Abstract
Background
Congenital heart surgery initiates a complex inflammatory response that can influence the post-operative course. However, broad integration of the cytokine and proteolytic cascades (matrix metalloproteinases: MMPs), which may contribute to post-operative outcomes has not been performed.
Methods/Results
Using a low volume (50 – 60 μL), high sensitivity, multiplex approach a panel of cytokines (IL-2, -4, -6, -8, -10, TNFα, IL-1β, GM-CSF) and MMPs (MMP-2, -3, -7, -8, -9, -12, -13) were serially measured in patients (n=9) pre-operatively and post-VSD repair. Results were correlated with outcomes such as inotropic requirement, oxygenation, and fluid balance. Serial changes in peri-operative plasma levels of the cytokines and MMPs exhibited distinct temporal profiles. Plasma levels of IL-2, -8, -10, and MMP-9 peaked within 4 hours, while MMP-3 and MMP-8 levels remained elevated at 24 and 48 hours following cross-clamp removal. Area-under-the-curve analysis of early cytokine levels were associated with major clinical variables, including inverse correlations between early IL-10 levels and cumulative inotrope requirement at 48 hours (r: −0.85, p<0.005) and late MMP-7 levels and cumulative fluid balance (r: −0.90, p<0.001).
Conclusions
The unique findings of this study were that serial profiling a large array of cytokines and proteolytic enzymes following congenital heart surgery can provide insight into relationships between changes in bioactive molecules to early postoperative outcomes. Specific patterns of cytokine and MMP release may hold significance as biomarkers for predicting and managing the post-operative course following congenital heart surgery.
Keywords: Ventricular septal defect repair, Inflammation, Cytokines, Matrix metalloproteinases
Introduction
The importance of post-cardiopulmonary bypass (CBP) inflammation in pediatric cardiac surgery is reflected in the many interventions directed at its reduction. Steroid administration, modification of pump circuit surfaces by heparin bonding, ultrafiltration strategies, leukocyte trapping filters, reduced post-bypass oxygen exposure, monoclonal antibody administration, have all been described to modify features of the post-CPB inflammatory response in newborns, infants, and children.1 Despite this, a survey of 36 centers performing pediatric CPB1 revealed that no anti-inflammatory strategy achieved the level of standard practice in the pediatric CPB population, reflecting absence of convincing data to guide therapies. CPB engenders inflammation through multiple mechanisms, including direct tissue injury, myocardial ischemia/reperfusion, neutrophil/platelet activation from the CPB circuit, and lipopolysaccharide exposure.2 The molecular events of inflammation include synthesis and release of cytokines, which in turn induce complex and context-dependent cellular and molecular events, including release of matrix metalloproteinases (MMPs).3 The characteristic pleotropy, redundancy, and complexity of the inflammatory response have complicated efforts to define this process in infants, due to the number of inflammatory biomarkers to consider in relation to relative volume of blood available for sampling. This study was performed in order to address this issue through the use of high-sensitivity multiplex assays to allow serial measurement of cytokine and MMP levels from repeated small volume blood sampling in a population of infants undergoing VSD repair. This study provided the first opportunity to perform a comprehensive cytokine/MMP profile and begin to identify potential relationships between the inflammatory response and clinical outcomes.
Methods
Patients
This study was approved by the MUSC Institutional Review Board (HR #16017 and HR#17161). Entry criteria for the VSD group were planned complete surgical repair of the anatomic defect, and age from 1 to 9 months. Exclusion criteria were recognized chromosomal anomalies, prior intracardiac surgery, or complex heart disease. Enrollment was from January 2006-June 2007, the cardiac surgeons remained constant during this interval (FAC, SMB). The conduct of CPB was performed in identical fashion and aprotinin was utilized in all cases. Standard non-pulsatile CPB was utilized, and the circuit primed with Plasmalyte A (Baxter Healthcare Corporation, Deerfield, IL, USA), and 1 unit of fresh-frozen plasma. Banked, packed red blood cells (PRBC) were added to achieve a hematocrit of approximately 28–30% during CPB. No steroids were administered preoperatively or in the CPB prime. Moderate (25°C to 28°C) hypothermia was employed, and myocardial pres ervation was obtained with cold blood cardioplegia at 20 min. intervals. The pH-stat regimen was used during cooling, and alpha-stat for rewarming. Modified ultrafiltration was performed after separation from CPB. Protamine was given at 0.6:1 protamine to heparin ratio. Blood product transfusions following CPB were administered as necessary to achieve satisfactory hemostasis and a target hematocrit of >30%. Standard transatrial closure of the VSD was employed in all patients. The aprotinin dose consisted of both an intravenous and pump prime load of 240 mg/m2 BSA (1.7 × 106 kIU/m2 BSA), and a continuous infusion at 56 mg/m2 BSA/hr (4 × 105 kIU/m2 BSA/hr) until the completion of the primary procedure. For the purposes of providing a referent control range, a group of ten infants were recruited from patients undergoing ambulatory non-cardiac surgery. In this group, a one-time venous blood collection (1 mL) was performed at the time of intravenous cannulation.
Clinical Data Collection
Clinical characteristics such as age, weight, and body surface area (BSA), and z-score for weight were recorded. Data recorded or calculated during the 48 hour study period included CPB and cross-clamp times, arterial blood gases, blood counts, and arterial-alveolar oxygen gradients at 4 hours. Inotrope score was calculated as dopamine [(mcg/kg/min) × hrs] + epinephrine [(mcg/kg/min) × hrs ×100] + milrinone [(mcg/kg/min) × hrs ×10]. Fluid balance was calculated as total fluid administered (all sources) minus total fluid out (all sources). Inotrope score, fluid balance and near infrared spectroscopy (NIRS) were analyzed as cumulative values from time of arrival in the post-operative care unit. In addition, blood product utilization was recorded during the operative and peri-operative period.
Blood Collection and Processing
Baseline blood (1 mL) was collected in the operating room pre-incision into a chilled EDTA tube. Plasma was isolated by centrifugation, decanted into aliquots and stored at −70°C until processed for immunoassays. Subsequent 1 mL samples were obtained following modified ultrafiltration and 4, 12, 24, and 48 hours post cross-clamp removal and processed identically. Cross-clamp removal (onset of myocardial reperfusion injury) was the reference time zero for post-operative measurements.
Quantitative Measures of Cytokines and MMPs
Plasma levels of cytokines and MMPs were determined by multiplex suspension array using calibrated and validated combinatorial immunoassays (R&D Systems, Minneapolis, MN). For cytokines, measurements were made from undiluted plasma. For MMP analysis, plasma was diluted 1:10 for MMP-3, -7, -8, -12, and -13, and 1:100 for MMP-2 and -9. The identification and quantification of the analyte/bead complexes were determined by flow cytometry with dual excitation lasers (the excitation and emission wavelengths are 532 nm and 575 nm respectively, Bio-Plex Suspension Array Workstation, Bio-Rad, Hercules, CA). Each analyte concentration was calculated from an analyte-specific five parameter logistic calibration equation (Bio-Plex Manager Software 4.1.1). Average sensitivities for cytokines were 0.3 pg/mL, and 5 pg/mL for MMP/TIMPs. The cytokine and MMP multiplex assays have less than 0.5% cross-reactivity and interference with the other measured analytes. Plasma values were corrected for hemodilution using simultaneously obtained hematocrit vales.
Data Analysis
Patient demographics and pre-operative values for plasma levels of cytokines and MMPs were compared between the control and VSD groups using Kruskal-Wallis test of medians. Comparisons were withheld if the analyte failed to be detectable in at least 25% of the samples for each group. For post-operative analysis, two area-under-the-curve (AUC) values were derived from the time course of expression for each analyte. Early AUC was determined as the dose x time product (summation of the areas of individual trapezoids) for each analyte from baseline to the 4 hours post-cross-clamp time point. Late AUC was computed in the same fashion for the 4 to 48 hour interval. For AUC determinations, an undetectable level was set at zero. Correlations between AUCs for each cytokine/MMP and clinical outcomes, and between early and late AUCs, were examined by Spearman correlation analysis. Statistical tests were performed using the Stata software package (Stata Intercooled, v8.0) or SAS (SAS, Cary, NC).
Results
Demographics
The age of the VSD patients were 4.3±0.5 months whereas the referent normal control subjects were older (7.8±1.3 months, p<0.05), but nevertheless could be utilized for the purposes of generating a referent normal range for the cytokine and MMP assays. The male to female ratio in the VSD group was (4/5) and similar in the referent control group (5/5). Preoperative white blood counts, platelet counts and serum measurements of renal function (electrolytes, BUN, and creatinine, not shown) were all within the normal range for the VSD patients. Cross-clamp time, CPB time, intubation times, cumulative introtrope score, fluid balance, NIRS and blood product utilization for the VSD patients are summarized in Table 1.
Table 1.
Intraoperative and perioperative characteristics in subjects with a ventricular septal defect (VSD)
| Cross clamp time (min) | 57.2±5.0 |
| CPB time (min) | 95.7±7.4 |
| Intubation time (hrs) | 36.2±3.3 |
| Inotrope score (mcg/kg/min × hrs) | 126±34 |
| Fluid balance (mL) | −5.3±12.5 |
| NIRS (%) | 66.3±2.3 |
| Blood product utilization | |
| Packed red blood cells (units) | 1 (1) |
| Fresh frozen plasma (units) | 0 (1) |
| Cryoprecipitate (units) | 0 (0) |
| Platelets (units) | 1 (0) |
| Sample size (n) | 9 |
Times, inotrope score, and fluid balance are presented as Mean ± SEM.
Blood product utilization is presented as Median (Interquartile range)
CPB: cardiopulmonary bypass
NIRS: Near infrared spectroscopy, average of values recorded in intensive care unit
Baseline Plasma Levels of Cytokines and MMPs
Baseline levels for tumor necrosis factor alpha (TNF), the interleukins (ILs), interferon gamma (INF), and granulocyte-macrophage colony stimulating factor (GM-CSF) for the referent control and VSD group are summarized in Table 2. IL-1β, IL-10, and GM-CSF were detectable, but to a variable degree, in each group. Other cytokines assayed (TNFα, interferon-γ (IFN-γ), IL-2, IL-6, IL-8) were detectable at baseline in 90% or more samples. Plasma levels of IL-2 were lower in the VSD group when compared to referent control values.
Table 2.
Baseline Plasma Cytokine and MMP levels in referent control subjects and subjects with ventricular septal defect (VSD).
| Control | IQR | VSD | IQR | p - value | |
|---|---|---|---|---|---|
| TNFα (pg/mL) | 4.25 (10) | 3.12–5.62 | 2.87 (9) | 2.38–3.89 | 0.19 |
| IL - 2 (pg/mL) | 4.5 (10) | 3.07–4.68 | 1.76 (8) | 1.09–2.64 | 0.03 |
| IL - 6 (pg/mL) | 0.57 (10) | 0.49–0.96 | 0.83 (9) | 0.49–2.66 | 0.59 |
| IL - 8 (pg/mL) | 2.68 (10) | 1.78–5.24 | 4.98 (9) | 3.94–5.17 | 0.33 |
| IL - 10 (pg/mL) | 0.81 (6) | 0.00–1.44 | 0.00 (4) | 0.00–1.03 | 0.63 |
| INFγ (pg/mL) | 1.11 (9) | 0.33–1.42 | 0.33 (8) | 0.17–0.62 | 0.13 |
| GM - CSF (pg/mL) | 0.30 (5) | 0.00–1.00 | 0.00 (3) | 0.00–0.39 | 0.23 |
| MMP - 2 (ng/mL) | 364 (10) | 328–471 | 401 (9) | 293–536 | 0.74 |
| MMP - 3 (pg/mL) | 1346 (10) | 821–2079 | 1080 (9) | 720–1816 | 0.68 |
| MMP - 7 (pg/mL) | 2615 (10) | 2320–3151 | 2275 (9) | 1355–2514 | 0.29 |
| MMP - 8 (pg/mL) | 1033 (10) | 810–1941 | 1722 (9) | 1664–2112 | 0.41 |
| MMP - 9 (ng/mL) | 26 (10) | 21–48 | 39 (9) | 30–44 | 0.37 |
| MMP - 13 (pg/mL) | (1) | 85 (5) | 0–318 | 0.03* |
Values presented as Median (number of samples with detectable levels). IQR = inter-quartile range. P-values from nonparametric 2-sample Kruskal-Wallis test of medians except *, which is Pearson Chi-square.
TNFα: tumor necrosis factor alpha
IL: interleukins
INFγ: interferon gamma
GM – CSF: granulocyte-macrophage colony stimulating factor
MMP: matrix metalloproteinase
Baseline MMP plasma levels for the referent control and VSD groups are shown in Table 2. MMP-12 was not detectable in either group, and therefore was excluded from further analysis. Plasma MMP levels at baseline were similar between groups, with one notable exception where MMP-13 was detectable in 5/9 VSD patients but 1/10 controls (p=0.033, Pearson chi-square).
Post-operative Cytokine and MMP Measurements
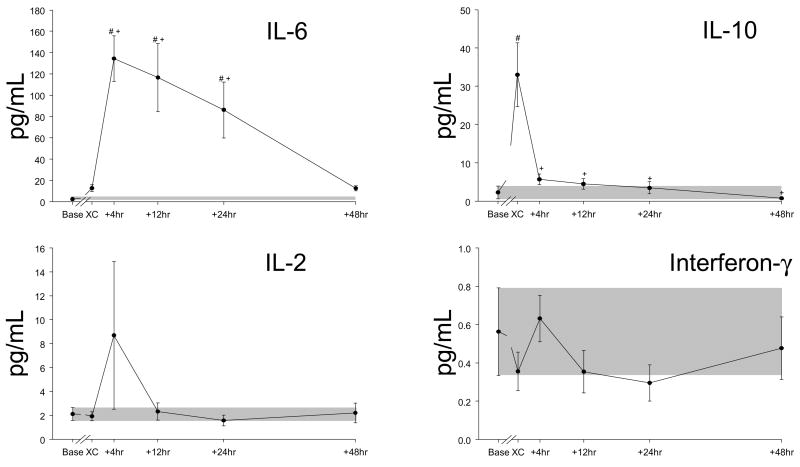
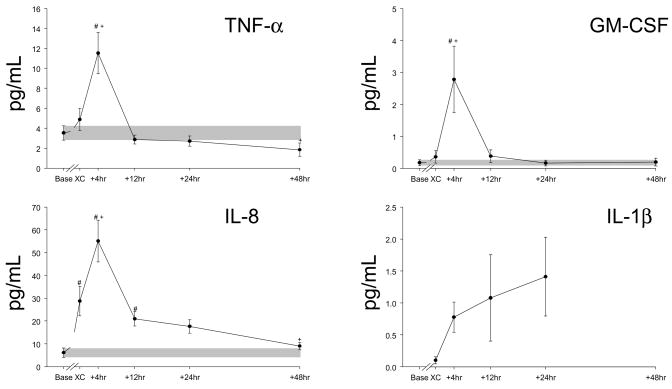
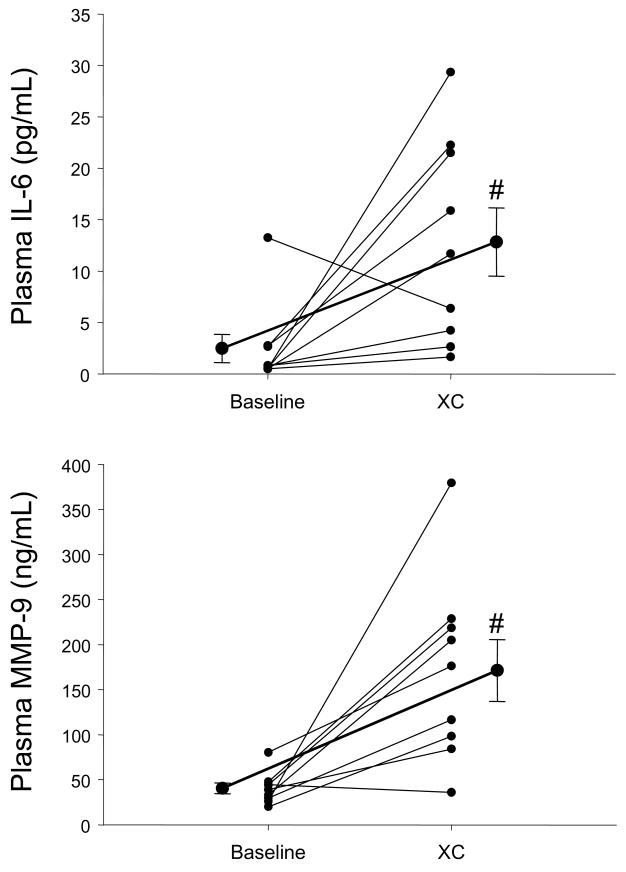
Using the multiplex array analysis, a full temporal profile for cytokines (Figures 1 and 2) and for MMPs (Figure 3) could be constructed. These temporal profiles were examined with respect to referent normal control ranges, baseline values, as well as in reference to the intra-operative event- cross-clamp release and completion of modified ultrafiltration. In general, with the exception of IFN and IL-2, cytokine values increased in the early peri-operative period. The cytokine IL-6 increased to the greatest relative degree, and IL-10 levels peaked the earliest. An individual response plot for IL-6 as a function of baseline values is shown in Figure 4. All other cytokines with significant elevations peaked at 4 hours post cross-clamp, with the exception of IL-1β, which was detected in only 3 of 9 subjects at the end of modified filtration, became more regularly detectable at 4,12, and 24 hours, and returned to undetectable in 7/8 samples at 48 hours. No cytokine remained significantly elevated over baseline levels at 48 hours.
Figure 1.
Time course for peri- and post-operative plasma levels (± SEM) of IL-6, IL-10, IL-2 and interferon gamma (INF-γ) in infants undergoing repair of a VSD. The Th1 cytokines (IL-2 and IFN-γ) never increase above baseline values. The shaded bars indicate the range of mean baseline values ± SEM. Baseline values were obtained pre-incision, “XC” samples were obtained following modified ultrafiltration, and the other time points are measured from cross clamp removal. Note the variation in the Y-axis scale (all in pg/ml). #, p<0.05 vs baseline values, +, p<0.05 vs post-MUF (“XC”) values.
Figure 2.
Time course for peri- and post-operative plasma levels of the cytokines TNFα, GM-CSF, IL-8 and IL-1β in infants undergoing repair of a VSD. TNFα, GM-CSF, IL-8 have similar time courses, peaking at 4 hours and declining rapidly. IL-1β increases from essentially undetectable levels to a peak at 24 hours before returning to generally undetectable levels at 48 hours. The shaded bars indicate the range of mean baseline values ± SEM. Baseline values were obtained pre-incision, “XC” samples were obtained following modified ultrafiltration, and the other time points are measured from cross clamp removal. Note the variation in the Y-axis scale (all in pg/ml). #, p<0.05 vs baseline values, +, p<0.05 vs post-MUF (“XC”) values.
Figure 3.
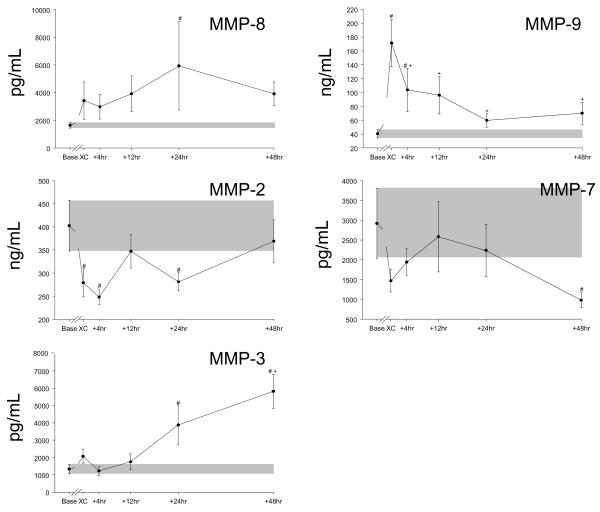
Time course for peri- and post-operative plasma levels of MMP-2, MMP-3, MMP-7, MMP-8, and MMP-9 in infants undergoing repair of a ventricular septal defect. MMP-2 and MMP-7 values drop below baseline, while MMP-8 and MMP-9 values increase with dissimilar time courses. MMP-3 increases steadily through the period from 4 to 48 hours post-operatively. The shaded bars indicate the range of mean baseline values ± SEM. Baseline values were obtained pre-incision, “XC” samples were obtained following modified ultrafiltration, and the other time points are measured from cross clamp removal. Note the variation in the Y-axis scale (all in pg/ml). #, p<0.05 vs baseline values, +, p<0.05 vs post-MUF (“XC”) values.
Figure 4.
TOP- Individual response plots for plasma IL-6 levels in the VSD patients at baseline and following cross-clamp removal (XC) and modified ultrafiltration. While the relative magnitude varied between patients, IL-6 levels increased at this early post-CPB time point. BOTTOM- Individual response plots for plasma MMP-9 levels in the VSD patients at baseline and at XC. Overall, a robust increase in plasma MMP-9 levels occurred at this early post-CPB time point. The darker solid lines indicate the mean baseline and XC values ± SEM. #, p<0.05 vs baseline values.
With respect to the full array of MMPs, a dynamic and diverse portfolio of changes occurred in the early peri-operative period. Plasma levels of MMP-8 and -9 increased in the early post-CPB period, whereas MMP-2 and MMP-7 levels actually fell from baseline values. Plasma MMP-3 levels increased in the later post-CPB time points and did not return to baseline values. All other MMPs changed significantly from baseline at some time point. A representative individual response plot for MMP-9 is presented in Figure 4. Plasma MMP-2 levels were significantly lower than baseline levels in the early post-operative period but returned to baseline levels at 48 hours.
Post-operative Inflammatory Markers and Clinical Outcomes
We examined the hypothesis that early or late cytokine/MMP AUCs were linked to specific operative events or post-operative outcomes such as cross-clamp time, inotrope score, net fluid balance, and blood product utilization. Early IL-10 AUC was negatively correlated with cumulative inotrope requirement at 48 hours (r: −0.85, p<0.005). Early GM-CSF was negatively correlated with cross-clamp time (r: −0.85, p<0.005). The late MMP-8 AUC was negatively correlated with cross-clamp time (r:−0.87, p<0.005), as was late MMP-9 AUC (r: −0.73, p<0.03). Late MMP-7 AUC was negatively correlated with cumulative fluid balance (r: −0.9, p<0.001). The early MMP-2 AUC as well as the late MMP-2 AUC, were negatively associated with packed red blood cell use (r=−0.88, r=−0.68, respectively, both p<0.05).
Discussion
While cardiopulmonary bypass (CPB) is essential for the conduct of a large number of cardiac surgical procedures, the post-CPB period is associated with a heightened inflammatory response as demonstrated by cellular and non-cellular indices of immune activation.4–8 This inflammatory response is characterized by the release of cytokines and through binding to cognate receptors, result in a number of cellular and extracellular events such as cell death an induction of proteolytic pathways. For example, cytokine activation is an upstream pathway for the induction of proteolytic enzymes such as the matrix metalloproteinases (MMPs).3,5 Increased MMP induction in and of itself can lead to significant changes in tissue structure and function, and have been demonstrated to play a contributory role in a number of acute and chronic cardiovascular disease states.3,9–13 Specifically, MMP induction can directly affect wound healing, alter vascular permeability and reactivity, and contribute to multi-organ dysfunction.9–13 Thus, cytokine activation and MMP induction would hold particular relevance in the context of surgical repair of congenital defects requiring CPB. Past studies have focused upon a small number of cytokines, at a limited number of time points following pediatric heart surgery requiring CPB.4,7,8 However, a full temporal cytokine and MMP plasma profile have not been simultaneously performed in this patient population previously and these values related to peri-operative outcomes. The unique results of the present study demonstrated in children following VSD repair, inflammatory cytokine induction occurs, which is temporally related to the emergence of a specific MMP profile. Moreover, this specific cytokine and MMP profile was associated with ischemic time (cross-clamp time), inotropic requirements, postoperative fluid balance, and blood product utilization. The present study provided the clinical proof of concept that profiling a large array of relevant bioactive molecules reveals a distinctive signature in infants undergoing congenital cardiac surgery which may hold relevance with respect to the underpinning pathophysiological events which occur in the early post-operative period as well as potential adjunctive use as biomarkers for prognosis and peri-operative management.
Temporal Profile of Cytokine Release Following VSD Repair
Past studies that have examined cytokine release, particularly that in congenital cardiac surgery, have been limited either by cytokine type or time points post-CPB.4,7,8,14 One likely rate limiting step in these past studies is that cytokines were performed using a high sample volume immunoassay approach, and therefore blood sample volumes would be problematic. In the present study, a validated high-sensitivity approach was utilized which provided the ability to measure multiple analytes using a single, small volume plasma sample.15 Through this approach, multiple cytokines could be measured simultaneously, which thereby reduces intrinsic assay variability and allows for sequential analysis. Moreover, these values were placed in context with referent normal values obtained from children without cardiovascular disease. The outcomes from this analysis demonstrated that a robust increase in certain cytokines occurred immediately following CPB, which included the interleukins (ILs); IL-2, IL-6, IL-8, IL-10, and IL-1β, tumor necrosis factor-α, (TNF), and granulocyte-macrophage colony stimulating factor (GM-CSF). While the peak levels of IL-6 and IL-10 which were observed in the present study are consistent with those reported previously following congenital cardiac surgery,4,8 the ability to perform a full cytokine array across time provided some unique insights. First, the pattern of cytokines released are likely reflective of the underlying biology of the immune response in these VSD patients. Specifically, a polarization of cytokine expression occurs early in development with subsets of lymphocytes such as T-helper 1 cells (Th1) express IL-2, TNF and INF, whereas T-helper 2 (Th2) cells do not.16 There were three relevant observations from the present study which would suggest that a Th2 polarization occurred in VSD patients. First, the Th1 cytokine IL-2 values were lower at baseline in VSD infants compared to referent normal values. Second, peri-operative values of the Th2 cytokines IL-10 and IL-6 were significantly increased. Third, the Th1 cytokines IFN-γ and IL-2, were reduced in the peri-operative period. This pattern of mild Th2 polarization has been observed previously in newborns.16 This specific cytokine profile remained elevated for a much greater period post-CPB when compared to adults undergoing cardiac surgery.17 These findings underscore the fundamental differences in the immune response in newborns undergoing cardiac surgery and that the subsequent inflammatory response may be much different than that which would be anticipated in older children or adults. Thus, profiling the unique cytokine signature which occurs in the peri-operative period following pediatric cardiac surgery may hold relevance when developing specific anti-inflammatory strategies for this particularly vulnerable patient population.1
Both IL-6 and IL-8 are potent pro-inflammatory cytokines which are chemoattractants for inflammatory cells,7,14 remained substantially elevated up to 48 hours post-CPB. While the present study was not designed nor powered to develop prediction models for cytokine profiles and post-operative outcomes, some relative associations could be made. First, heightened release of pro-inflammatory cytokines such as IL-6 and IL-8 following congenital cardiac surgery has been associated previously with pulmonary dysfunction, capillary leak syndrome, and oxidative stress resulting in changes in endothelial and vascular reactivity and function.7,14 Second, IL-10 which can be considered an anti-inflammatory molecule,18 increased early in the post-CPB period, but fell towards baseline values at later time points. Indeed, the present study demonstrated that the relative magnitude of the early release of IL-10 was associated with a decreased inotrope score, suggestive of the potentially protective effects of this specific cytokine. Third, the release of IL-1β into the circulation is due to cleavage by caspase-1,19 suggestive of a gradual increase and rapid shut-down of caspase-1 dependent inflammatory pathways in infants following VSD repair. Finally, robust burst of TNF release occurred in the VSD patients post-CPB and the myocardium has been shown to be a potent source of this pleotropic cytokine.20 In the present study, the relative magnitude of the TNF response in comparison to the IL-6 response following CPB is characteristic of neonatal monocytes.21 One unique bioactive molecule measured in the present study was GM-CSF, which increased transiently in the early peri-operative period was inversely correlated to cross-clamp time. GM-CSF plays a fundamental role in the maturation of the monocyte/macrophage lineage and transfusion of GM-CSF has been utilized for neonates with neutropenia and sepsis.22 However, mechanistic and clinical relationships of changes in GM-CSF in pediatric patients following cardiac surgery will require a much greater sample size and longer follow-up periods.
Temporal Profile of MMP Release Following VSD Repair
A common downstream biological event following cytokine release is the induction and activation of MMPs. Indeed, past studies in adults following cardiac surgery have clearly demonstrated a significant increase in certain MMP types following CPB.5,6 Specifically, past studies in adults have identified a robust increase of plasma MMP-8 and -9 following CPB.5,6 The present study is the first to examine and compare large portfolio of MMPs in referent normal pediatric subjects as well as in patients following congenital cardiac surgery. This approach yielded several important observations. First, detectable plasma MMP-13 levels occurred with greater frequency in VSD patients compared to referent normal subjects. MMP-13 is elevated in the myocardium of patients with LV failure,23 and the presence or absence of plasma MMP-13 may hold relevance as to the etiology of the cardiovascular pathophysiological process which underlies adult patients with LV failure. Second, not all MMP types were uniformly increased in the peri-operative period, and the temporal pattern of release appeared to be also different between MMP types. For example, relative MMP-2 and MMP-7 levels actually decreased following CPB. This would imply that there are different regulatory pathways for specific MMP types, which would be operative in children undergoing cardiac surgery. Whether the relative reduction in MMP-2 levels which were observed in the early peri-operative period in the present study were due to a relatively low capacity to upregulate this particular MMP with respect to transcriptional activity, relatively low stores of preformed pro-MMP-2, increased consumption and degradation of this MMP type prior to spill-over into the circulation, or a combination of these factors, remains to be established. Potential cardiac sources of MMP-7 include macrophages and cardiomyocytes.3,20 Thus, the relative reduction of this specific MMP type may be indicative of the relative immaturity of the immune system and myocardium. While the mechanisms which underlie the relative reduction in MMP-2 and MMP-7 remain to be established, the present study identified that MMP-2 levels were negatively correlated with packed red blood cell utilization and MMP-7 levels were negatively correlated with peri-operative fluid retention. These observations while associative, underscore the functional diversity of MMPs, whereby some MMP types may play a predominant pathological role whereas other MMP types may contribute to beneficial biological processes.
MMP types which are most associated with an inflammatory process, such as MMP-8 and MMP-9 increased dramatically in the early post-CPB time points and were temporally related to the release of pro-inflammatory cytokines such as IL-6, IL-8 and TNF. Thus, while only associative, this would suggest that the early and robust increase in these cytokines would facilitate the egress of neutrophils and subsequent release of MMP-8 and MMP-9. Indeed, the primary source of MMP-8 is neutrophils, and has been consequently termed neutrophil collagenase. The increased levels of these specific MMP types would likely have significant consequences on tissue structure and function in the early post-CPB period. For example, changes in MMP levels can directly affect the wound healing response, and changes in levels of MMP-8 can directly affect the time course of wound healing.2,11,24 Indeed, levels of MMP-3 and MMP-9, both of which changed in the present study, have been associated with wound healing following burn injury in children.13 Since the relative magnitude of MMP release would likely play a contributory factor in endothelial-matrix interaction and stability, then it is likely that this proteolytic system contributes to the loss of capillary integrity which can often occur following congenital cardiac surgery.2,11,24
Limitations and Conclusions
While the present study provided the proof of principle that a large portfolio of cytokines and MMPs can be serially examined in pediatric patients undergoing cardiac surgery, there are several limitations which must be recognized. First, this study was performed in a small number of patients and therefore robust relationships between a specific cytokine/MMP profile to critical outcomes such as major morbidity and mortality endpoints could not be established. Second, the study was performed in the context of a surgical repair of a relatively simple congenital malformation-VSD, and therefore extension of these findings to more complex congenital malformations with respect to cytokine/MMP profiles would be an appropriate future direction. Third, this study was performed in which the non-specific serine protease inhibitor aprotinin was administered, which in and of itself can potentially alter inflammatory pathways.17,25 Since aprotinin is no longer available for routine clinical use, then whether and to what degree the cytokine/MMP profile may be altered in the early post-operative period in the absence of this anti-fibrinolytic therapy remains to be established. Nevertheless, a unique cytokine profile emerged which appears to be Th2 polarized in infants with VSD, with a dominant IL-6 response, a transient IL-10 response and an absent IL-4 response. The only cytokine/MMP with an apparent relationship to cross-clamp time in this study was GM-CSF, which was suppressed by long CPB times. For the MMPs, the neutrophil-associated proteases MMP-8 and MMP-9 showed marked increases in early post-bypass levels and later sustained elevations that were inversely correlated with cross-clamp times. MMP-3 showed a smaller post-CPB spike in expression and a steady late increase that we attribute to wound healing activity. In contrast, circulating levels of MMP-2 and MMP-7 were not elevated above baseline at any time point post-CPB. Taken together, these findings suggest that the neutrophil-mediated MMP response following VSD repair in infants is robust (MMP-8 and MMP-9), but that early and late significant macrophage, endothelial and myocyte compartment responses are largely suppressed or absent (MMP-2, MMP-7, MMP-12). The mechanisms behind these unique patterns, the dissection of beneficial from deleterious effects, and the benefits of therapeutic manipulation of the inflammatory state engendered by CPB remain to be determined.
Acknowledgments
We are grateful to Nikole O’Quinn for her able assistance in the preparation of this manuscript.
Funding Sources
This work was supported in part by NIH grants R01-HL059165, HL-057952 (FGS), a VA Merit Award (FGS), and elements of the work were conducted in a facility constructed with support from NIH C06 RR015455.
Footnotes
Disclosures
Tim C. McQuinn: Grant recipient from NIH
Rachael L. Deardorff: Nothing to disclose
Rupak Mukherjee: Nothing to disclose
Anna Greta B. Taylor: Nothing to disclose
Eric M. Graham: Nothing to disclose
Andrew M. Atz: Nothing to disclose
Geoffrey A. Forbus: Nothing to disclose
Stacia M. DeSantis: Nothing to disclose
Jennifer B. Young: Nothing to disclose
Robert E. Stroud: Nothing to disclose
Fred A. Crawford: Nothing to disclose
Scott M. Bradley: Nothing to disclose
Scott T. Reeves: Nothing to disclose
Francis G. Spinale: Grant recipient from the NIH and a VA Merit Award
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Checchia PA, Bronicki RA, Costello JM, Nelson DP. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: an international survey of 36 centers. Pediatr Crit Care Med. 2005;6:441–444. doi: 10.1097/01.PCC.0000163678.20704.C5. [DOI] [PubMed] [Google Scholar]
- 2.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81:S2347–54. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 3.Spinale FG. Matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 4.Berdat PA, Eichenberger E, Ebell J, Pfammatter JP, Pavlovic M, Zobrist C, Gygax E, Nydegger U, Carrel T. Elimination of proinflammatory cytokines in pediatric cardiac surgery: analysis of ultrafiltration method and filter type. J Thorac Cardiovasc Surg. 2004 Jun;127(6):1688–96. doi: 10.1016/j.jtcvs.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Lin TC, Li CY, Tsai CS, Ku CH, Wu CT, Wong CS, et al. Neutrophil-mediated secretion and activation of matrix metalloproteinase-9 during cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2005 Jun;100(6):1554–60. doi: 10.1213/01.ANE.0000154307.92060.84. [DOI] [PubMed] [Google Scholar]
- 6.Joffs C, Gunasinghe HR, Multani MM, Dorman BH, Kratz JM, Crumbley AJ, 3rd, et al. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001 May;71(5):1518–23. doi: 10.1016/s0003-4975(01)02442-0. [DOI] [PubMed] [Google Scholar]
- 7.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006 Jun;81(6):S2347–54. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 8.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006 Jul-Aug;27(4):408–13. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 9.Lorente L, Martín MM, Solé-Violán J, Blanquer J, Páramo JA. Matrix metalloproteinases and their inhibitors as biomarkers of severity in sepsis. Crit Care. 2010 Jan 19;14(1):402. doi: 10.1186/cc8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cena JJ, Lalu MM, Cho WJ, Chow AK, Bagdan ML, Daniel EE, Castro MM, Schulz R. Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H45–51. doi: 10.1152/ajpheart.00273.2009. [DOI] [PubMed] [Google Scholar]
- 11.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009 Nov-Dec;17(6):832–9. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Pirilä E, Korpi JT, Korkiamäki T, Jahkola T, Gutierrez-Fernandez A, Lopez-Otin C, Saarialho-Kere U, Salo T, Sorsa T. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 2007 Jan-Feb;15(1):47–57. doi: 10.1111/j.1524-475X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 13.Dasu MR, Spies M, Barrow RE, Herndon DN. Matrix metalloproteinases and their tissue inhibitors in severely burned children. Wound Repair Regen. 2003 May-Jun;11(3):177–80. doi: 10.1046/j.1524-475x.2003.11305.x. [DOI] [PubMed] [Google Scholar]
- 14.Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand. 2001 Jul;45(6):671–9. doi: 10.1034/j.1399-6576.2001.045006671.x. [DOI] [PubMed] [Google Scholar]
- 15.Ford RL, Mains IM, Hilton EJ, Reeves ST, Stroud RE, Crawford FA, Jr, et al. Endothelin-A receptor inhibition after cardiopulmonary bypass: cytokines and receptor activation. Ann Thorac Surg. 2008 Nov;86(5):1576–83. doi: 10.1016/j.athoracsur.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 17.Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilon-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003 Nov;126(5):1498–503. doi: 10.1016/s0022-5223(03)00946-2. [DOI] [PubMed] [Google Scholar]
- 18.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 19.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 21.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNFα production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 22.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009 Aug 13;114(7):1289–98. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 24.Székely A, Cserép Z, Sápi E, Breuer T, Nagy CA, Vargha P, Hartyánszky I, Szatmári A, Treszl A. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009 Jan;87(1):187–97. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 25.Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001 Feb;71(2):745–54. doi: 10.1016/s0003-4975(00)02218-9. [DOI] [PubMed] [Google Scholar]