Abstract
Objectives
MIDUS is a national study of health and aging among individuals aged 25 to 74 at baseline(1995/96). Longitudinal survey assessments (2004/05), were followed by biological assessments on a subsample aged 35–85. To facilitate public use, we describe the protocol, measures, and sample.
Methods
Respondents traveled to clinics for a two-day data collection protocol that included fasting blood specimens, 12-hour urine specimen, medical history, physical exam, bone densitometry, a laboratory challenge (heart rate variability, blood pressure, respiration, salivary cortisol).
Results
Response rates for the biological protocol (N = 1,255) were 39.3%, or 43.1% (adjusting for those who could not be located or contacted). Reasons for non-participation were travel, family obligations, and being too busy. Respondents were comparable to the recruitment pool on most demographic characteristics and health assessments.
Discussion
Strengths of the protocol vis-à-vis other similar studies include opportunities to link biological factors with diverse content from other MIDUS projects.
MIDUS (Midlife in the U.S.) is a national sample of continental U.S. residents, aged 25 to 74, who were first interviewed in 1995/96. The original study was conceived by a multidisciplinary team of investigators interested in the influence of psychological and social factors on health, broadly defined, as people age from early adulthood to later life (see Brim, Ryff, & Kessler, 2004). The sample included over 7,000 individuals on whom extensive psychosocial assessments (e.g., personality traits, well-being, affect, sense of control, quality of social relationships) were obtained. Such constructs received extensive attention in prior studies of adult development and aging, but the prior work was based on small, select samples with limited generalizability to the larger population. Including comprehensive psychosocial content in MIDUS afforded new directions for demography, epidemiology, and sociology, by allowing linkage of diverse “individual difference” variables to core demographic factors and broad-ranging assessments of health.
With support from the National Institute on Aging, a longitudinal follow-up of the MIDUS sample was launched in 2004/05. The objective was to investigate long-term change (9–10 years) across the sociodemographic, psychosocial, behavioral, and health domains assessed at baseline. A further objective was to extend the scientific scope of the study by adding comprehensive biological assessments on a subsample of respondents. In its longitudinal extension, MIDUS thus became a forum for investigating health as an integrative process, which involved combining the behavioral and social sciences together with bio-medically oriented research. The research was not disease-specific, given that psychosocial factors have relevance across multiple diseases. The broad aim was to “delineate the biopsychosocial pathways through which converging processes contribute to diverse health outcomes” (Singer & Ryff, 2001, p. 18). A further guiding theme was to investigate protective roles that behavioral and psychosocial factors have in delaying the onset of morbidity and mortality, or in fostering resilience and recovery from health challenges once they occur (Ryff & Singer, 1998).
Comprehensive bioindicator and health assessments data were collected on a sample of 1,255 adults. Here, we describe the data collection protocol, the specific biological measures and physical health assessments, and sociodemographic characteristics of the sample. Because MIDUS data are in the public domain (see http://www.icpsr.umich.edu/NACDA/), with over 400 publications generated by scientists from diverse fields to date, numerous research opportunities accompany the new bioindicators. To facilitate understanding of these prospects, the five projects that comprise the MIDUS II data collection are described briefly below, followed by an overview of the major systems covered in the biological protocol.
The MIDUS II Program Project
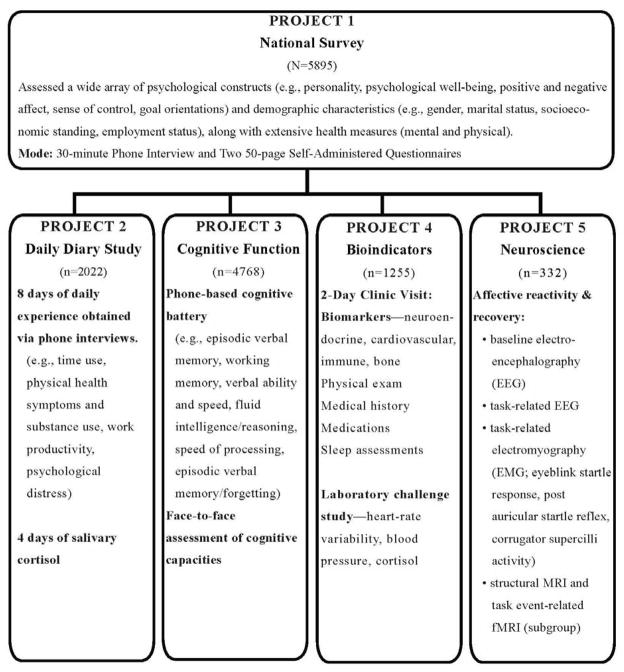
Figure 1 illustrates the five data collection projects that comprise MIDUS II (i.e. the first longitudinal follow-up). Survey assessments that replicated the MIDUS I baseline comprised Project 1, which consisted of a phone interview and self-administered questionnaires. Thus, a second round of extensive psychosocial, demographic, and health data were obtained from the original MIDUS I samples. Originally recruited in 1995/96, the respondents included: a national sample, obtained through random-digit dialing procedures (Main RDD); siblings of many respondents, for the purpose of investigating familial factors in health and well-being; and a national sample of twins, of the same age range as the national RDD sample (for the purpose of investigating genetic influences on health and well-being). See Radler & Ryff, 2010 for information on sample retention. Added to Project 1 (survey assessments) at MIDUS II was a new city-specific sample of African Americans from Milwaukee, Wisconsin. The objective was to investigate health in a highly segregated U.S. city in close proximity to collection of biological data in Madison, Wisconsin. Sample details (size, sociodemographic characteristics) at baseline and at the longitudinal follow-up are available at http://www.icpsr.umich.edu/NACDA/.
Figure 1.
Overview of the Content of the MIDUS II Projects. (Note, Samples from Projects 2–5 are not exclusive, see Table 5 for details about overlap).
All of the additional projects were based on subsamples from Project 1. That is, participation in the national survey was an eligibility criterion for participation in Projects 2–5. Many participants in the national survey completed more than one of these additional projects. The daily diary assessments (Project 2) involved 8 days of phone assessments about multiple aspects of daily life, including stressful experiences at work and with family, and emotional reactions to them. Daily stress assessments were also obtained on a large subsample at MIDUS I and thus constitute longitudinal assessments for part of the Project 2 sample. For MIDUS II, the sample was expanded, and assessments of daily salivary cortisol were added to the protocol (Almeida, McGonable, & King, 2009). Assessments of cognitive function (Project 3) were obtained by phone interviews. All participants in Project 1 were invited to participate in Project 3 cognitive assessments (see Tun & Lachman, 2008 for a description of some measures). The biological protocol (Project 4) consisted of the bioindicator and health assessments, which are the focus of this article. Details of the protocol are described below along with eligibility criteria, response rates, and sample characteristics. The neuroscience assessments (Project 5) were carried out on a subsample of the Project 4 respondents at one data collection site (University of Wisconsin-Madison). Assessments focused on affective reactivity and recovery and include multiple electroencephalography (EEG) and electromyography (EGM) indicators as well as structural magnetic resonance imaging (MRI) and task event-related functional imaging (fMRI) on a subgroup of respondents.
Taken as a whole, the five projects illustrate the MIDUS emphasis on aging as a biopsychosocial process. The broad intent of data collection across the five projects was to assemble in-depth assessments across diverse content areas on the same respondents to facilitate cross-cutting analyses. We return to this theme in Results where we show the extent, defined in terms of sample sizes, of cross-project participation in MIDUS II.
The MIDUS II Biological Protocol
Figure 2 provides an overview of the major categories of data collection in the MIDUS biomarker project. Our specimens (fasting blood draw, 12-hour urine, saliva) allow for assessment of major biological systems: cardiovascular, neuroendocrine, inflammatory, musculoskeletal, and anti-oxidants. After each system, we list the specific measures/assays that were obtained. In addition, the Project 4 biological protocol included multiple assessments obtained by clinicians or trained staff, including vital signs, morphology, functional capacities, bone densitometry, medication usage, and a physical exam (details provided in Figure 2). We also obtained indicators of heart-rate variability, beat to beat blood pressure, respiration, and salivary cortisol assessments during an experimental protocol that included both a cognitive and orthostatic challenge. Finally, to augment the extensive self-reported data collected in Project 1 survey assessments, participants in the biological protocol (Project 4) completed a medical history and self-reported sleep assessments.. For respondents at one site (UW-Madison), objective sleep assessments were also obtained with an Actiwatch® activity monitor.
Figure 2.
Detailed Summary of Bioindicators and Health Assessments in Project 4.
Methods
This section reviews eligibility for participation in the MIDUS II bioindicator and health assessments (Project 4) as well as the procedures for contacting respondents and arranging for clinic visits. The specifics of what occurred over the two-day visits are then described.
Eligibility and Initial Contacts
The overarching objective was to allow for broad participation in the biomedical assessments. Thus, all living Project 1 (national survey) respondents were considered eligible for participation if their existing health information indicated an ability to travel to the clinic without excessive risk to the respondent or project staff. Siblings of main sample respondents were not part of the recruitment pool (primarily because of cost), but members of the twin sample were included. Members of the Milwaukee sample of African Americans, newly recruited at MIDUS II, were also part of the recruitment pool. Eligible respondents were first sent a letter explaining what the biological project was about. A brochure sent with the letter sketched the key objectives of the biomedical assessments, outlined what would be included in the clinic visit, and explained how financial matters related to respondents’ time and travel would be handled. Follow-up phone calls were then made to provide additional details and answer any questions the respondent might have. All travel expenses to and from the clinics were covered, and project staff also helped arrange travel itineraries. For aged individuals, or those concerned about traveling alone, an option was provided to travel to the clinic with a companion. Respondents were given $200 in consideration of their two-day visit to the medical clinic. For some, childcare costs were also provided. The study was approved by the Institutional Review Board at each participating center, and informed written consent was obtained for all participants.
Two-Day Protocol
After arriving at one of the three participating sites, respondents were escorted by project staff to the clinic where they were checked in, and were then escorted to the room where they would stay overnight. In most cases, respondents arrived mid-afternoon of Day 1 of their visit and ended their stay by noon of Day 2. On Day 1, with staff assistance, they completed the medical history, the bone densitometry scan, and physical exam, each of which required 30–45 minutes. They were also given the self-administered questionnaire (SAQ) to complete that evening (see www.midus.wisc.edu for copies of assessment instruments, which are included under descriptions of the MIDUS II projects) Clinic nursing staff began collecting the 12 hour urine specimen (collection period 7 p.m. to 7 a.m.). On Day 2 nursing staff collected the fasting blood specimen and completed the 12 hour urine specimen collection.
After breakfast, project staff carried out an experimental protocol assessing physiological response to, and recovery from, cognitive and orthostatic challenges similar to stressors people experience in their daily lives. The protocol consisted of a series of two randomized 6 minute cognitive challenges, one involving a math task and the other a Stroop-like test (decision-making about stimuli in which letters and colors are in conflict), followed by a 6 minute orthostatic (standing) challenge. Each challenge was followed by a 6 minute recovery period. Physiological reactivity throughout the experimental protocol was monitored via measures of blood pressure, heart rate variability and respiration, and salivary cortisol. Completed SAQs were then collected, and respondents were debriefed. At the UW-Madison data collection site, information was given about completing objective sleep assessments, to be returned by mail, after returning home. At the end of their visits, respondents were given a report about their blood pressure, body mass index (BMI), and waist-hip ratio. They were sent letters reporting cholesterol, HAlc, and bone density 1–2 months after the clinic visit.
To ensure consistency across sites and optimize the pace and quality of data collection, project staff and clinic nursing staff at all three sites followed standardized procedures that were detailed in a general Manual of Procedures, as well as more specific Guidelines for Collecting and Processing Biomarkers, and a Psychophysiology Manual. An administrative database was used to facilitate management and tracking of cross-project participation as well as tracking of participation at the three Project 4 sites. This information allowed review of participation information and quality control assessments, including identifying areas where additional staff training was required. Monthly conference calls with staff and investigators from all sites provided a forum to discuss issues or problems. Prior to these calls, each site generated a “Progress Report”, using report queries built into the administrative database; the reports were circulated for review by all on the conference call.
Results
Information about response rates for Project 4 is summarized below, followed by a description of primary reasons for refusal. To assess possible selection bias, we then examine the demographic and health characteristics of the participants in Project 4 compared with the pool from which they were drawn. Finally, we provide information about the scope of cross-project participation in MIDUS II, illustrating the scope of research opportunities to link bioindicator and health data with other areas of assessment in MIDUS.
Participation in the Bioindicators and Health Protocol (Project 4)
Table 1 summarizes participation rates in the MIDUS II biological protocol. Among those eligible (N = 3,191), 39.3% (n = 1,255) participated in Project 4, with higher rates among twins, compared with main sample respondents. Participation rates for the Milwaukee sample of African Americans, newly recruited at MIDUS II, were similar to the longitudinal sample. After adjusting for respondents who could not be located or contacted, the response rates were 43.1% for the longitudinal sample (main RDD and twins), and 50.5% for the new Milwaukee sample. Overall, 45.1% of eligible respondents refused to participate in Project 4, while 6.9% never made a final decision about whether to participate (i.e., they indicated some interest, but never scheduled a visit). We were unable to locate or contact 8.7% of eligible respondents.
Table 1.
Disposition of Eligible Participants for Project 4 (Bioindicators and Health)1
| Sample | Participants | Non-Participants |
|||
|---|---|---|---|---|---|
| Refused | No Decision2 | Unable to Locate3 | No Contact4 | ||
| All Cases (n=3191) | 1255 (39.3%) | 1439 (45.1%) | 221 (6.9%) | 197 (6.2%) | 79 (2.5%) |
| % after Exclusion5 (n=2915) | (43.1%) | (49.4%) | (7.6%) | ||
| Main RDD (n=1809) | 666 (36.8%) | 947 (52.3%) | 75 (4.1%) | 86 (4.8%) | 35 (1.9%) |
| % after Exclusion5 (n=1688) | (39.5%) | (56.1%) | (4.4%) | ||
| Twins (n=871) | 388 (44.5%) | 353 (40.5%) | 88 (10.1%) | 31 (3.6%) | 11 (1.3%) |
| % after Exclusion5 (n=829) | (46.8%) | (42.6%) | (10.6%) | ||
| Milwaukee (n=511) | 201 (39.3%) | 139 (27.2%) | 58 (11.4%) | 80 (15.7%) | 33 (6.5%) |
| % after Exclusion5 (n=398) | (50.5%) | (34.9%) | (14.6%) | ||
Notes:
Eligible cases includes those who are living and whose existing health information indicated ability to travel to the clinic without excessive risk to respondent or project staff;
No Decision – respondent was interested but was unable to schedule date to visit clinic before study ended.
Unable to Locate – respondent moved after completing Project 1 and contact information could not be re-established despite multiple attempts;
No Contact – recruitment letter mailed to valid address with working phone but recruitment staff were unable to reach anyone in household despite numerous attempts;
Excluded cases are those designated as Unable to Locate or No Contact.
Table 2 summarizes reasons for refusal. The primary explanations given by respondents were that they: (1) did not want to travel to the clinic, (2) had other family obligations (such as caregiving), (3) were too busy, or (4) were not interested in the biological part of MIDUS II. Personal health problems and work obligations were also mentioned, but less often. Most respondents reported one primary barrier to participation; some reported multiple reasons.
Table 2.
Reasons for Refusals (n=1439, categories not exclusive)
| Respondent Defined Barriers to Participationa | Frequency | Percentage |
|---|---|---|
| Not Interested | 318 | 22.1 |
| Too Busy | 323 | 22.4 |
| Travel | 460 | 32.0 |
| Hospital Aversion | 74 | 5.1 |
| Family Obligations (caregiving, other issues) | 323 | 22.5 |
| Personal Health | 269 | 18.7 |
| Work/School Obligations | 237 | 16.5 |
| Other (incentive too small, age, pet care, etc.) | 69 | 4.8 |
Note:
Respondents report 1–4 barriers to participations as follows: 62% (897) – 1 Barrier; 31% (447) - 2 Barriers; 6% (87) - 3 Barriers; 1% (8) - 4 Barriers.
An important question in evaluating the biological subsample is how comparable it is to the pool of respondents from which it was recruited. Table 3 summarizes information on the demographic and health characteristics of the Project 4 sample compared with those who completed the survey assessments in Project 1, separately by those who completed only the phone interview as well as by those who completed both the Project 1 phone interview and self-administered questionnaire (SAQ).. The Project 4 sample was not significantly different from either Project 1 sample on age, sex, race, marital status, or income, although respondents in the biological protocol were significantly more likely to have a college degree and significantly less likely to have only high school or some college compared with the national sample (Project 1). Nonetheless, more than half of the biological participants came from the lower educational category. This result, combined with the similarity on income, indicates that the MIDUS II biological sample is useful for inquiries related to social inequalities in health – a major thematic focus in publications from the study thus far.
Table 3.
Comparison of Demographic and Health Characteristics for Project 1 (National Survey) and Project 4 (Bioindicator) Samples.
| Demographic Characteristics | MIDUS Project |
|||
|---|---|---|---|---|
| Project 1 Interview Sample1 (n=5,500) | Project 1 Interview & SAQ Sample2 (n=4,006) | Project 4 Bioindicator Sample (n=1,255) | Project 4 Non-Respondents (n=1,992) | |
| Age (M, SD) | 55.0 (12.4) | 55.4 (12.4) | 54.5 (11.7) | 55.8a (12.9) |
| Female (%) | 54.3 | 56.1 | 56.8 | 56.7 |
| Education | ||||
| High School/Some College (%) | 57.9 | 57.4 | 52.2a,b | 61.4a,b |
| College grad or more (%) | 34.5 | 34.3 | 42.1a,b | 28.4a,b |
| White (%) | 81.0 | 77.9 | 78.3 | 75.3a,b |
| Married (%) | 70.5 | 68.6 | 69.2 | 67.4a |
| Personal Income (M) | 39,842 | 39,755 | 41,538 | 36,871b |
|
Health Characteristics | ||||
| Subjective Physical Health (M, SD: range:1=Excellent, 5=Poor) | 2.52 (1.0) | 2.54 (1.0) | 2.41 (0.9) | 2.63a,b (1.1) |
| Body Mass Index (M, SD: range:14.2–82.3) | 28.3 (6.3) | 28.4 (6.3) | 28.5 (6.1) | 28.5 (6.4) |
| Instr. Activities of Daily Living (M, SD, range:1=Limited, 4=Not At All Limited) | 1.82 (.90) | 1.83 (.90) | 1.75 (.86) | 1.87 (.93) |
| Use of at least one Alternative Therapy (%) | 32.7 | 32.6 | 37.6a,b | 30.5 |
| Health Insurance Coverage(%) | 81.2 | 81.0 | 79.9 | 82.0 |
| Currently smoking cigarettes (%) | 16.8 | 16.6 | 13.8a,b | 18.5 |
| Ever drank 3+ days/week (%) | 38.1 | 37.1 | 37.8 | 35.2 |
| # Physician Visits -12 mo (M, SD) | 4.4 (8.3) | 4.4 (8.7) | 4.8 (12.8) | 4.2 (6.2) |
| # of Chronic Conditions (M, SD) | 3.3 (2.5) | 3.3 (2.5) | 3.1 (2.4) | 3.4 (2.6) |
| # of times exercise vigorously/month (M, SD) | 3.6 (3.36) | 3.7 (3.38) | 3.7 (3.34) | 3.6 (3.42) |
Significantly different from Project 1 Interview Sample at p < .01;
Significantly different from Project 1 Interview & SAQ Sample at p < .01
Notes.
Respondent completed a phone interview, or for Milwaukee, a personal interview;
Recruitment pool for Project 4, respondents completed an interview and Self-Administered Questionnaire (SAQ).
With regard to health characteristics, the Project 4 sample was also strongly comparable to the Project 1 national samples from which it was recruited. There were no significant differences in ratings of subjective health, chronic conditions, instrumental activities of daily living, exercise, alcohol use, health insurance coverage, or physician visits in the past 12 months. Biomarker respondents were, however, significantly less likely to smoke than Project 1 participants, and they were more likely to use alternative therapies (e.g., herbal remedies, spiritual practices) than Project 1 respondents.
A key objective in the MIDUS II program project was to facilitate linkage of biological data with numerous other domains of assessment. All Project 4 respondents had to have completed the survey assessments in Project 1. Completion of the survey assessments (Project 1) was also a prerequisite for participating in any of the other MIDUS II projects as well (as illustrated in Figure 1). Table 4 provides information on cross-project participation, first by showing the number of participants in MIDUS II who completed Project 1 (survey assessments), Project 4 (bioindicators and health) and at least one other project. In each instance of 3-way participation, respondents are further disaggregated into those from the main sample, the twin sample, or the Milwaukee sample of African Americans. Next listed in Table 4 is the number of cases participating in at least 4 MIDUS II projects. For example, the table shows that 960 members of the MIDUS II sample completed Projects 1 (survey assessments), Project 4 (bioindicators and health), Project 2 (daily diaries), and Project 3 (Cognitive Function). Among those completing the neuroscience assessments (Project 5) with at least 3 other projects, sample sizes ranged from 221 to 296. Such overlap underscores the richness of the MIDUS II data collection, and further documents the extensive degree of time and effort contributed to the study by the MIDUS respondents. We attribute their active involvement to the high level of commitment they expressed about the study as well as to the care with which project staff across all aspects of data collection worked to ensure a positive experience for members of the sample.
Table 4.
Summary of Cross-Project Participation.
| All Cases Below Completed Project 1 National Survey Assessments | ||||
|---|---|---|---|---|
| Completed Project 4 (Bioindicators) and: | Number of cases | Number of Project 4 Cases By Subsample |
||
| Main RDD | Twin | Milwaukee | ||
| Project 2 (Daily Diaries) | 1011 | 588 | 285 | 135 |
| Project 3 (Cognitive Function) | 1152 | 636 | 374 | 136 |
| Project 5 (Neuroscience) | 332 | 134 | 88 | 109 |
| Project 2 & 3 | 960 | 576 | 279 | 102 |
| Project 2 & 5 | 238 | 125 | 35 | 77 |
| Project 3 & 5 | 296 | 132 | 85 | 78 |
| Project 2, 3, & 5 | 221 | 124 | 35 | 61 |
Discussion
The purpose of this article is to provide a description of the biological data collection in MIDUS II and the sample on which such measures were obtained. The work is aligned with other publications whose intent is to introduce public-use data sets to the research community. Examples in aging research include descriptions of the Taiwan SEBAS (Social Environment and Biomarkers of Aging Study) (Chang, Glei, Goldman, & Weinstein, 2008); the AGES (age, gene/environment susceptibility) Reykjavik study (Harris, Launer, Eiriksdottir et al., 2007); the WHAS (Women’s Health and Aging Study) (Kasper, Shapiro, Guralnik, Bandeen-Roche, & Fried, 1999); the Rotterdam Elderly Study (Hofman, Grobbee, De Jong, & VanDenOuweland, 1991); the Cardiovascular Health Study (Cushman, Cornell, Howard, Bovill, & Tracy, 1995); the LSADT (Longitudinal Study of Aging Danish Twins) (Christensen, Bathum, & Christiansen, 2008); and the Whitehall II and ELSA (English Longitudinal Study of Aging) studies (Marmot & Steptoe, 2008).
Viewed in the context of these other investigations, MIDUS has a demanding biological protocol: there are no directly comparable studies with which to evaluate participation rates. Respondents had to travel sometimes lengthy distances to one of three medical clinics around the country as well as stay overnight to enable two days of biomedical assessments. Among other epidemiological studies of aging in the U.S. involving a visit to a health clinic, such as the Cardiovascular Health Study (CHS), response rates were 57% (Fried et al., 1998). Our response rates are lower (39.3% overall; 43% among those we were able to contact and invite), but the differences in protocol demands are notable. In the CHS, sample members traveled to a nearby clinic and did not stay overnight. Many MIDUS respondents had extensive travel time to and from the clinics in addition to committing two full days of time to their participation. Given the mid-life focus of the study, most MIDUS respondents are also middle-aged (mean age 55.4 years) and thus dealing with active demands of work and family life, whereas CHS was recruiting largely among retired individuals.
Importantly, those who did agree to participate are sociodemographically similar to the national sample (Project 1) from which they were recruited, although they are somewhat better educated. Nonetheless, a sufficiently large proportion of Project 4 (bioindicators and health) participants (25%) are in the lowest education category (HS or less), while more than 50% did not complete college. The biological sample also did not differ from the Project 1 sample on income. For multiple indicators of health status and health behaviors, Project 4 respondents were also comparable to the pool from which they were recruited. Only for two measures were differences noted: Project 4 respondents were significant less likely to smoke and significantly more likely to use alternative therapies. Overall, our efforts to collect comprehensive bioindicator data, via a uniquely demanding protocol, fared well: we succeeded in assembling a large, sociodemographically diverse sample on which comprehensive biomedical assessments are now available.
Equally important is the fact that respondents in the MIDUS II biological protocol also participated in multiple other MIDUS II projects. All members of the Project 4 sample completed the extensive survey assessments from Project 1, and for all but the Milwaukee respondents, these detailed data on sociodemographic, psychosocial, and health characteristics represent repeat assessments over a 9–10 year period. Long-term profiles of psychosocial strengths and vulnerabilities can thus be created and used to investigate variation in biological assessments obtained at MIDUS II. Measures of biological regulation in multiple systems can further be used to illuminate reports of health conditions and symptoms, also assessed longitudinally. Nearly all (92%) biomarker respondents completed the comprehensive cognitive assessments (Project 3), and 81% of biomarker respondents completed the daily diary assessments (Project 2). Although the neuroscience sample (Project 5) in MIDUS II is notably smaller, it represents one of the largest samples of brain-based measures ever assembled. All respondents participating in the neuroscience project completed Project 1 (survey assessments) and Project 4 (bioindicators and health) assessments. In sum, the scope of cross-project participation in MIDUS II is high, which bodes well for scientific analyses that take seriously the biopsychosocial integration that motivated the study.
MIDUS is not unique in including biological assessments. Numerous major surveys of aging (e.g., English Longitudinal Study of Aging, Health and Retirement Survey, MacArthur Study of Successful Aging, National Long-Term Care Survey, Normative Aging Study, Social Environment and Biomarkers of Aging Study in Taiwan, Swedish Adoption Twin Study of Aging, Wisconsin Longitudinal Study, Women’s Health and Aging Studies) now include biological measures (see Weinstein, Vaupel, & Wachter, 2008). To place our effort in the context of these other studies, Table 5 provides comparative data from 22 major longitudinal studies with biomarkers. Two primary websites served as sources for information presented in the table. The first is the National Institute on Aging Database of Longitudinal Studies (http://www.nia.nih.gov/ResearchInformation/ScientificResources/LongitudinalStudies.htm) and the second is a listing of population studies collecting biomarkers through the University of Chicago (http://biomarkers.uchicago.edu/studiescollectingbiomarkers.htm).
Table 5.
Longitudinal Studies with Bioindicators
| Study Name (Sample Size) Acronym |
Cardiovascular Metabolic |
Immune/Inflammatory |
Musculo-Skeletal |
Neuroendocrine |
||
|---|---|---|---|---|---|---|
| Tissue Marker | Clinician Assessment | Tissue Marker | Clinician Assessment | |||
| Midlife in the US (n=5,555) MIDUS |
Blood -Cholesterol (HDL, LDL, Total), Triglycerides, HA1c | BP, Pulse, Respiration Rate | Blood - CRP, ICAM, IL6, s-IL6r, Fibrinogen, E-Selectin | Blood - P1NP, BSAP, NTx, Vitamin D | Bone Densitometry | Blood - DHEA, DHEA-S; Urine - Cortisol, Epinephrine, Norepinephrine, Dopamine; Saliva - Cortisol |
| Age, Gene/Environment Susceptibility Study (n=9,500) AGES |
Blood -Cholesterol (HDL, LDL, Total), Triglycerides, HA1c; Fasting Insulin & Glucose | BP, ElectroCardioGram, Carotid Ultrasound, Echocardiography, Arterial Tomography & Tonometry, Cardiac MRI; | Blood - CRP, CBC including white cell count | INAPP | Scout film (hip, spine, femur); Photography (hand, foot); Tomography of L1/2 (lumbar spine) & hip, muscle mass in thigh and abdominals, fat deposits; Assessment of hip joint space, hip & knee; Heel Bone Densitometry; Bioimpedence, X-ray assessment of bone marrow | Saliva - Cortisol |
| Women’s Health and Aging Study III (n=1,002) WHAS III |
Blood -Cholesterol (HDL, LDL, Total), Triglycerides, Glucose | BP, Electrocardiogram, Treadmill | Blood - CRP | Blood - Creatinine, Calcium, Osteocalcin, Parathyroid Hormone, TSH, Vitamin D | Bone Densitometry, Bioimpedence | Saliva - Cortisol |
| The Study of Women’s Health Across the Nation (n=3,302) SWAN |
Blood - Cholesterol (HDL, LDL, Total), Triglycerides, Apolipoprotein B, lipoproteins Lp(a) & Lp (A1); Glucose, Insulin | BP, Vascular Stiffness (Dyna Pulse technique) | Blood - CRP, Fibrinogen, TPA (tissue plamsinogen activator), PAI1 (Plasminogen activator inhibitor 1) | INAPP | Bone Densitometry, Vertebral Morphometry, Bioimpedance | Blood – DHEA-S |
| Cardiovascular Health Study (n=5,888) CHS |
Blood - Cholesterol (HDL, Total), Trigylcerides, Insulin, Glucose | BP, Electrocardiogram, Echocardiography, Carotid & Abdominal Aortic Ultrasound, Ankle Arm Index | Blood -CRP, Fibrinogen, Hemostatic Factor VII & VIII, IL-6 | INAPP | Bone Densitometry, Bioimpedence | INAPP |
| Health, Aging & Body Composition Study (n=3,075) Health ABC |
Blood - Cholesterol (HDL, LDL, Total), Fasting Glucose and Insulin, HA1c | BP, Arterial Venous Blood Gas, Arterial Pulse Wave Velocity, ECG | Blood - CRP, IL-6, Leptin, PAI-1, TNFa | INAPP | Bone Densitometry, Joint Evaluation, Osteo- arthritis Assessment, Ultrasound of the Heel, Computed Tomography (Body Composition) | INAPP |
| Normative Aging Study (n=2,280) NAS |
Blood - Cholesterol (HDL, LDL, Total), Triglycerides, Glucose Tolerance (fasting & 2 hour); SGOT (glutamic oxalacetic transaminase) | BP & Heart Rate (sitting, standing & supine); Electrocardiogram | Blood - Erythrocyte Sedimentation Rate | INAPP | Tibia & Patella Lead Content | INAPP |
| NHANES/NHEFS NHANES & NHANES I Epidemiologic Followup Study (n=14,407) | Blood- HA1c, Fasting Glucose, Insulin, Oral Glucose Tolerance, Cholesterol (HDL, LDL, Total), Triglycerides, Apolipoprotein B | BP, Pulse | Blood-HLA-B27, Hepatitis Viruses; CRP | Blood- Vitamin D, Calcium, Creatinine | Bone Densitometry | INAPP |
| Rotterdam Study (n=7,983) | Blood -Non-Fasting Glucose Tolerance Test, Total & HDL Cholesterol, Platelets, Various Coagulation & Anticoagulation Factors, Blood Vessel Wall Protein | BP (Regular, Doppler), Echocardiograph, Cardiac Ultrasound, ECG, Arterial Stiffness | Blood - Erythrocyte Sedimentation Rate, Fibrinogen & Other Fibrinolytic Measures | Blood - Calcium, Creatinine, 25 OH Cholecalciferol, Parathormone, TSH, Osteocalcin, Urine - Sodium, Calcium, Proline, Creatinine | Bone Densitometry, X- Ray (hands, thoracol-lumbar spine, hips & knees) | INAPP |
| Baltimore Longitudinal Study on Aging (n=3,000) BLSA |
Blood -Cholesterol (HDL, LDL, Total), Triglycerides, Lipoproteins, Glucose tolerance | BP, Resting, Treadmill & Ambulatory ECG, Arterial Stiffness, Thallium Scan, Echocardiography, Cardiac MRI, Basal Metabolism | Blood- Cell Counts (white cells, lymphocytes, eosinophils), Mitogen Response tests (anti- EBVCA, EBEA, pneumonia, tetnus etc.), Monoclonal Antibody, Histocompatibility etc., Prostate Specific Antigens | INAPP | Bone Densitometry - very detailed, Body composition | INAPP |
| Established Populations for Epidemiologic Studies of the Elderly (n=14,456) EPESE |
Blood - Cholesterol (HDL, LDL, Total) Triglycerides, Glucose, HA1c | INAPP | Blood - Leukocytes, IL-6, Alpha-2 globulin, Fibrin-d- dimers, Lymphocytes | Blood -Calcium, Creatinine | INAPP | INAPP |
| Italian Longitudinal Study of Aging (n=5,493) ILSA |
Blood - Cholesterol (HDL & Total), Triglycerides, Glucose, HA1c, Insulin | Examination of Heart, Lungs, Pulses, Bruits, ECG, BP, Spirometry | Blood - Fibrinogen, Factor VII (proconvertin), Factor VIII (clotting factor) | INAPP | INAPP | INAPP |
| Longitudinal Aging Study Amsterdam (n=3,017) LASA |
Blood - Cholesterol (HDL, LDL, Total), Triglycerides, D19 Glucose | BP, Heart Rate | Blood - Leukocytes, CRP | Blood - Calcium, Creatinine | Bioimpedence | INAPP |
| The Women’s Health Initiative (n=68,132) WHI |
Blood - Tryglycerides | Resting Pulse, BP, 12 lead ECG | Blood - White Cell Count, Platelet Count | Urine - Bone Metabolites | Bone Density Scan | INAPP |
| Honolulu-Asia Aging Study (n=3,734) HAAS |
Blood - Cholestero (HDL, LDL, Total), Triglycerides, Glucose and Insulin (fasting 1 & 2 hour post load); Urine - Glucose | Heart rate, BP (multiple measures - sitting, standing, ankle-arm etc), Resting Electrocardiogram | Blood - Fibrinogen | INAPP | INAPP | INAPP |
| The Health and Retirement Study (n=22,000) HRS |
Blood - Cholesterol (HDL, LDL, Total), HA1c, | BP | Blood - CRP | INAPP | INAPP | INAPP |
| English Longitudinal Study of Aging (n=12,100) ELSA |
Blood -Cholesterol (HDL, Total), Triglycerides, Apolipoprotein E, Fasting Glucose, HA1c | BP | Blood-CRP, Fibrinogen | INAPP | INAPP | INAPP |
| MacArthur Study of Successful Aging (n=1,189) | Blood - HDL & total Cholesterol, HA1c, SGOT (glutamic oxalacetic transaminase), | Seated and Postural BP, Pulse | CRP, IL-6 | INAPP | INAPP | Blood -DHEA-S; Urine - Cortisol, Epinephrine, Norepinephrine, Dopamine, Creatinine |
| Australian Longitudinal Study on Aging (n=2,087) ALSA |
Blood -Cholesterol (HDL, LDL, Total), Triglycerides, Glucose | BP | INAPP | Blood - Creatinine, Calcium, Urine -Calcium, Parathyroid Hormone, Vitamin D | Bone Densitometry | Urine – DHEA |
| Social Enviromnent and Biomarkers of Aging Study in Taiwan (n varies) SEBAS |
Blood - Cholesterol (HDL, Total), Triglycerides, HA1c, Fasting Glucose | BP, Heart Rate, Peak Flow, Abdominal Ultrasound | Blood -IGF-1, IL-6, IL-6sr, ICAM-1, E-Selectin, High Sensitivity CRP, Fibrinogen, Leukocytes, Lymphocytes, Platelet Counts | Blood - Creatinine | INAPP | Blood -DHEA-S; Urine - Cortisol, Epinephrine, Norepinephrine, Dopamine, Creatinine |
| Natinal Social Life, Health and Aging Project (n=3,005) NSHAP |
Blood - HA1c | BP | Blood - CRP, EpsteinBarr Virus | INAPP | INAPP | Saliva - DHEA |
The information in Table 5 extends previous endeavors (Harris, Gruenewald, & Seeman, 2008) to provide an overview of biological content across multiple major investigations1. What is evident is that the MIDUS II biological protocol shares similarities with those included in other major population-based studies in the US and abroad. Areas of greatest similarity include what might be termed the “basic” assessments of lipid profiles, glucose metabolism, blood pressure, inflammation and weight along with assessments of functional status (e.g., grip strength, walking speed). Where MIDUS (and a smaller number of other studies) differ from a majority of population studies is the inclusion of assessments of hypothalamic-pituitary-adrenal and sympathetic nervous system activity, bone (including both bone density as well as peripheral bone turn-over markers from blood), and data on antioxidant profiles. Perhaps the most unique feature of the MIDUS II biological protocol is the inclusion of a standardized “response to challenge” protocol that includes data on system dynamics with respect to sympathetic/parasympathetic activity (from heart rate variability data) and hypothalamic-pituitary-adrenal axis activity (from salivary cortisol data).
Thus, while some studies offer expanded samples sizes for investigating, for example, socioeconomic differences in cardiovascular risk factors, MIDUS can probe such questions on a reduced subsample, but this constraint is offset by the unusual breadth of data that have been collected on the same respondents across the MIDUS II projects. Those interested in social inequalities in health can bring together analyses that involve a rich array of psychosocial factors, daily stress assessments, cognitive function, comprehensive biomarkers, and neuroscience assessments. Unlike MIDUS, many population-based studies begin with people in their 50’s or 60’s. An important feature of MIDUS is that a broad spectrum of measures has been assembled on respondents in midlife (aged 25–74 at baseline, aged 35–85 at MIDUS II); thus, the study is well-situated to investigate pre-disease pathways – i.e., precursors to later life health problems.
In sum, the MIDUS II biological data obtained on a relatively large sample co-exist with rich data obtained on psychosocial factors, daily stress, cognitive function, and neuroscience. Such data afford unique opportunities to those in the scientific community who are interested in interdisciplinary questions that link cumulative experience to biological processes known to affect multiple major health outcomes over the life course.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The research also received support from the following grants: M01-RR023942 (Georgetown) and M01-RR00865 (UCLA) from the General Clinical Research Centers Program; and 1UL1RR025011 (UW-Madison) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
We thank members of the sample for their many contributions to the MIDUS study. We also thank Project 4 staff and the staff of the Clinical Research Centers at the University of Wisconsin-Madison, UCLA, and Georgetown University for their assistance in conducting the study. We also thank Nancy Davenport and Kris Hansen for their assistance in compiling information about reasons for refusal and barriers to participation.
Footnotes
Twelve of the studies listed, including MIDUS, collected specimens (blood, saliva, buccal) for genotyping while MIDUS, and two others, also included anti-oxidant assessments.
References
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. The MIDUS National Survey: An overview. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we?: A national study of well-being at midlife. Chicago: University of Chicago Press; 2004. pp. 1–36. [Google Scholar]
- Chang M, Glei D, Goldman N, Weinstein M. The Taiwan biomarker project. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial Surveys. Washington, DC: The National Academies Press (Committee on Population, Division of Behavioral and Social Sciences and Education); 2008. pp. 60–77. [Google Scholar]
- Chicago Core on Biomarkers in Population-Based Aging Research Database of Studies Collecting Biomarkers. available at http://biomarkers.uchigao.edu/studiescollectingbiomarkers.htm.
- Christensen K, Bathum L, Christiansen L. Biological indicators and genetic information in Danish Twin and Oldest-Old Survey. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial Surveys. Washington, DC: The National Academies Press (Committee on Population, Division of Behavioral and Social Sciences and Education); 2008. pp. 42–59. [Google Scholar]
- Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory Methods and quality assurance in the Cardiovascular Health Study. Clinical Chemistry. 1995;41:264–270. [PubMed] [Google Scholar]
- Harris JR, Gruenewald TL, Seeman T. An overview of biomarker research from community and population-based studies on aging. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Washington, DC: The National Academies Press (Committee on Population, Division of Behavioral and Social Sciences and Education); 2008. pp. 96–135. [Google Scholar]
- Harris TB, Launer LJ, Eiriksdottir G, et al. Age, gene/environment, susceptibility Reykjavik Study: Multidisciplinary applied phenomics. American Journal of Epidemiology. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman A, Grobbee DE, De John PTVM, VanDenOuweland FA. Determinants of disease and disability in the elderly: The Rotterdam Elderly Study. European Journal of Epidemiology. 1991;7:403–422. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- Inter-university Consortium for Political and Social Research (ICPSR) MIDUS data. doi: 10.1111/j.1360-0443.2011.03564.x. Available at http://www.icpsr.ucmich.edu/NACDA/ [DOI] [PMC free article] [PubMed]
- Kasper JD, Shapiro S, Guralnik JM, Bandeen-Roche KJ, Fried LP. Designing a community study of moderately to severely disabled older women: The Women’s Health and Aging Study. Annals of Epidemiology. 1999;9:498–507. doi: 10.1016/s1047-2797(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Marmot M, Steptoe A. Whitehall II and ELSA: Integrating epidemiological and psychobiological approaches to the assessments of biological indicators. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Washington, DC: The National Academies Press (Committee on Population, Division of Behavioral and Social Sciences and Education); 2008. pp. 15–41. [Google Scholar]
- Midlife in the United States (MIDUS) available at http://www.midus.wisc.edu.
- National Institute on Aging Database of Longitudinal Studies. available at http://www.nia.nih.gov/ResearchInformation/ScientificResources/LongitudinalStudies.htm.
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS (Midlife in the U.S.) National Study of Health and Well-Being. Journal of Aging and Health. 2010;22:xx–xx. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9:1–28. [Google Scholar]
- Singer B, Ryff CD. New horizons in health: An integrative approach. Washington, D.C: National Academy Press; 2001. [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Developmental Psychology. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Washington, DC: The National Academies Press (Committee on Population, Division of Behavioral and Social Sciences and Education); 2008. [PubMed] [Google Scholar]