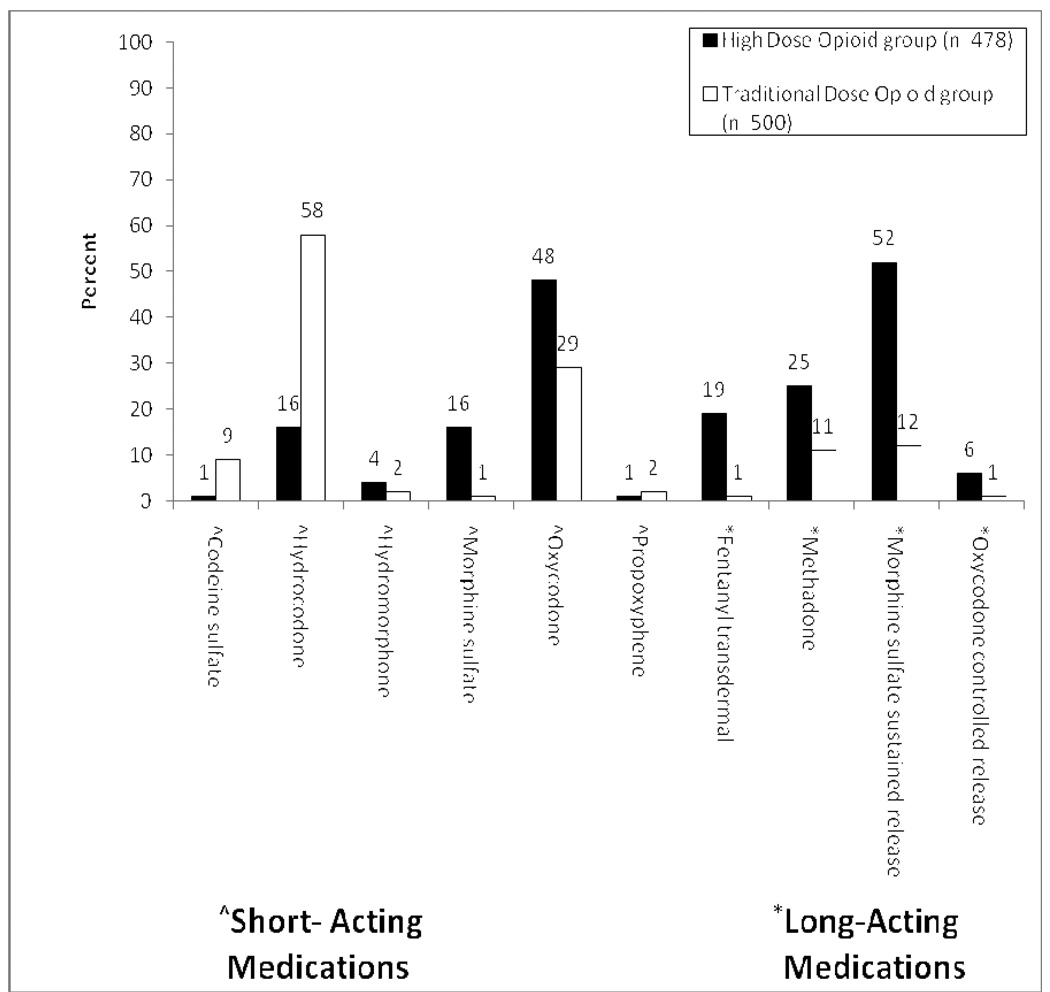
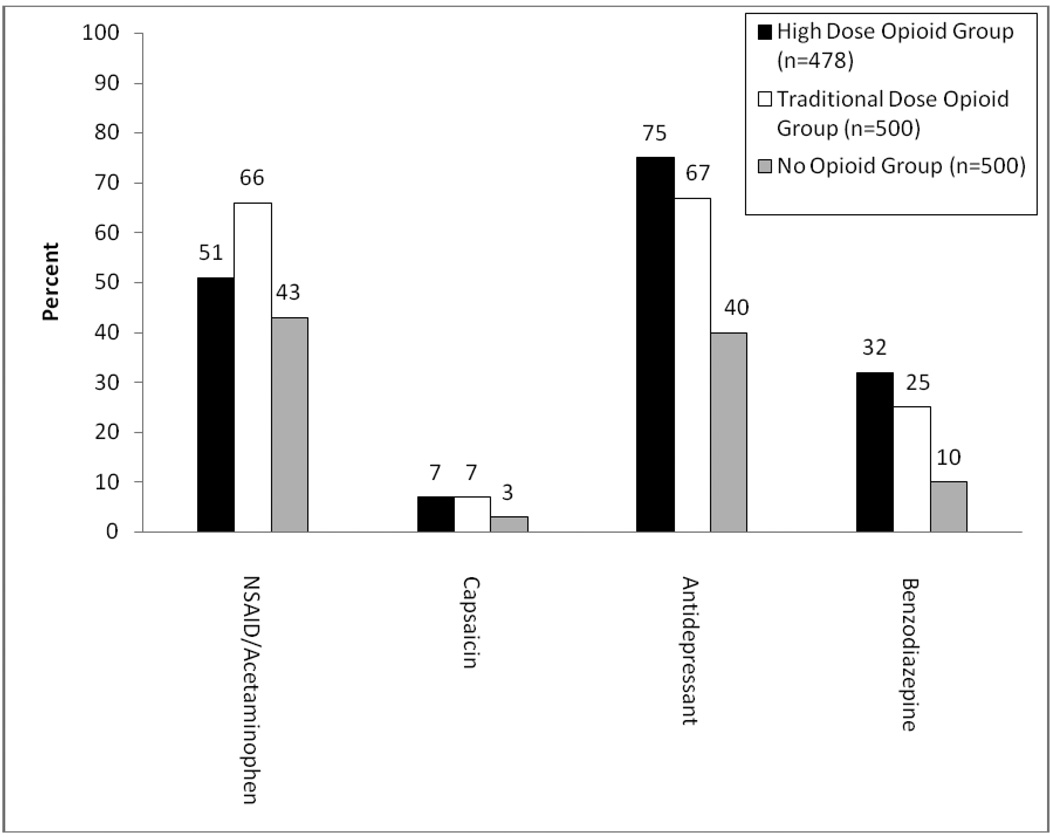
Patients in the high-dose group had an average daily opioid dose of 324.9 (SD=285.1) mg morphine equivalent per day (range = 180 – 5,496; median = 273.2). Patients in the traditional-dose group had an average daily dose of 32.4 (SD=20.1) mg morphine equivalent per day (range = 5 – 128; median = 27.5). Figure 2a displays comparisons between the high-dose group and traditional-dose group on types of opioid medications that were prescribed at the time of their index date. There were statistically significant differences between the two opioid groups with respect to the proportion of patients prescribed at least one of each opioid. The high-dose group had greater proportions of prescriptions for all of the long-acting medications (transdermal fentanyl, methadone, morphine sustained-release, and oxycodone controlled-release). There were also statistical differences in the proportions of the various short-acting medications prescribed and a statistically significant difference between groups in the proportion of patients prescribed two or more different types of opioids: 74.3% of the high-dose group versus 26.6% of the traditional-dose group (χ2 (1) = 222.1, p < 0.001). However, there was no difference between the high-dose group and the traditional-dose group in the proportion of patients prescribed two or more short-acting opioids (9.0% versus 12.2%, χ2 = 2.64, p = 0.10). Patients in the high-dose group were most likely to be prescribed the combination of only one long-acting opioid and no short-acting opioids (23.2% versus 7.6%; χ2 = 46.2, p < 0.001). There was a statistically significant difference among the three groups in current prescriptions for non-steroidal anti-inflammatory drugs (NSAID) and/or acetaminophen, capsaicin, antidepressants, and benzodiazepines (Figure 2b). The two opioid groups had higher rates of prescriptions for all medications than the no opioid group, with the high-dose group having the highest rate for all medications except NSAID/Acetaminophen.
Figure 2. Proportions of Patients Prescribed Certain Medications.
Panel A. Current Opioid Medications. Statistically significant differences occurred between the two groups in the proportion of patients prescribed each of these medications (p < 0.05).
Panel B. Current Non-Opioid Medications. Statistically significant differences occurred among the three groups in the proportion of patients prescribed each of these medications (p < 0.01).
Urine Drug Screen Results
We examined the frequency of past-year administrations of urine drug screen by group status; 39.9% (n=590) of all patients prescribed opioids were administered a urine drug screen in the past year. There was no significant difference among groups in the rate of administrations (p = 0.85) or the proportion of patients with a positive screen (p = 0.44). Twenty-two total patients had a positive urine drug screen for an illicit substance (1.7% high-dose group, 1.2% traditional-dose group, and 1.6% no opioid group). The most common illicit substance detected via urine drug screen was marijuana (n=19, 86.4% of positive tests).
Correlates of High-Dose Opioid Use
The results of the forward stepwise logistic regression analysis examining factors associated with prescriptions of high-dose of opioids are displayed in Table 5. After controlling for the effects of age, sex, and VA facility, diagnoses of neuropathy (OR = 1.95, 95% CI = 1.28 – 2.97), low back pain (OR = 1.88, 95% CI = 1.32 – 2.70), or nicotine disorder (OR = 1.36, 95% CI = 1.00 – 1.86) were associated with increased likelihood of being prescribed high doses of opioids. Conversely, a diagnosis of hypertension (OR = 0.54, 95% CI = 0.39 – 0.75) was associated with a significantly decreased likelihood of being in the high-dose opioid group. No other variables were significantly associated with being prescribed high doses of opioids.
Table 5.
Correlates of High-Dose Opioid Group (n=774).
| Beta (Standard Error) |
Wald | p-value | Odds Ratio (95% Confidence Interval) |
|
|---|---|---|---|---|
| Age | −0.01 (.01) | 1.30 | 0.25 | 0.99 (0.98 – 1.01) |
| Male Gender | −1.52 (.26) | 0.33 | 0.56 | 0.86 (0.51 – 1.44) |
| VA Facility | 0.01 (.01) | 0.19 | 0.67 | 1.00 (0.99 – 1.00) |
| Neuropathy | 0.67 (.21) | 9.79 | 0.002 | 1.95 (1.28 – 2.97) |
| Low Back Pain | 0.63 (.18) | 11.98 | 0.001 | 1.88 (1.32 – 2.70) |
| Hypertension | −0.62 (.17) | 13.35 | <0.001 | 0.54 (0.39 – 0.75) |
| Nicotine Disorder | 0.31 (.16) | 4.00 | 0.048 | 1.36 (1.00 – 1.86) |
Note. This analysis includes only those patients who had complete data and were currently prescribed at least one opioid medication. Variables that were potentially eligible to be included in the analysis, but ultimately were not included were: race, migraine headache, inflammatory bowel disease, neck or joint pain, fibromyalgia, congestive heart failure, chronic pulmonary disease, paraplegia and/or hemiplegia, major depressive disorder, dysthymic disorder, panic disorder, posttraumatic stress disorder, other anxiety disorder, any sleep disorder, any substance use disorder, Charlson Comorbidity Index score, number of pain diagnoses, and number of psychiatric diagnoses.
Discussion
High-dose opioid therapy was prescribed for 2–3% of veterans with chronic non-cancer pain who were prescribed opioids long-term. Patients in the high-dose opioid group were prescribed an average daily opioid dose of 325 mg per day morphine equivalent, a dose 10 times greater than that of patients in the traditional-dose opioid group. High-dose patients had pain intensity scores similar to patients in the traditional-dose group and significantly higher than patients in the no opioid group. Specific reasons why patients in the high-dose group had the highest pain intensity scores were not identified, but may be due to greater medical morbidity, increased tolerance to opioids, central sensitization, or to other factors not assessed (e.g., severity of illness).
In this study, certain demographic and clinical factors were associated with high-dose opioid use. Black patients were less likely to be in the high-dose group, a finding which is consistent with prior research indicating patients who are black receive fewer services for the treatment of chronic pain [36,38] and find pain treatment less effective [20]. Patients in the high-dose group were more likely to be diagnosed with four or more pain diagnoses, as well as a variety of medical, psychiatric, and substance use disorder diagnoses. These results are consistent with prior studies indicating that patients with chronic pain and comorbid psychiatric disorders are more likely to receive opioids than pain patients without comorbid psychopathology [7,48,50], and extend prior literature by showing that these diagnoses are also associated with prescription of high opioid doses.
Results from the regression analysis identified several factors associated with being in the high-dose group relative to patients in the traditional-dose group. Significant results were found for certain pain diagnoses (neuropathy, low back pain) and nicotine dependence, which increased the likelihood of being prescribed high-doses of opioids. Contrary to our initial hypotheses, there were no statistically robust predictors of high-dose opioid use, as the significant odds ratios were all less than 2.0. The finding that a diagnosis of nicotine dependence was associated with an increased likelihood of receiving high doses of opioids is consistent with recent research which found that nicotine use predicted long-term opioid use among patients with painful spine conditions [29]. We also found that a diagnosis of hypertension was associated with a decreased likelihood of being in the high-dose group. While hypertension may be a marker for some other illness or treatment factor, previous literature has also documented a relationship between hypertension and pain sensitivity, showing a negative correlation between blood pressure and pain regulation [1,9,33].
Prior research indicates that opioid prescribing varies considerably by provider and that several factors inhibit prescription of opioid medications, including perceived regulatory scrutiny, knowledge gaps, concern about risk of dependence or addiction, and negative views about patients with chronic pain [39,41,54,55]. In a recent study of VA primary care providers, a large degree of variation in rates of prescribing opioids was associated with clinician panel size, job and resource satisfaction, and professional training [19]. Future research might also evaluate the relationship between opioid prescriber and opioid dose. In our study, we adjusted for practice variation using VA facility, though this variable was not significantly associated with prevalence of high-dose opioid prescriptions.
Guidelines have been published which provide recommendations for managing patients prescribed opioids for chronic non-cancer pain [2,3,13]. Although the primary purpose of the present study was not to evaluate the extent to which patients prescribed high doses of opioids received guideline-concordant care, some of our findings address these issues. Consistent with recommended guidelines was the small proportion of patients taking two or more short-acting opioids, which did not differ by group status (9.0% in the high-dose group versus 12.2% in traditional-dose group). Patients in the high-dose group were most likely to be prescribed a long-acting opioid; 96.9% in high-dose group versus 22.6% in the traditional-dose group. Patients in the high-dose group were more likely to be prescribed the combination of only one long-acting opioid and no short-acting opioids (23.2% versus 7.6%). Treatment recommendations generally support the use of long-acting opioids for chronic pain, though it has been suggested that this pattern of prescribing could also contribute to tolerance and possibly dose escalation [5].
Patients in the high-dose opioid group had the highest rates of alcohol or substance use disorders (36.6%). Although individuals with a substance use disorder should not necessarily be denied access to opioid therapy, this group is at greater risk for misuse of prescription medications and should receive more intensive structure and monitoring [13,35]. The overall rate of past-year administrations of urine drug screens was low, 39.9% in the entire sample, which did not differ by group status. Seventy-five percent of patients in the high-dose group were prescribed at least one short-acting opioid, and 9.4% were concurrently prescribed two or more short-acting opioids, rates which are higher than guideline-recommended care. Patients in the high-dose group had the highest rate of benzodiazepine prescriptions (32.0% versus 25.2% in traditional-dose group and 9.6% in no opioid group). The frequent use of short-acting opioids, high rates of sedative-hypnotic (benzodiazepine) prescribing, and relatively low rates of urine drug screen monitoring suggests that patients prescribed high doses of opioids may be at greater risk of not receiving guideline-level chronic pain care [13,46]. Other settings have identified a similar pattern of care among patients prescribed opioids for low back pain [17], suggesting these results are not unique to a specific geographical area or health system. More explicit opioid treatment guidelines regarding high-dose opioid use may be needed, as well as better system support to assist providers.
There are several limitations that are important to consider when reviewing the results from this study. Due to our research methodology, we were unable to identify reasons why these patients were prescribed high-dose opioid therapy or the effectiveness of treatment. Future studies using similar datasets may be able to assess the effectiveness of opioid therapy by tracking opioid dose and pain intensity (or pain-related function) scores over time. All data for this study were obtained from electronic medical records as part of standard clinical care. Medical and psychiatric diagnoses were not confirmed with laboratory data or via structured clinical interviews. Further, diagnoses were obtained over the past five years, and some disorders may have not been problematic at the time opioid use was assessed. This methodology provides a comprehensive assessment of medical comorbidity, but may not fully assess severity, which could be a better predictor of opioid dose. Participants included were veterans receiving medical care in the Pacific Northwest portion of the United States and results may not be generalizable to non-veterans or to veterans seeking care elsewhere. In addition, consistent with a general VA population, the majority of participants in our study were male, though chronic pain may be more common among women, and research suggests women may have different responses to opioid analgesia than men [23], further limiting the generalizability of our results. Our definition of chronic non-cancer pain was dependent upon having pain numeric rating scores ≥ 4 documented in the medical record in three separate months in 2008. This methodology is consistent with some prior research [25], but may skew our sample toward a cohort that utilizes medical services at a high rate and to patients with a moderate to high degree of pain. Finally, we limited our examination to dose of opioid medication and did not assess other potentially relevant aspects of opioid exposure (e.g., duration).
Opioid medications are widely used in the management of chronic pain. Findings from this study indicate that two to three percent of patients with moderate to severe chronic non-cancer pain are prescribed high doses of opioids on a long-term basis and that certain clinical factors are associated with high-dose opioid use. Patients receiving high doses of opioids may not be receiving some aspects of guideline recommended care in that patients frequently receive short-acting opioids, there are high rates of sedative-hypnotic use, and relatively low rates of urine drug screen monitoring [13,46]. Additional empirical data are needed to replicate these findings, explore alternative factors that result in patients being prescribed high doses of opioids, and to assess the utility and safety of taking high doses of opioids.
Acknowledgments
This study was supported in part by award K23DA023467 from the National Institute on Drug Abuse to Dr. Morasco. Jonathan Duckart, MPS, was supported by a Research Enhancement Award Program grant from the VA Health Services Research and Development service. The authors appreciate assistance from Sharon Medley and for support provided from the Oregon Clinical and Translational Research Institute, grant number Ul1RR024140 from the National Center for Research Resources, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Institute on Drug Abuse, or the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author reports having any potential conflict of interest with this study.
References
- 1.al’Absi M, France C, Harju A, France J, Wittmers L. Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosom Med. 2006;68:292–298. doi: 10.1097/01.psy.0000203240.64965.bd. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pain Medicine. The use of opioids for the treatment of chronic pain: A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6–8. [PubMed] [Google Scholar]
- 3.American Pain Society. Guideline for the management of pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis. Chicago, IL: American Pain Society; 2002. [Google Scholar]
- 4.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;329:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: A review of the evidence. Clin J Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 7.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31:564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–350. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 9.Bruehl S, Chung OY, Ward P, Johnson B, McCubbin JA. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: The effects of opioid blockade. Pain. 2002;100:191–201. doi: 10.1016/s0304-3959(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 10.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: Findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Fanciullo Chou R, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts FG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. NEJM. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 15.Cleeland CS, Schall M, Nolan K, Reyes-Gibby CC, Paice J, Rosenberg JM, Tollett JH, Kerns RD. Rapid improvement in pain management: The Veterans Health Administration and the Institute for Healthcare Improvement Collaborative. Clin J Pain. 2003;19:298–305. doi: 10.1097/00002508-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Cruciani RA, Sekine R, Homel P, Lussier D, Yap Y, Suzuki Y, Schweitzer P, Yancovitz SR, Lapin JA, Shaiova L, Sheu RG, Portenoy RK. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29:385–391. doi: 10.1016/j.jpainsymman.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Smith DH, Johnson ES, Dobscha SK, Donovan M, Yang X, Petrik A. Patterns and quality of opioid prescribing for patients with low back pain; Presentation at the Annual Meeting of the North American Primary Care Research Group; Montreal. November 2009. [Google Scholar]
- 18.Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of non-malignant pain. J Pain. 2008;9:28–36. doi: 10.1016/j.jpain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Dobscha SK, Corson K, Flores JA, Tansill EC, Gerrity MS. Veterans Affairs primary care clinicians’ attitudes toward chronic pain and correlates of opioid prescribing rates. Pain Med. 2008;9:564–571. doi: 10.1111/j.1526-4637.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 20.Dobscha SK, Soleck GD, Dickinson KC, Burgess DJ, Lasarev MR, Lee ES, McFarland BH. Associations between race and ethnicity and treatment for chronic pain in the VA. J Pain. 2009;10:1078–1087. doi: 10.1016/j.jpain.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: A cohort study. Annal Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Fillingim RB, Gear RW. Sex differences in opioid analgesia: Clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. CMAJ. 2006;174:1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskell SG, Brandt C, Krebs EE, Skanderson M, Kerns R, Goulet JL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom: Do women and men differ? Pain Med. 2009;10:1167–1173. doi: 10.1111/j.1526-4637.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 27.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs EE, Lurie JD, Fanciullo G, Tosteson TD, Blood EA, Carey TS, Weinstein JN. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain. 2010;11:44–52. doi: 10.1016/j.jpain.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137:501–504. doi: 10.7326/0003-4819-137-6-200209170-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kuehn BM. Opioid prescriptions soar. JAMA. 2007;297:249–251. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 32.Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy and associated with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 33.McCubbin JA, Helfer SG, Switzer FS, Galloway C, Griffith WV. Opioid analgesia in persons at risk for hypertension. Psychosom Med. 2006;68:116–120. doi: 10.1097/01.psy.0000195742.24850.79. [DOI] [PubMed] [Google Scholar]
- 34.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: Systematic review of randomized trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–R1051. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morasco BJ, Dobscha SK. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen Hosp Psychiatry. 2008;30:93–99. doi: 10.1016/j.genhosppsych.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J Pain. 2006;7:225–235. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed July 7, 2009];Oregon Health Sciences University Chronic Pain Management Manual. Available at: http//www.ohsu.edu/ahec/pain/painmanual.html.
- 38.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 39.Ponte CD, Johnson-Tribino J. Attitudes and knowledge about pain: An assessment of West Virginia family physicians. Fam Med. 2005;37:477–480. [PubMed] [Google Scholar]
- 40.Portenoy RK, Farrar JT, Backonja M, Cleeland CS, Yang K, Friedman M, Colucci SV, Richards P. Long-term use of controlled-release oxycodone for noncancer pain: Results of a 3-year registry study. Clin J Pain. 2007;23:287–299. doi: 10.1097/AJP.0b013e31802b582f. [DOI] [PubMed] [Google Scholar]
- 41.Potter M, Schafer S, Gonzalez-Mendez E, Gjeltema K, Lopez A, Wu J, Pedrin R, Cozen M, Wilson R, Thom D, Croughan-Minhane M. Opioids for chronic nonmalignant pain: Attitudes and practices of primary care physicians in the UCSF/Stanford Collaborative Research Network. J Fam Practice. 2001;50:145–151. [PubMed] [Google Scholar]
- 42.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Medical Care. 2002;40:675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby FE, Ghali WA. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Medical Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 44.Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Saunders KW, Dunn KM, Merrill JO, Sullivan M, Weisner C, Braden JB, Psaty BM, Von Korff M. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010 Jan 5; doi: 10.1007/s11606-009-1218-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streltzer J, Ziegler P, Johnson B. Cautionary guidelines for the use of opioids in chronic pain. Am J Addiction. 2009;18:1–4. doi: 10.1080/10550490802544508. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: The TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: Association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 50.Turk DC, Okifuji AP. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? Clin J Pain. 1997;13:330–336. doi: 10.1097/00002508-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 51. [Accessed 7/13/09];Veterans Integrated Service Network-20 Data Warehouse. http://moss.v20.med.va.gov/v20dw/default.aspx.
- 52.Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry. 2005;7:86–88. doi: 10.4088/pcc.v07n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Von Roenn JH, Cleeland CS, Gonin R, Hatfield AK, Pandya KJ. Physician attitudes and practice in cancer pain management: A survey from the Eastern Cooperative Oncology Group. Ann Intern Med. 1993;119:121–126. doi: 10.7326/0003-4819-119-2-199307150-00005. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein SM, Laux LF, Thornby JI, Lorimor RJ, Hill CS, Jr, Thorpe DM, Merrill JM. Physicians’ attitudes toward pain and the use of opioid analgesics: Results of a survey from the Texas Cancer Pain Initiative. South Med J. 2000;93:479–487. [PubMed] [Google Scholar]