Abstract
Few predictive markers exist for response to adjuvant chemotherapy in breast cancer. The 78-kD glucose-regulated protein (GRP78) is a potent anti-apoptotic factor, conferring drug resistance. Recently, we reported that high GRP78 expression in breast cancer specimens predict a shorter recurrence-free survival in patients who received doxorubicin-based adjuvant chemotherapy. Interestingly, the opposite effect was observed in 25 patients who additionally received a taxane. To confirm this potentially paradigm shifting finding, we investigated whether GRP78 is associated with recurrence-free survival in an independent cohort of taxane-treated breast cancer patients. Immunohistochemical staining of GRP78 was performed on archival paraffin-embedded formalin-fixed tumor specimens obtained from 48 female breast cancer patients before chemotherapy treatment. These patients received doxorubicin and cyclophosphamide, followed by paclitaxel or docetaxel on a clinical trial. GRP78 expression level was evaluated by a pathologist, masked to all clinical and outcome data. Association between GRP78 expression and recurrence-free survival was evaluated. GRP78 positivity predicts a better recurrence-free survival, independent of other prognostic factors [hazard ratio (HR) for moderate positivity: 0.40 (95% confidence interval (CI): 0.087–1.83); HR for strong positivity: 0.16 (95% CI: 0.018–1.50); Ptrend=0.053]. In a pooled analysis with the previous 25 patients, almost identical HRs were obtained with Ptrend=0.024. This provides further evidence that GRP78 is a potential independent predictor for response to taxane-based adjuvant chemotherapy in breast cancer.
Introduction
Approximately 182,000 women are diagnosed with breast cancer each year in the US.1 Over 90% are found at an early stage without distant metastasis.2 Adjuvant chemotherapy has been an important treatment strategy3, and addition of a taxane to anthracyclin-containing regimens has significantly improved patient survival.4, 5 Currently, prognostic factors including patient age, tumor stage, and estrogen receptor status are used to decide the need of adjuvant chemotherapy.6 However, there are few predictive markers of benefit from taxane-containing adjuvant chemotherapy. One candidate marker is HER2 in which positivity was shown to predict benefit from addition of paclitaxel to doxorubicin treatment7 although these results may not be applicable given the current targeted treatment for HER2-positive breast cancer (ie, trastuzumab).
The 78-kD glucose-regulated protein (GRP78), also known as BiP, is a multi-functional Ca2+ binding protein primarily residing in the endoplasmic reticulum controlling endoplasmic reticulum homeostasis, stress signaling and protein quality control.8 In vitro studies established that GRP78 possesses potent anti-apoptotic properties and confers drug resistance to tumor cells as well as tumor associated endothelial cells.9–12 GRP78 suppresses doxorubicin-mediated apoptosis in part through inhibition of BAX and caspase-7 activation.13, 14 A retrospective analysis of 127 breast cancer patients who received adjuvant doxorubicin-based chemotherapy directly links GRP78 positivity to a shorter recurrence-free survival independent of other prognostic factors.15 Interestingly, an unplanned subset analysis revealed that this association was reversed among patients who also received adjuvant taxane (n=25) such that GRP78 positivity appeared to be associated with a better outcome (HR=0.15; P=0.072; p for interaction=0.012). As taxanes are now widely prescribed as adjuvant chemotherapy,16 investigation of the predictive value of GRP78 in sequential doxorubicin and taxane regimens is of great importance.
Here we report the results of a follow-up retrospective study, designed to examine the predictive value of one specific marker, GRP78, in response to adjuvant doxorubicin followed by taxanes.
Material and methods
Participants
This study includes female patients with breast cancer who had archived prior to treatment formalin-fixed paraffin-embedded tumor specimen in LAC+USC Medical Center and who received doxorubicin, cyclophosphamide, followed by either paclitaxel or docetaxel between 2000 and 2002 on a clinical trial. Among the 60 patients enrolled in the trial, 48 had invasive tumor samples that were suitable for GRP78 analysis; 46 specimens were primary breast tumor specimens; 2 specimens were from lymph node metastasis (their primary tumors were not available). This study was approved by USC institutional review board (IRB). A waiver of informed consent was justified and granted by the IRB consistent with the waiver criteria of the common rule.
Immunohistochemical staining of GRP78
Paraffin embedded formalin fixed tissues were immunohistochemically stained for GRP78 using anti-GRP78 antibody (H129, Santa Cruz Biotechnology, Santa Cruz, CA) as previously described.15, 17 Briefly, five micron sections prepared from formalin-fixed, paraffin embedded tissues were mounted on poly L-lysine-coated slides. The slides were deparaffinized in xylene, washed in 100% ethanol and rehydrated in 95% ethanol. After pre-incubation in 3% hydrogen peroxide in absolute methanol, antigen retrieval was performed using citrate buffer (pH 6). After protein blocking with normal horse serum, incubation with 1:100 dilution of the primary antibody, anti-GRP78 H129 antibody (Santa Cruz Biotech, Santa Cruz, CA), in phosphate-buffered saline was performed. The GRP78 (H129) antibody is a rabbit polyclonal antibody raised against amino acids 525 to 653 of human GRP78, and only recognizes a single protein band GRP78 in human cell lysates. Biotinylated horse anti-rabbit antibody was used as a secondary antibody at a 1:200 dilution. The slides were then incubated with avidin-biotin-conjugate (ABC, Vector Laboratories, Inc., Burlingame, CA), which was followed by incubation with 0.03% diaminobenzidine. Counterstaining was performed with hematoxylin.
Evaluation of GRP78 staining
It is known that plasma cells express high levels of GRP78, which facilitates immunoglobulin chain assembly.18 Plasma cells were present on all GRP78-stained slides and, as expected, showed intense immunoreactivity with the anti-GRP78 antibody. Therefore, plasma cells were used as internal positive controls. We also included additional controls across immunohistochemistry staining batches: two breast cancer samples (one positive; and one negative for GRP78) from the previous study.15 These additional controls were used to decide whether to accept an entire batch. One pathologist (P.N.) who was masked to all clinical data, reviewed all the immunohistochemically stained slides, and evaluated: (1) intensity of staining (1 = weak; 2 = moderate; 3 = strong) and (2) percentage of cells stained (1 = 0–<10%; 2 = 10–<50%; 3 = 50–100%) as previously described.18 The overall index of GRP78 expression was determined based on the previous two variables: positive when both scores were 2 or above; negative otherwise. The positive group was further divided into moderate positive (when both scores were 2) or strong positive (when either score was 3). The evaluation of the GRP78 level by the same pathologist (PN) in a previous study indicated substantial agreement, with the kappa coefficient for reader reproducibility of 0.73 [95% confidence interval (95% CI): (0.50, 0.98)].15 Therefore, no additional evaluation of the reader reproducibility was performed in the current study.
Statistical analyses
Time to recurrence (TTR), calculated as start of chemotherapy until the date of documented recurrence, was used as the outcome, censoring patients who had not experienced a recurrence at the date of last follow-up (death or last contact) up to February 28, 2008. The association between TTR and GRP78 immunostaining was evaluated using Kaplan-Meier plots and the Cox Proportional hazards model.19 All p-values reported are two-sided and are based on the likelihood ratio test associated with the Cox model. Multivariable analyses included covariates selected a priori, based on associations observed in the initial study: tumor size, lymph node status, and grade.15
Results
The mean age of the participants was 49.5 (SD 9.2). The median follow-up time was 5.5 years. Seventy-one percent were of Hispanic origin. Distribution of clinical and prognostic factors of the patient population is listed in Table 1. Many variables in Table 1, such as age, tumor size, lymph node status, and tumor grade, histology, ER/PR, HER2 status, are known prognostic factors, although these associations did not reach statistical significance in this study due to the limited sample size. Twenty-nine of 48 (60.4%) patients showed GRP78 positivity. This was based on immunohistochemical staining of paraffin embedded formalin fixed tissue, obtained prior to the start of chemotherapy. Examples of negative and positive staining are shown in Figure 1. As expected for a protein residing in endoplasmic reticulum, GRP78 staining was primarily in the perinuclear/cytoplasmic region. Plasma cells, which expressed high level of GRP78, were present on all slides. GRP78 was intensely stained in these cells, which served as internal positive control for staining evaluation. GRP78 positivity was not associated with other clinical or prognostic factors, such as age, ethnicity (Hispanic origin), tumor size, lymph node positivity, grade, and ER/PR or Her2/neu status (Table 1).
Table 1.
Association between GRP78 immunostaining and clinical/prognostic factors
| Total | GRP78 Positive |
||||
|---|---|---|---|---|---|
| Number | Percent | P-value1 | |||
| Age | <40 | 5 | 3 | 60% | >0.99 |
| 40–<50 | 22 | 13 | 59% | ||
| 50–<60 | 13 | 8 | 62% | ||
| ≥60 | 8 | 5 | 62% | ||
| Menopause | Pre | 30 | 17 | 57% | 0.55 |
| Post | 18 | 12 | 67% | ||
| Ethnic | Hispanic | 34 | 22 | 65% | 0.52 |
| Others | 14 | 7 | 50% | ||
| Size | ≤2cm | 9 | 7 | 78% | >0.99 |
| >2cm–≤5cm | 30 | 15 | 50% | ||
| >5cm | 9 | 7 | 78% | ||
| N of Positive Lymph Nodes | 0 | 12 | 7 | 58% | 0.78 |
| 1–3 | 16 | 10 | 62% | ||
| 4–9 | 12 | 6 | 50% | ||
| ≥10 | 8 | 6 | 75% | ||
| Grade | 1 | 1 | 1 | 100% | 0.58 |
| 2 | 16 | 10 | 62% | ||
| 3 | 30 | 17 | 57% | ||
| Histology | Infiltrating ductal | 44 | 25 | 57% | 0.14 |
| Others | 4 | 4 | 100% | ||
| ER/PR | −/− | 12 | 5 | 42% | 0.17 |
| (−/+) or (+/−) or (+/+) | 34 | 23 | 68% | ||
| Her2/neu | Negative | 25 | 15 | 60% | >0.99 |
| Positive | 21 | 13 | 62% | ||
| Radiation Therapy (RT)/Surgery | Lumpectomy | 1 | 0 | 0% | 0.73 |
| Mastectomy | 18 | 11 | 61% | ||
| Lumpectomy/RT | 12 | 8 | 67% | ||
| Mastectomy/RT | 17 | 10 | 59% | ||
| Treatment | Doxorubicin/Paclitaxel | 24 | 14 | 58% | >0.99 |
| Doxorubicin/Docetaxel | 24 | 15 | 62% | ||
| Tamoxifen or Aromatase | No | 10 | 6 | 60% | 0.71 |
| Yes | 32 | 22 | 69% | ||
P-values are based on Mantel-Haenszel chi-square test (for ordinal or continuous variables) or Fisher’s exact test (for categorical variables).
Figure 1.
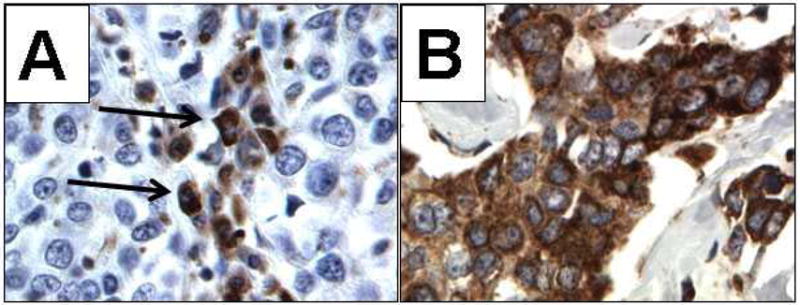
Photomicrographs of immunohistochemical staining of GRP78 (400X). A, Negative staining for GRP78 in neoplastic cells of an infiltrating ductal carcinoma; plasma cells stain intensely (arrows). B, Intense staining (3+) for GRP78 in neoplastic cells of an infiltrating ductal carcinoma.
Consistent with our findings from a subgroup analysis in the initial study,15 here we observed that GRP78 positivity was associated with longer TTR, a surrogate marker for response to treatment regimen (Figure 2 and Table 2). This association did not change after adjusting for prognostic factors selected a priori including tumor size, number of positive lymph nodes, and grade (multivariable hazard ratio (HR): 0.29; 95% confidence interval (CI): 0.072–1.17; p=0.077; Table 2). While the association has not reached statistical significance in this study, our current results directly confirm the previous study; patients treated with doxorubicin followed by taxane whose tumor stained positively for GRP78 have a better outcome than patients treated similarly but with negative staining (HR=0.24; 95% CI: 0.020–3.00; P=0.24).15 When GRP78 positivity was further categorized into moderate positivity and strong positivity, the HR for moderate positivity of GRP78 was 0.40 (95% CI: 0.087–1.83), and the HR for strong positivity of GRP78 was 0.16 (95% CI: 0.018–1.50; Ptrend=0.053).
Figure 2.
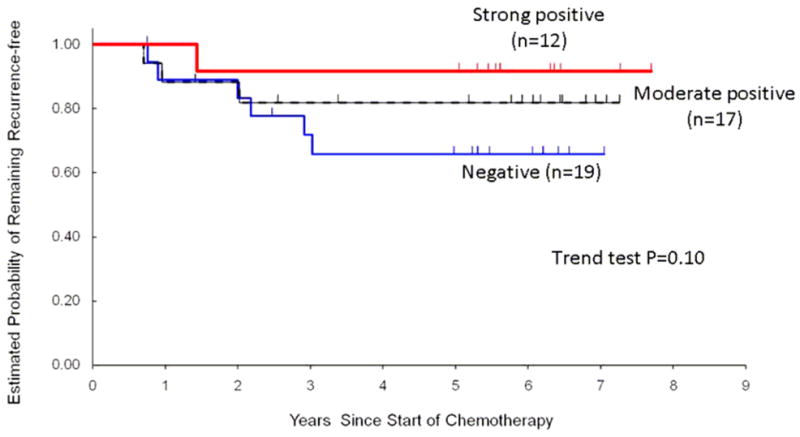
Probability of remaining recurrence-free according to GRP78 immunostaining in 48 patients who received adjuvant doxorubicin and cyclophosphamide, followed by either paclitaxel or docetaxel.
Table 2.
Association between GRP78 expression and time to recurrence in taxane-treated patients in the current study and in a previous study
| Univariate Analysis |
Multivariable Analysis1 |
|||||
|---|---|---|---|---|---|---|
| GRP78 | n | Events2 | HR (95% CI)3 | P value4 | HR (95% CI) 3 | P value4 |
| Current data | ||||||
| Negative | 19 | 6 | 1 | 1 | ||
| Positive | 29 | 4 | 0.40 (0.11–1.42) | 0.15 | 0.29 (0.072–1.17) | 0.077 |
| Moderate positive | 17 | 3 | 0.54 (0.14–2.18) | 0.40 (0.087–1.83) | ||
| Strong positive | 12 | 1 | 0.22 (0.027–1.87) | 0.16 (0.018–1.50) | ||
| Trend P | 0.10 | 0.053 | ||||
| Previous data5 | ||||||
| Negative | 8 | 3 | 1 | 1 | ||
| Positive | 17 | 1 | 0.15 (0.016–1.46) | 0.072 | 0.24 (0.020–3.00) | 0.24 |
| Moderate positive | 8 | 1 | 0.33 (0.034–3.19) | 0.47 (0.028–7.86) | ||
| Strong positive | 9 | 0 | 0 | 0 | ||
| Trend P | 0.038 | 0.15 | ||||
Model for current data was adjusted for tumor size (≤2cm, >2–≤5cm, >5cm, and unknown), number of positive lymph nodes (0, 1–3, 4–9, and 10 or more), and grade (well or moderately differentiated, poorly differentiated, and unknown). The model for previous data was stratified by propensity score (based on T stage, lymph node status, and grade) divided into quintiles as described in original report.15
Number of recurrences.
Hazard ratio (95% confidence interval).
P–values from likelihood ratio test based on Cox model.
Data from Lee et al.15 on a subgroup of women who received doxorubicin and taxane.
When we pooled the current data with the previous series of 25 patients who received adjuvant doxorubicin-based chemotherapy combined with or followed by taxanes, the multivariable HR for moderate positivity of GRP78 was 0.42 (95% CI: 0.12–1.50), and the HR for strong positivity of GRP78 was 0.13 (95% CI: 0.015–1.16; P for trend=0.024; Table 3).
Table 3.
Pooled analyses of association between GRP78 expression and time to recurrence in taxane-treated patients
| Univariate Analysis1 |
Multivariable Analysis2 |
|||||
|---|---|---|---|---|---|---|
| Variable | n | Events3 | HR (95% CI)4 | P value5 | HR (95% CI)4 | P value5 |
| GRP78 | ||||||
| Negative | 27 | 9 | 1 | 1 | ||
| Positive | 46 | 5 | 0.31 (0.10–0.92) | 0.030 | 0.30 (0.090–1.02) | 0.050 |
| Moderate positive | 25 | 4 | 0.47 (0.15–1.53) | 0.42 (0.12–1.50) | ||
| Strong positive | 21 | 1 | 0.13 (0.016–1.02) | 0.13 (0.015–1.16) | ||
| Trend P | 0.013 | 0.024 | ||||
| Other covariates in the multivariable model6 | ||||||
| Grade | ||||||
| 1 or 2 | 27 | 6 | NA | 1 | ||
| 3 | 39 | 8 | 1.03 (0.30–3.49) | |||
| Unknown | 7 | 0 | - | |||
| T stage | ||||||
| 1 | 17 | 3 | NA | 1 | ||
| 2 | 43 | 9 | 0.58 (0.12–2.64) | |||
| 3 | 11 | 1 | 0.62 (0.054–7.14) | |||
| Unknown | 2 | 1 | - | |||
| Number of positive lymph nodes | ||||||
| 0 | 14 | 2 | NA | 1 | ||
| 1–3 | 32 | 5 | 0.96 (0.15–6.29) | |||
| 4–9 | 17 | 2 | 0.89 (0.12–6.76) | |||
| 10 or more | 10 | 5 | 5.32 (0.88–32.3) | |||
Models were stratified by data source.
Multivariable analysis were stratified by data source and further adjusted for tumor size (≤2cm, >2–≤5cm, >5cm, and unknown), number of positive lymph nodes (0, 1–3, 4–9, and 10 or more), and grade (well or moderately differentiated, poorly differentiated, and unknown).
Number of recurrences.
Hazard ratio (95% confidence interval).
P-values from likelihood ratio test based on Cox model.
Estimates for other covariates come from the multivariable model for GRP78 (negative, moderate positive, and strong positive).
Discussion
The discovery of predictor markers for adjuvant breast cancer chemotherapy remains critical towards improving the efficacy of breast cancer treatment. This study reveals a potentially paradigm shifting finding that GRP78, a well established drug resistance effector in human cancer,8–11, 20 may serve as a novel positive predictor for breast cancer sensitivity to doxorubicin/taxane-based adjuvant chemotherapy. By using an independent cohort of patients of primarily Hispanic origin, this study directly replicates the results of the initial novel observation first reported in a pilot study in 2006 among primarily non-Hispanic white population.15 Pooled analysis of the two studies yielded nearly identical hazard ratios with a statistically significant Ptrend (0.024), which is strongly supportive of the validity of our discovery that GRP78 could be a true positive predictor for response to doxorubicin/taxane-based adjuvant chemotherapy. This study was specifically designed to evaluate one potential predictive marker, and therefore, avoids complication arising from studies involving a large number of candidate markers where chance associations can potentially pose a problem. Furthermore, we directly proved here that GRP78 expression level was independent of other established clinical or prognostic factors. Since GRP78 is readily detectable by immunohistochemical staining in biopsy samples and highly specific antibodies to GRP78 are already commercially available, GRP78 could be developed expeditiously as a novel biomarker for prediction of response to adjuvant chemotherapy with doxorubicin followed by taxane in the clinic.
The new results reported here, coupled with our earlier finding that GRP78 positivity is associated with shorter recurrence-free survival following doxorubicin-based treatment alone,15 implies that GRP78 status may serve as an independent predictor for responsiveness for specific adjuvant chemotherapy drugs for breast cancer patients. Consistent with the results reported here, in our in vitro studies of human breast cancer MCF-7 cells we have observed the same trends, such that cells with enforced overexpression of GRP78 showed higher resistance to doxorubicin treatment alone, but greater sensitivity to sequential doxorubicin and taxane treatment (our unpublished results). While the mechanism whereby GRP78 modulates the sensitivity of cancer cells to adjuvant chemotherapy awaits further investigations, our results warrant confirmation in larger cohort studies as the need for improving adjuvant therapy for breast cancer patients remains critical.
Acknowledgments
This study was supported by Whittier Foundation and National Institutes of Health grants CA026706 to ASL and CA014089 to SG (co-investigator on grant).
References
- 1.American Cancer Society. Breast Cancer Facts & Figures. Atlanta, GA: American Cancer Society, Inc; 2007–2008. [Google Scholar]
- 2.Ries LA, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, et al. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: 2008. from http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Bria E, Nistico C, Cuppone F, Carlini P, Ciccarese M, Milella M, Natoli G, Terzoli E, Cognetti F, Giannarelli D. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer. 2006;106:2337–44. doi: 10.1002/cncr.21886. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2007:CD004421. doi: 10.1002/14651858.CD004421.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Marchionni L, Wilson RF, Wolff AC, Marinopoulos S, Parmigiani G, Bass EB, Goodman SN. Systematic review: gene expression profiling assays in early-stage breast cancer. Ann Intern Med. 2008;148:358–69. doi: 10.7326/0003-4819-148-5-200803040-00208. [DOI] [PubMed] [Google Scholar]
- 7.Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, Broadwater G, Goldstein LJ, Martino S, Ingle JN, Henderson IC, Norton L, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 8.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 10.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–11. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–16. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 12.Virrey JJ, Dong D, Stiles C, Patterson JB, Pen L, Ni M, Schönthal AH, Chen TC, Hofman FM, Lee AS. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–75. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–24. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen-starvation induced apoptosis. Cancer Res. 2007;67:3734–40. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 15.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 16.Vahdat L. Choosing a taxane for adjuvant treatment of breast cancer: more than a flip of the coin? Nat Clin Pract Oncol. 2008;5:570–1. doi: 10.1038/ncponc1215. [DOI] [PubMed] [Google Scholar]
- 17.Shi SR, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry: past, present, and future. J Histochem Cytochem. 1997;45:327–43. doi: 10.1177/002215549704500301. [DOI] [PubMed] [Google Scholar]
- 18.Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 19.Kalbfleish J, Prentice R. The statistical analysis of failure time data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 20.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, Bode AM, Dong Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]


