Abstract
Understanding deep muscle pain is of increasing importance for evaluating clinical pathologic pain states. Previously, a central role of deep muscle tissue in the development of ongoing pain behavior after incision was demonstrated. The underlying mechanisms, however, remain unclear. Using a new in vitro plantar flexor digitorum brevis (FDB) muscle-nerve preparation, we investigated properties of mechanosensitive group III and IV afferents innervating incised and unincised muscle, and explored potential mediators of afferent excitation after incision. Afferents of uninjured muscle had a low incidence (14.3%) of ongoing activity. A high proportion (65.8%) of afferents responded to heat and a minority, 20.8%, were activated by pH 6.0 lactic acid. Incision increased the prevalence of afferents with ongoing to 54.7%. A greater proportion of group III and IV afferents responded to pH 6.0 lactic acid after incision compared to control (55.4% vs. 20.8%). Sensitization of afferents to heat and mechanical stimulation was prominent in group IV afferents after incision; both heat (38.0 °C vs. 40.5 °C in control) and mechanical response threshold (median: 5.0 mN vs. 22.0 mN in control) were decreased. The finding that incision increased ongoing activity of muscle afferents is consistent with our previous in vivo studies and supports the idea that deep muscle tissue has a prominent role in the genesis of ongoing pain after incision. The enhanced chemosensitivity of muscle afferents to lactic acid after incision suggests an increased response to an ischemic mediator may contribute to pain and hyperalgesia caused by surgery in deep tissues.
Keywords: incision, peripheral sensitization, chemosensitive, hyperalgesia
Introduction
Much of our knowledge of pain mechanisms and hyperalgesia has relied on tests for cutaneous nociception and examination of post-injury models using cutaneous stimuli. Injuries to other tissues including muscle, bladder, and bone are often associated with pathologic pain states. Accumulating evidence suggests that different tissues and their sensory afferents respond uniquely to certain stimuli. For example, tourniquet-induced ischemic pain is described in terms like aching and cramping; these are deep tissue descriptors rather than terms that describe cutaneous symptoms [6; 26]. Furthermore, specific tissues and their sensory afferents respond distinctly to injury or disease. For instance, pain from tumor metastases to bone is severe; yet, soft tissue metastases can be less painful [32; 29].
Sensory afferents innervating muscle have unique characteristics compared to those innervating other structures. For example, compared with neurons innervating skin, a greater proportion of rat small dorsal root ganglion (DRG) neurons innervating muscle (51% vs. 28%) expressed acid-sensing ion channel 3 (ASIC3) [28]. DRG neurons innervating muscle were also more likely to be capsaicin-responsive (42% vs. 25%) than those innervating skin [13]. Clinically, muscle afferents contribute to cardiovascular responses during exercise triggering fatigue [16] and are thought to be activated by metabolites from muscle to signal pain in patients with claudication [6]. Certainly, muscle makes a significant contribution to chronic disease states like myofascial pain and fibromyalgia [36].
Deep muscle also has a prominent role in post-traumatic pain. Clinical studies indicate that the extent of muscle rather than skin injury influences the magnitude of pain at rest after surgery [30; 8]. Contraction of injured muscle during cough or active mobilization evokes much greater pain than pain at rest in postoperative patients [27; 22].
The specific contribution of different tissues to nociceptive signaling after injury is important for our understanding of postoperative and posttraumatic pain mechanisms. Recent studies indicate that unprovoked, ongoing pain behaviors occur after injury that include deep muscle [42; 43]. Correspondingly, compared with skin incision, incision of skin plus deep muscle caused a greater prevalence of ongoing activity in nociceptive transmitting dorsal horn neurons and primary afferent fibers after injury. These studies indicate that the strongest form of sensitization after injury, unprovoked ongoing activity, originates largely from injured deep muscle, whereas cutaneous injury is sufficient to signal hyperalgesia. The characteristics of the afferent fibers that are activated by injury and transmit ongoing pain have not been examined.
In the present study, we hypothesized that muscle incision would increase chemosensitivity in sensory afferent fibers. We tested this hypothesis by characterizing muscle afferents under normal conditions and comparing them to those from incised muscle using a novel in vitro muscle-nerve preparation. Chemosensitivity was tested using lactic acid; ongoing activity and afferent responses to mechanical stimuli and heat were also studied under both injured and uninjured conditions.
Methods
General
All experiments were reviewed and approved by The University of Iowa Animal Care and Use Committee. Rats were treated in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals as issued by the International Association for the Study of Pain [44]. A total of eighty-five adult male Sprague-Dawley rats (250–300 g, Harlan, Indianapolis, IN) were used in the present study. Rats were housed on a 12-h light-dark schedule; food and water were available ad libitum.
Plantar incision
A plantar incision through the skin, underlying fascia and the flexor digitorum brevis (FDB) muscle was made as described in our previous papers [42; 43]. Briefly, rats were anesthetized with 1.5–2% isoflurane delivered through a nose cone. The right hindpaw of rat was sterilized with 10% povidone-iodine. Beginning 0.5 cm from the proximal edge of the heel, a 1-cm longitudinal incision was made through the skin, underlying fascia and the FDB muscle with a #11 surgical blade (Feather Co., Osaka, Japan). Blunt curved forceps were then inserted through the incision into FDB to further divide and retract the muscle. The muscle origin and insertion remained intact. This method was similar as described previously [4] except that the FDB muscle was not elevated at its dorsal surface. The wound was then closed with three subcutaneous mattress sutures with 6-0 nylon on a P-1 needle (Ethicon, Somerville, NJ) and covered with antibiotic ointment.
A sham incision, which only included anesthesia with isoflurane, sterile preparation of the hindpaw, and topical antibiotics, but no incision, served as the control.
Electrophysiological studies
Preparation
One day after plantar incision or sham incision, rats were anesthetized with isoflurane. For this muscle-nerve preparation, the tibial nerve from the medial malleolus to the mid-thigh was dissected free from the surrounding tissue under anesthesia. Then, the rat was euthanized with CO2 and the FDB muscle was isolated from the skin and other connective tissue. It was dissected at its tendons proximally at the heel and distally at the metatarsal phalangeal joints. The FDB muscle is innervated by the medial plantar nerve, a branch of the tibial nerve. The medial plantar nerve was located as it entered the FDB muscle and the muscle and nerve were carefully excised together. The isolated muscle together with proximal and distal tendons was approximately 25 mm in length (the muscle itself was approximately 15 mm long), 2 to 5 mm in width, and about 1 to 2 mm thick. The length of the tibial nerve after dissection was 15 to 25 mm.
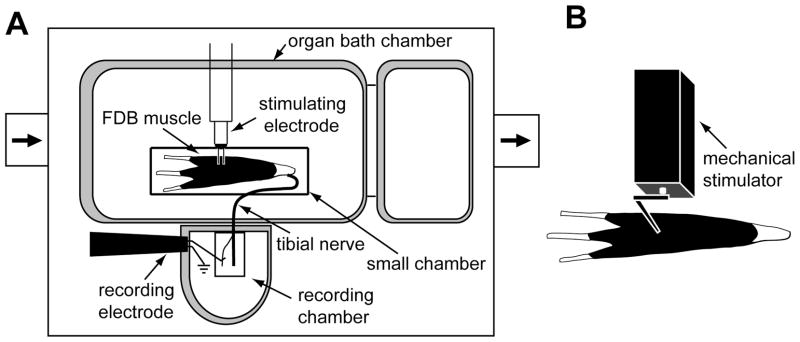
The isolated muscle-nerve preparation was placed in an organ bath (Medical Instrument, University of Iowa, Iowa City, IA; Fig. 1A), which was designed based on the one used for skin-nerve recordings [33; 1]. The FDB muscle was positioned with its plantar side down and pinned at the proximal tendon and three distal tendons approximating its rest length in vivo. It was superfused with modified Krebs-Henseleit solution (in mM: 110.9 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2SO4, 24.4 NaHCO3, and 20.0 glucose, pH 7.4), which was saturated by bubbling with 95% O2 and 5% CO2. The temperature of the bath solution was maintained at 36 ± 1.0 °C. The tibial nerve was threaded through a small hole into the recording chamber filled with mineral oil. On a mirrored stage immersed in the mineral oil, the nerve was desheathed and fine filaments of the nerve were repeatedly teased and placed on a platinum electrode until single-unit activity could be discriminated and recorded. Afferent activity was amplified (DAM50, Harvard Apparatus, Holliston, MA), filtered, and displayed on a digital oscilloscope. All data were recorded and stored into a PC computer with a data acquisition system (1401 Plus Laboratory Interface and spike 2 program, Cambridge Electronic Design, Cambridge, UK). The FDB muscle-nerve preparation was usually viable for approximately 5 h.
Figure 1.
Schematic of the flexor digitorum brevis (FDB) muscle-nerve preparation and the afferent fiber recording apparatus. (A) The isolated FDB muscle and tibial nerve were placed in the organ bath chamber, which was continuously perfused with oxygenated Krebs solution. The distal part of tibial nerve was threaded through a small hole into the recording chamber which was filled with mineral oil. Following repeated dissection, single fibers or thin bundles of the tibial nerve were attached to the recording electrode and afferent activities were recorded. A bipolar stimulating electrode was positioned on the receptive field to evoke afferent action potentials to measure conduction velocity. (B) Computer-controlled mechanical stimulation was applied to the receptive field on the dorsal side of the muscle.
Recording protocol
Mechanosensitive afferents were indentified by probing the dorsal aspect of the FDB muscle with a blunt glass rod. Afferents with a receptive field on the proximal or distal tendons were excluded. After a mechanosensitive unit was found, the muscle was gently stretched at each distal tendon for approximately 3 to 5 mm with blunt forceps. Stretch receptive afferents were excluded based on their responses to muscle stretch and conduction velocity in the group I and II afferent range (greater than 30 m/sec). Units accepted for further study also had a clearly distinguished signal to noise ratio (greater than 2:1). No more than two afferents were recorded in the same preparation.
Ongoing activity
Once an afferent that fulfilled the search criteria was identified, ongoing activity of the unit was first recorded for 10 min. The activity during the latter 5-min period was averaged and analyzed. An afferent fiber with ongoing activity was defined as one with a mean ongoing discharge rate of at least 0.1 imp/sec (a minimum of 30 impulses during the 5-min period). Ongoing activity in this preparation may be related to a variety of factors; these include not only the plantar incision, but also the dissection and preparation of the muscle, and prolonged after discharge following the mechanical search stimulus.
Mechanical responses
Following evaluation for ongoing activity, mechanosensitivity of the afferent was evaluated with a servo-controlled mechanical stimulator (Series 300B dual mode servo system, Aurora Scientific, Canada). A flat-ended cylindrical metal probe (tip diameter, 0.7 mm) attached to the stimulator arm was placed at the most sensitive spot of the receptive field as illustrated in Fig. 1B. First, a series of three ramp-shaped force stimuli were applied until the mechanical response threshold of the afferent was obtained. Each force ramp started from 0 and proceeded to 40, 80, and 120 mN, respectively, in 5 sec, with a 60-sec interstimulus interval. The mechanical response threshold of each afferent fiber was defined as the lowest force that caused either activation of the afferent if no ongoing activity was present or an increase in afferent discharge rate by at least 2 standard deviations above mean ongoing activity for 10 sec (1-sec bins) before mechanical stimulation. After the mechanical threshold was measured, an ascending series of sustained force stimuli (5, 10, 20, 40, 80, 120 mN) were applied to determine suprathreshold mechanosensitivity. Each stimulus had a rise time of 0.1 sec, sustained force plateau for 1.9 sec and inter-stimulus interval of 60 sec. To obtain a stimulus-response function, the total discharges during the 1.9-sec sustained force stimulus were counted and plotted versus force. The ongoing activity was included in the total discharge measurement. In a few fibers (n=5), threshold was determined, but the stimulus response function was not completed. Complete data on mechanical responses were successfully measured in 58 of 70 and 65 of 75 units from the control and incision group, respectively. We were unable to measure mechanosensitivity in some fibers because the positioning of cylindrical probe on the receptive field was not always successful given the relatively uneven shape of edges of the muscle.
Lactic acid responses
After mechanical stimulation, the chemosensitivity of muscle afferents was assessed with lactic acid. Baseline afferent activity was recorded for 5 min. Then the FDB muscle was isolated with a rectangular small chamber (30 mm long, 15 mm wide) and the attached tibial nerve was placed beneath the chamber through a notch on the sidewall. The small chamber made a seal at the bottom of the organ bath by its own weight and inert silicone grease (Dow Corning Corporation, Midland, MI) added to its rim. After a waterproof seal was ensured, the Krebs solution inside the small chamber was removed with a syringe. Then either lactic acid (15 mM, pH 6.0, 36 °C) or the control solution (Krebs-Henseleit solution, pH 7.4, 36 °C) was added and immersed the muscle for 5 min. Both solutions were equilibrated with room air. Following the lactic acid application, the small chamber was removed and the muscle was subjected to a 5-min washout. For chemical responses, an afferent was defined as responsive to lactic acid if its mean discharge rate during the 5-min application was greater than 0.1 imp/sec in the absence of ongoing activity or there was an increase in afferent discharge rate by at least 2 standard deviations above mean ongoing activity 5 min (60-sec bins) before the chemical application. Overall, 53 of 70 afferents from the control group and 56 of 75 afferents from the incision group were tested with pH 6.0 lactic acid. We were unable to measure chemosensitivity in some fibers because a tight seal of the small chamber with the organ bath was not always possible.
In 42 muscle afferents (26 units from the control group and 16 units from the incision group), 15 mM lactic acid with decreasing pH (pH 6.5, 6.0, and 5.5, 36 °C) was sequentially tested to examine the acid-response relation. The protocol for each pH test was the same as described above and the interval between each application was 5 min.
We examined 7 acid responsive units from those tested for the acidity-response relation for tachyphylaxis. These 7 units (4 group III and 3 group IV) were subjected to repeated application of pH 6.0 lactic acid. Three of these units were from control rats and 4 were from the incision group.
We also examined the effect of an incision made in vitro on acid responsiveness. In a separate group of 9 afferents (3 Group III and 6 group IV) recorded from unincised muscle, response to lactic acid pH 6.0 was measured first. Then a 3 mm longitudinal incision was made approximately 1 mm from the receptive field with a #11 blade though the FDB muscle. The incised muscle was further divided and retracted carefully with micro-dissecting forceps. Ten minutes later, the same unit was tested with pH 6.0 lactic acid again. The responses before and after the incision were compared.
Heat responses
After chemical application, heat responses of the muscle afferents were examined. The muscle was isolated with the small chamber and the Krebs solution inside was removed. Then a thermocouple was targeted to the receptive field and gently touched the dorsal side of the FDB muscle to measure the temperature. A radiant heat lamp was placed underneath the translucent floor of the organ bath and the light beam was adjusted to focus onto the plantar side of the FDB muscle. The receptive field was exposed to a baseline temperature (32 °C) for 30 sec with a feedback-controlled heat stimulator (Bioengineering, University of Iowa, Iowa City, IA). Then a heat ramp (from 32 to 48 °C, in 17 sec) was delivered to the receptive field. The afferent activity was continuously recorded during heat testing. We measured the temperature of two sides of the FDB muscle during heat stimulation in several preparations. The plantar side was approximately 1 °C higher than the dorsal side during the heat stimulation.
The unit was designated as responsive to heat if activity was evoked when there was no ongoing activity present before heating, or the discharge rate during heating was increased by at least two standard deviations above the baseline ongoing discharge (10 sec, 1-sec bin) before heating. The heat response threshold was defined as the temperature that elicited the first action potential in the absence of ongoing activity, or the temperature that increased the discharge rate by at least 2 standard deviations greater than the ongoing discharge rate 10 sec (1-sec bins) before heating. To obtain a heat-response function, activity during 2 °C bins was counted and plotted versus the temperature. The ongoing discharge was included in the activity. We did not measure heat responses in all units. After an extended protocol, recordings can become tenuous. Conduction velocity was measured before heat testing was completed to avoid losing the fiber recording. Heat responses were measured in 38 of 70 and 52 of 75 units from the control and incision group, respectively.
Conduction velocity
At the end of each recording, the afferent conduction velocity was measured. Electrical stimulation (5 – 20 V, 0.1 – 0.2 ms duration, 0.2 – 1.0 Hz) was applied to the receptive field through a bipolar electrode to evoke action potentials in the afferent. The conduction velocity was calculated by dividing the distance between the receptive field and the recording electrode by the latency of the evoked action potential. Group III and group IV mechanosensitive afferents were included in this study. An afferent was classified as a group III unit if the conduction velocity was greater than 2.5 m/sec and equal or less than 30 m/sec, as a group IV afferent if the conduction velocity was equal or less than 2.5 m/sec [5; 25].
Statistical analysis
For continuous data, normal distribution of values was determined by the Kolmogorov-Smirnov test. χ2-test was performed to analyze prevalence of active, lactic acid-responsive, and heat-responsive units between groups. Relationships among ongoing activity, lactic acid responsiveness, and heat responsiveness of muscle afferents were also examined with χ2-test. Student’s t test was used for comparing mean rate of ongoing discharge, heat and mechanical response thresholds between groups. Mann-Whitney test was used for comparing median rates during lactic acid application between groups. Afferent responses to repeated pH 6.0 lactic acid application was analyzed with Friedman’s test. Repeated one-way ANOVA with Tukey’s post hoc test was performed to examine pH-dependent responses to lactic acid. Two-way ANOVA with repeated measures on one factor with student’s t test was used to analyze mechanical and heat stimulus-response functions at each force range between groups. All results are expressed as mean ± standard error of the mean (SEM) or median with range. A P value less than 0.05 was considered statistically significant. All tests were performed with GraphPad Prism software (GraphPad, San Diego, CA).
Results
General properties of Group III and Group IV FDB afferents in vitro
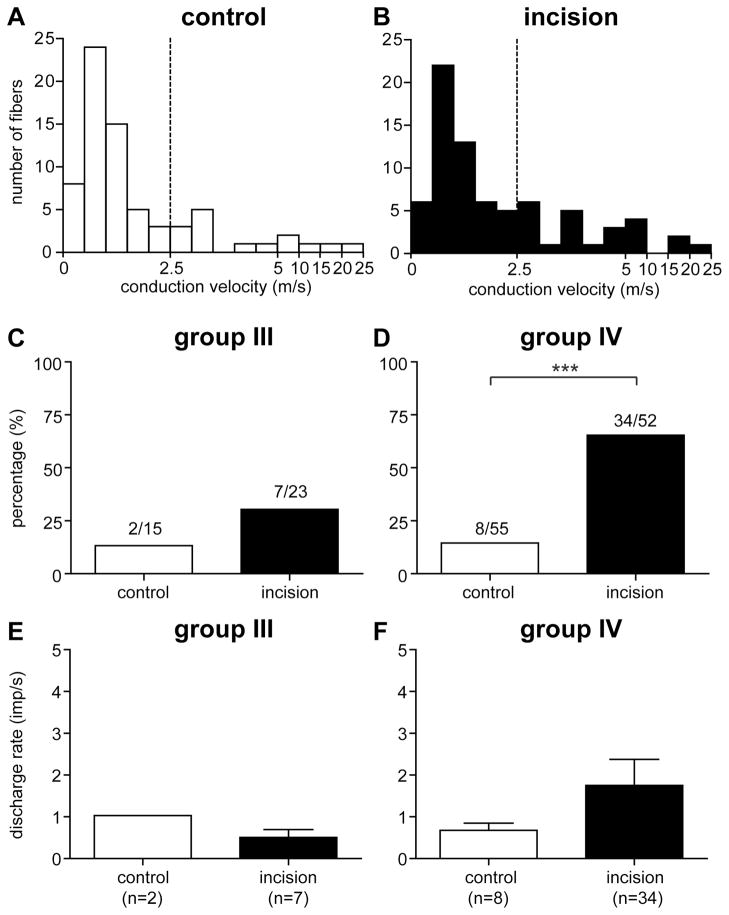
A total of 145 mechanosensitive afferents were recorded from 85 rats. There were 70 units (15 group III and 55 group IV) in the control group, 75 units (23 group III and 52 group IV) in the incision group. The distribution of conduction velocity is shown in figure 2A and 2B for the control and incision groups, respectively. The average conduction velocity of group III afferents was 7.6 ± 2.3 m/s and 6.1 ± 1.1 m/s in the control and incision group, respectively. The average conduction velocity of group IV afferents was 1.0 ± 0.1 m/s and 1.1 ± 0.1 m/s in the two groups, respectively. No difference in conduction velocity was found between groups for either group III or group IV afferents.
Figure 2.
The conduction velocity distribution histogram of muscle afferents recorded in the control (A) and incision (B) group. The percentage of group III (C) and group IV (D) afferents with ongoing activity. Mean rate of spontaneously active group III (E) and group IV (F) afferents. Results are expressed as mean and SEM. χ2-test for comparing the prevalence of active units between groups. ***, P < 0.001.
Ongoing activity
Ongoing activity was present in both group III and group IV afferents. For group III fibers, the prevalence of afferents with ongoing activity was 13.3% (2 of 15) and 30.4% (7 of 23) in the sham control and incision group, respectively (Fig. 2C). For group IV afferents, the percentage with ongoing activity was 14.5% (8 of 55) and 65.4% (34 of 52) in the control and incision group, respectively (Fig. 2D). The prevalence of afferents with ongoing activity in the incision group was greater than that in the sham control for group IV (P < 0.001) but not for group III afferents.
For group III afferents, the mean rate of ongoing activity was 1.0 ± 0.3 imp/sec and 0.5 ± 0.2 imp/sec in the control and incision group, respectively (Fig. 2E). For group IV afferents, the mean rate was 0.7 ± 0.2 imp/sec and 1.8 ± 0.6 imp/sec in the two groups, respectively (Fig. 2F). For both group III or group IV units, no difference in the average rate of ongoing activity was present between the control and incision groups.
Response to acid
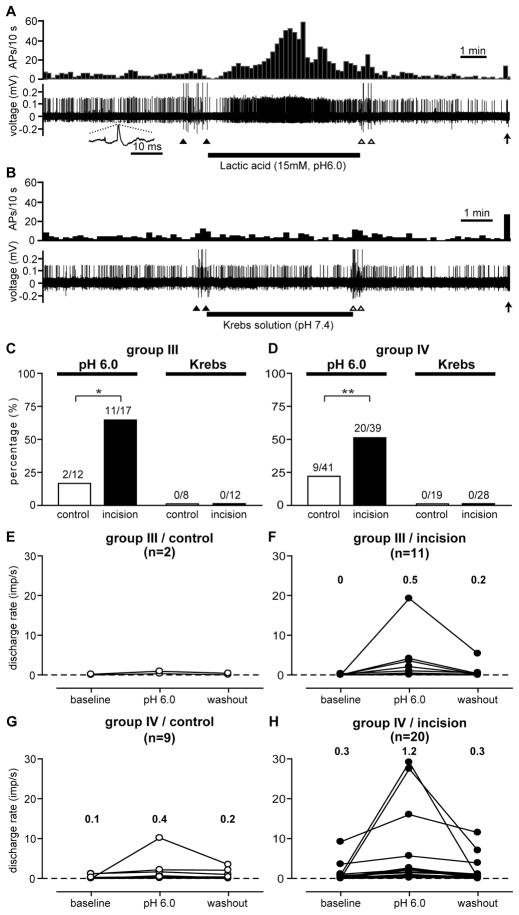
Lactic acid pH 6.0 was tested in a total of 12 group III and 41 group IV afferents in the sham control group, and 17 group III and 39 group IV afferents in the incision group. An example of a lactic acid-responsive group IV unit from the incision group is shown in figure 3A and 3B. In general, most lactic acid-responsive afferents increased activity approximately 30 to 60 sec after application; a few units responded almost immediately, within 10 sec. Their responses were, in general, sustained throughout the acid application and usually peaked at the middle or latter part of the application. Except in a few units, lactic acid-evoked activity resolved within 60 sec after washout. There was no obvious difference in acid response pattern between the sham control and the incision group. For group III afferents (Fig. 3C), the prevalence of lactic acid-responsive units in the incision group (64.7%, 11 of 17, P < 0.05) was greater than that in the control group (16.7%, 2 of 12). For group IV afferents (Fig. 3D), there was also a greater proportion of lactic acid-responsive units in the incision group (51.3%, 20 of 39, P < 0.01) than in the control group (22.0%, 9 of 41). Among those units tested with pH 6.0 lactic acid, 8 group III and 19 group IV afferents from the control, and 12 group III and 28 group IV afferents from the incised muscle were also tested using the small chamber with the control solution (pH 7.4 Krebs-Henseleit solution) applied. None of these units from either the control or incision group were excited after muscle isolation and application of the control solution (Fig. 3C and D).
Figure 3.
Example recordings for testing the lactic acid-responsiveness of muscle mechanosensitive afferents. Reponses of a group IV afferent from the incision group to 15 mM lactic acid (pH 6.0) (A) and to control Krebs solution (B), respectively. In each figure, the upper panel shows the discharge rate histograms (bin width = 10 sec) and the lower panel shows the digitized oscilloscope traces of action potentials. Inset: a representative single action potential. Filled and open arrowheads: artifacts generated by placing and removal of the small chamber, respectively. Filled arrows: responses of the afferent to mechanical stimulation via a glass probe. AP = action potential. Percentage of lactic acid-responsive group III (C) and group IV (D) afferents. No unit was activated by the control Krebs solution (C and D). Discharge rates of each lactic acid-responsive group III (E, control; F, incision) and group IV (G, control; H, incision) afferents before, during, and after lactic acid application. The median discharge rates were presented. χ2-test for the prevalence of lactic acid-responsive units between the control and incision group. * P < 0.05, ** P < 0.01.
For group III afferents, only two responded to lactic acid in the control group (Fig. 3E); in the incision group, the median activity before, during, and after pH 6.0 lactic acid application was 0 imp/sec (0 – 0.3 imp/sec, range), and 0.5 imp/sec (0.1 – 19.3 imp/sec), and 0.2 imp/sec (0 – 5.4 imp/sec, Fig. 3F), respectively. There were not a sufficient number of group III fibers responsive to acid in the control group to test for differences versus incision.
For group IV afferents, the median discharge rate before, during, and after lactic acid application was 0.1 imp/sec (0 – 1.2 imp/sec), 0.4 imp/sec (0.3 – 10.2 imp/sec), and 0.2 imp/sec (0 – 3.5 imp/sec) in the control group (Fig. 3G), respectively. For the incised muscle, activity was 0.3 imp/sec (0 – 9.2 imp/sec), and 1.2 imp/sec (0.2 – 29.2 imp/sec), and 0.3 imp/sec (0 – 11.5 imp/sec) before, during, and after lactic acid application (Fig. 3H), respectively. The increase in discharge rate during acid application was not significantly different between the control and incision groups.
In unincised muscle, 9 (3 group III and 6 group IV) afferents were tested with pH 6.0 lactic acid, then an incision was made adjacent to the receptive field in vitro. The prevalence of lactic acid-responsive units remained the same before (22.2%, 2 of 9) and after incision (22.2%, 2 of 9). The same units responsive before incision remained responsive afterwards and no unresponsive units became responsive after incision. This was similar to the prevalence of acid responsiveness in all group III and IV afferents from the control group (20.8%). In the two acid-responsive afferents, discharge rate during lactic acid application was 0.2 imp/sec and 0.7 imp/sec before incision and 0.2 imp/sec and 0.5 imp/sec after incision, respectively. These results indicate that incision does not likely increase the acid–responsiveness of afferents by increasing exposure of nerve terminals to acid.
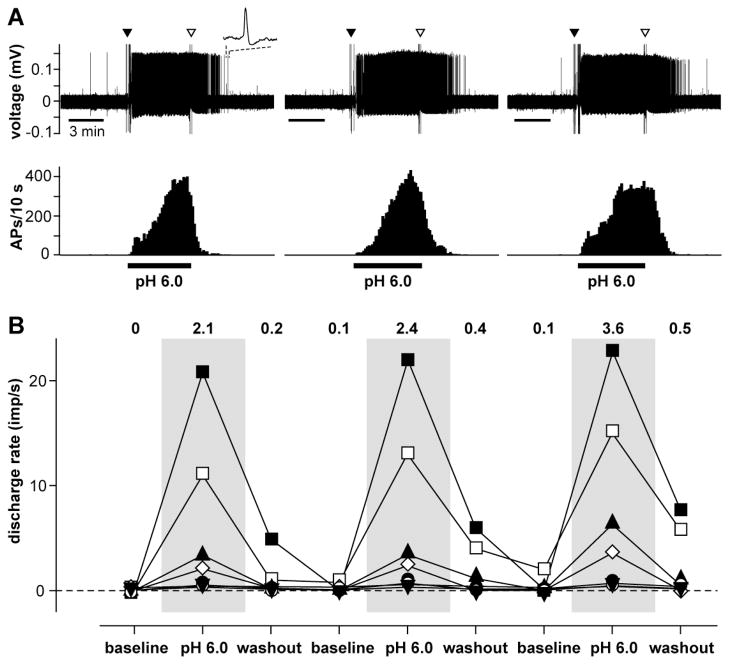
In 7 acid responsive afferents, pH 6.0 lactic acid was repeatedly tested using three consecutive applications (Fig. 4). The median response rate for each of the three applications was 2.1 (0.3 – 19.3 imp/sec), 2.4 (0.4 – 22.2 imp/sec), and 3.6 imp/sec (0.4 – 22.4 imp/sec). There was no difference among applications in terms of sensitization or tachyphylaxis.
Figure 4.
Repeated 15 mM lactic acid (pH 6.0) application to lactic acid-responsive afferents. (A) Example recordings showing responses of an afferent to repeated lactic acid application. The upper panel is the digitized oscilloscope traces of action potentials. Inset: a representative single action potential. Filled and open arrow heads: artifacts generated by placing and removal of the chemical chamber, respectively. The lower panel shows the discharge rate histograms of the unit. Bin width = 10 sec. AP = action potential. (B) Mean discharge rate of each unit during repeated lactic acid application. The discharge rate of individual unit was obtained based on the mean from 5-min periods each of baseline, lactic acid application, and washout recordings. The numbers showed the median discharge rate (n=7).
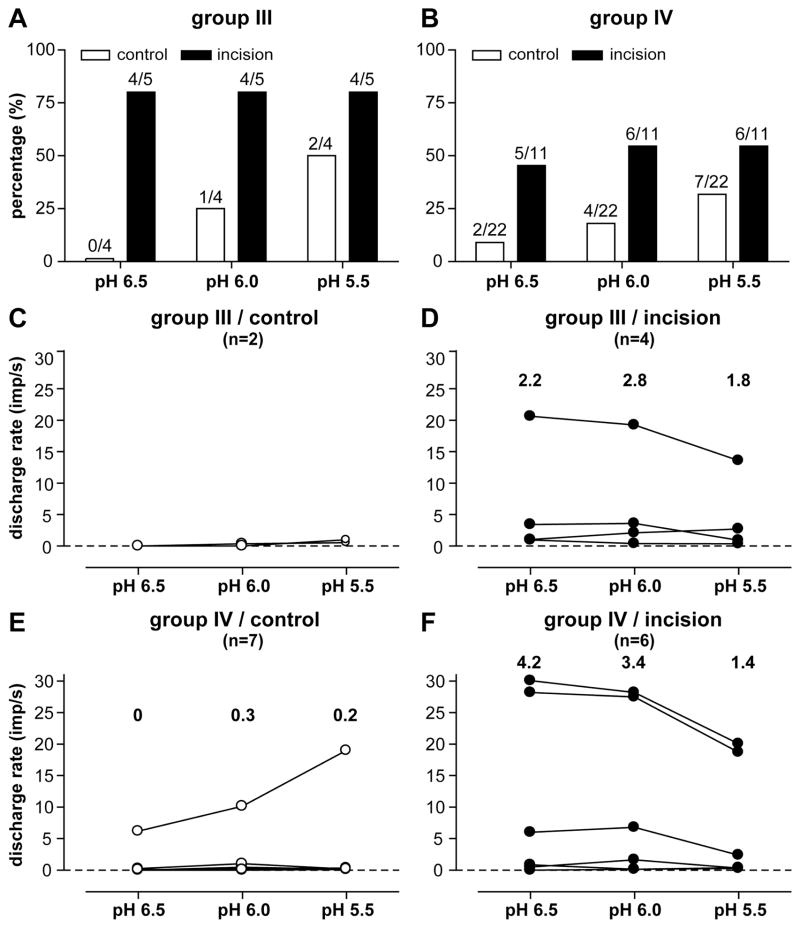
After noting that more afferents from incised muscle responded to pH6.0 lactic acid than the sham preparation (Fig. 3C–D), afferent responses to lactic acid with varying pH were tested in 4 group III and 22 group IV fibers from the sham control, and 5 group III and 11 group IV from the incised muscle, respectively. All fibers were tested whether they were responsive to lactic acid pH 6.0 or not. In the sham control group, greater acidity tended to activate more group III (Fig. 5A) or group IV (Fig. 5B) afferents. When the proportion of activated group III and IV afferents were combined, there was not an acid related recruitment of fibers (P = 0.054) in the control tissue. In the incision group, decreasing pH did not increase the proportion of acid responsive fibers; however, there was a pH-dependent decrease in the magnitude of group IV afferent responses (P < 0.05, Fig. 5F) to decreasing pH. This acid-response relationship was also evident in group III afferents from the incised muscle (Fig. 5D), but this was not statistically significant. Incision sensitized muscle afferents to lactic acid because the proportion of acid responsive units was greater. A limited number of fibers were tested with varying acid concentrations; the small number of fibers is likely underpowered to determine statistical significance.
Figure 5.
Responses of muscle afferents to 15 mM lactic acid with varying pH (pH 6.5, pH 6.0, and pH 5.5). Percentage of group III (A) and group IV (B) afferents responding to lactic acid at each pH level. The discharge rates of individual responsive group III (C, control; D, incision) and group IV (E, control; F, incision) afferents during each lactic acid application. The numbers represent the median discharge rates of these units.
Response to mechanical stimulation
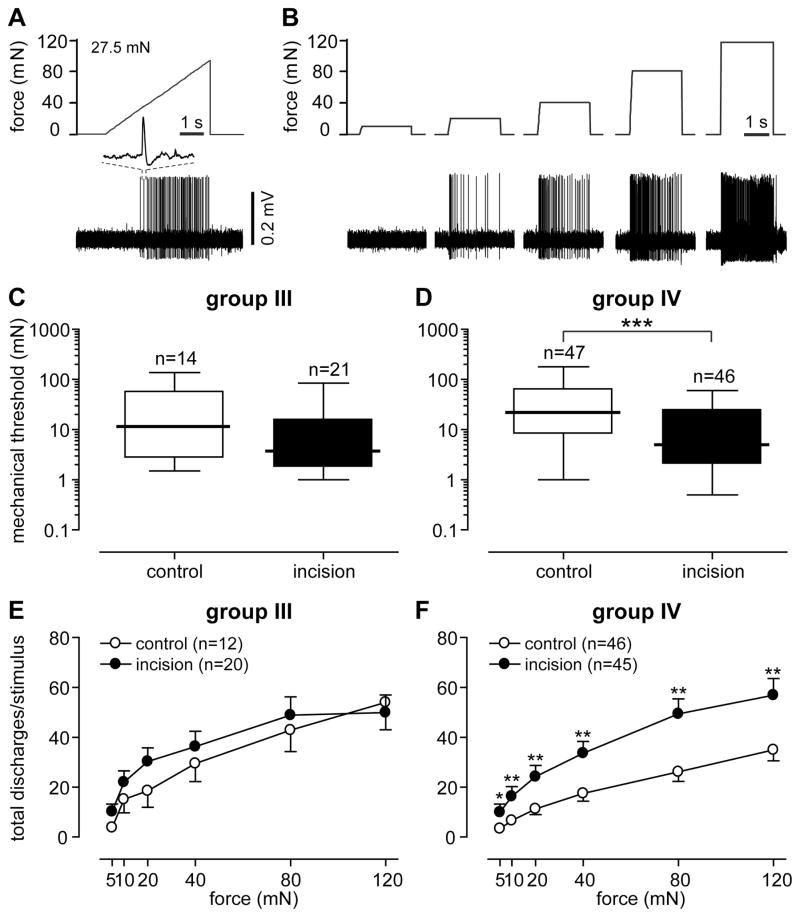
Most of the mechanical muscle receptive fields were located in the midportion of the muscle. Units with a receptive field on the proximal or distal tendons were not included. Receptive fields were often in round or oval shape and varied in size; we estimate most were less than 4mm2. The median mechanical threshold for group III afferents was 11.5 mN and 3.8 mN in the sham control and incision group, respectively (Fig. 6C). No difference in threshold was present between the groups. The incision group had a lower median threshold in group IV afferents (5.0 mN) compared with the control group (22.0 mN, P < 0.001, Fig. 6D).
Figure 6.
Responses of muscle afferents to mechanical stimulation. (A) The upper and lower panels represent the force ramp applied and digitized oscilloscope trace of action potentials during the mechanical stimulation, respectively. Inset: a representative single action potential. (B) The response of the same unit to sustained force stimuli in an ascending order. The upper and lower panels represent the sustained forces applied and digitized oscilloscope trace of action potentials. Mechanical response threshold of group III (C) and group IV (D) afferents generated from the force ramp. The stimulus-response function of group III (E) and group IV (F) afferents was drawn by counting the total activity during each sustained force applied. The results of response threshold are expressed as median (thick horizontal line) with 1st and 3rd quartiles (box), and 10th and 90th percentiles (thin horizontal lines). The results of stimulus-response function are expressed as mean and SEM. Mann-Whitney test for comparing mechanical response thresholds between groups. Two-way ANOVA followed by repeated t-test at each force level for comparing stimulus-response functions between groups. * P < 0.05, ** P < 0.01, *** P < 0.001.
The stimulus-response functions of group III afferents showed increased responses to greater forces (Fig. 6E), but there was no difference in stimulus-response functions between the control and incision groups. The stimulus-response function of group IV afferents also exhibited increases in responses to greater forces (Fig. 6F). Compared with the control group, units in the incision group had greater responses to 5 mN (P < 0.05), 10 mN (P < 0.01), 20 mN (P < 0.01), 40 mN (P < 0.01), 80 mN (P < 0.01) and 120 mN (P < 0.01).
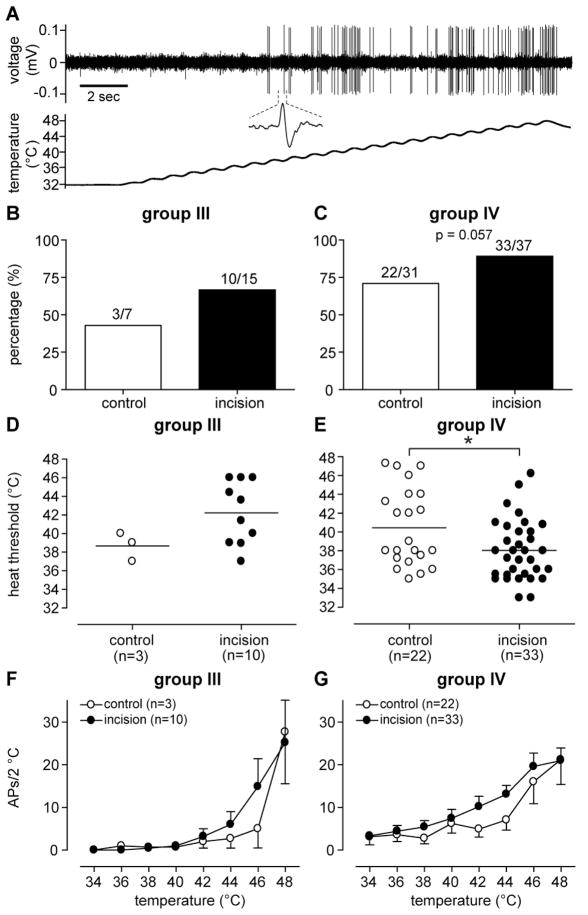
Response to heat stimulation
Heat was tested in a total of 38 and 52 units in the control and incision group, respectively. For group III afferents, 42.9% (3 of 7) and 66.7% (10 of 15) units responded to heat in the control and incision group, respectively (Fig. 7B); for group IV afferents, 70.9% (22 of 31) and 89.2% (33 of 37) units were heat-responsive in the control and incision group, respectively (Fig. 7C). There was no significant difference in the proportion of heat responsive afferents between the sham control and incision for either group III and group IV fibers. The average heat response threshold for group III afferents was 38.9 ± 0.9 °C and 42.2 ± 1.1 °C in the control and incision group, respectively (Fig. 7D); this was not statistically significant. For group IV afferents (Fig. 7E), the heat threshold of the incision group (38.0 ± 0.6 °C, P < 0.05) was less than that of the sham control (40.5 ± 0.9 °C). When heat-response functions were analyzed, both group III (Fig. 7F) and group IV (Fig. 7G) afferents showed temperature-dependent increases in heat responses. However, differences in the heat-response functions were not present between the sham control and incision for either group III or group IV afferents.
Figure 7.
Responses of mechanosensitive muscle afferents to heat stimulation. (A) An example recording showing the response of a group IV afferent from an incised muscle to heat stimulation. The upper panel is the digitized oscilloscope trace of action potentials during heat stimulation. The lower panel is the temperature recording from the thermode. Inset: a representative single action potential. Percentage of heat-responsive group III (B) and group IV (C) afferents. Heat response threshold of group III (D) and group IV (E) afferents. The stimulus-response functions of group III (F) and group IV (G) afferents. Results are expressed as mean and SEM. Student’s t-test for compared heat response thresholds between groups. * P < 0.05.
Interrelations of response properties of muscle afferents
We evaluated the relationships between acid responses, heat responses and ongoing activity in group IV afferents (Table 1A). Group IV fibers with ongoing activity were more likely to respond to acid; this was significant in the sham group (57.1 vs 14.7 %, P = 0.0135) but not in the incision group (61.5% vs. 30.8%, P = 0.0699). Group IV fibers with ongoing activity had a lower mean heat response threshold than those without ongoing activity in the incised group (37.0 ± 0.6 °C vs. 39.6 ± 0.8 °C, P = 0.0171). There was no relation between heat responsiveness or heat threshold and acid responsiveness in group IV fibers (Table 1B). There were too few group III fibers to examine these relationships.
Table 1.
Relations between ongoing activity, acid responses and heat responses.
| (A) | Group III | Group IV | ||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Incision | Sham | Incision | |||||
| Activity − | Activity + | Activity − | Activity + | Activity− | Activity + | Activity − | Activity + | |
| Acid-responsive units | 2/11 (18.2%) | 0/1 (0%) | 7/12 (58.3%) | 4/5 (80.0%) | 5/34 (14.7%) | 4/7 * (57.1%) | 4/13 (30.8%) | 16/26 (61.5%) |
| Heat threshold (°C) | § | 42.8 ± 1.4 | 40.9 ± 1.4 | 40.9 ± 1.0 | 37.4 ± 0.3 | 39.6 ± 0.8 | 37.0 ± 0.6 * | |
| Heat-responsive units | 2/6 (33.3%) | 1/1 (100%) | 7/10 (70.0%) | 3/5 (60.0%) | 19/27 (70.4%) | 3/4 (75.0%) | 13/14 (92.9%) | 21/23 (91.3%) |
| (B) | Group III | Group IV | ||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Incision | Sham | Incision | |||||
| Acid− | Acid + | Acid − | Acid + | Acid − | Acid + | Acid − | Acid + | |
| Heat threshold (°C) | § | § | 40.6 ± 1.5 | 37.2 ± 0.6 | 37.8 ± 1.2 | 38.1 ± 0.9 | ||
| Heat-responsive units | 3/5 (60.0%) | 0/0 | 2/4 (50.0%) | 3/6 (50.0%) | 9/15 (60.0%) | 3/3 (100%) | 11/12 (91.7%) | 13/13 (100%) |
Values for hest threshold are mean ± SEM; other values are proportions of afferents with percentage in parentheses.
P < 0.05 vs. SA- by χ2 test for comparing acid-responsiveness or by student t test for comparing heat response threshold;
no values are presented due to the small number of units. Activity=ongoing activity.
Discussion
The present study, for the first time, examined the properties of mechanosensitive group III and IV afferents using an in vitro FDB muscle-nerve preparation. The major finding of this study is that 1 day after incision, lactic acid activated a greater proportion of units than the control group. Also, there was a greater prevalence of afferents with ongoing activity compared with the control. Injury sensitized muscle afferents to both heat and mechanical stimulation. In injured muscle, decreased heat threshold was correlated to increased ongoing activity; units responsive to acid also tended to possess ongoing activity. These relations were most prominent in group IV afferents.
Ongoing activity in muscle afferents
The present study found a low prevalence of ongoing activity in group III and IV afferents of the uninjured FDB muscle. The low incidence of afferents with ongoing activity in uninjured muscle was also reported by others both in vitro [10] and in vivo [3; 35]. Injury increased the incidence of ongoing activity of muscle afferents. In agreement, the prevalence of afferents with ongoing activity was greater after skin plus deep muscle incision but was not changed by skin incision only in vivo [43]. There are some limitations of investigating ongoing activity of afferents using an excised in vitro preparation. Under this condition, some mediators present in vivo are likely removed and alteration of muscle integrity may also occur. Ongoing activity in this preparation may be related to a variety of factors; these include not only the previous plantar incision but also the dissection and preparation of the muscle, and possibly prolonged after discharge following the mechanical search stimulus. However, the agreement between our in vivo studies after muscle injury suggests that the activation of afferents remains in the in vitro condition. Unprovoked guarding pain was increased only after skin plus deep muscle injury but not after skin incision, suggesting ongoing activity in muscle afferents contributes to unprovoked pain behavior.
Response of muscle afferents to lactic acid
In the present study, a small proportion (20.8%) of group III and IV muscle afferents responded to 15 mM lactic acid (pH=6.0) under normal conditions. In agreement, 21.4% group IV afferents of the rat EDL muscle were activated by acid (pH 5.5) in vitro [38]. The incidence of acid-responsive units was greater in some in vivo studies: 56.0% group IV afferents were excited when acidic phosphate buffer (pH 6.0) was injected in to the mechanical receptive field of the rat gastrocnemius muscle [12]; 42.1% group III and 30.0% group IV afferents of the cat triceps surae muscle responded to 24mM lactic acid administered intra-arterially [34]. The different testing system (in vitro versus in vivo), route of administration, or method for acidification may account for these differences among studies. In the control group, decreasing pH tended to increase the prevalence of responsive units (P=0.054) but not the magnitude of response.
An important finding of the present study is that a greater percentage (55.4%) of group III and IV afferents responded to lactic acid after injury. In the injured group, increasing acidity did not change the proportion of responsive units and tended to decrease the response magnitude. These data indicate that nerve terminals in the injured muscle are sensitized to lactic acid. We did not identify the minimum pH reduction to elicit responses in injured muscle but the results suggest that pH greater than 6.5 may activate muscle afferents after injury. Previously we found that decreased pH (pH = 6.8) and increased lactate (approximately 5mM ) were present in the incised plantar hindpaw at the same time guarding pain behavior was evident [41; 18]. The current data showing direct evidence of increased chemosensitivity to lactic acid of muscle afferents after incision further supports the possibility that lactic acid in incised tissue activates muscle afferents and contributes to the development of ongoing activity in nociceptive sensory pathways and perhaps unprovoked guarding pain [42; 43].
Response of muscle afferents to heat and mechanical stimulation
We found that a large percentage of mechanosensitive group III and IV afferents of the FDB muscle respond to heat in both uninjured (65.8%) and injured (82.7%) groups. This high prevalence is in agreement with studies by others [19; 38]. It has been suggested that heat is noxious to muscle when the temperature exceeds 41 °C [19; 25; 10]. In the current study, incision reduced the heat threshold of group IV afferents. TRPV1 has been suggested to mediate heat sensitization of cutaneous afferents after a similar injury [2]; whether it also contributes to incision-induced heat sensitization of muscle afferents requires further study. In our previous behavioral study [43], incision of skin alone is sufficient to induce heat hyperalgesia after incision; the role for muscle afferents in heat hyperalgesia is not clear, a contribution of temperature to ongoing activity is possible.
We found no difference in mechanical threshold between group III and IV afferents in normal muscle, as has been reported previously both in vitro [10] and in vivo [24]. In the FDB muscle, the mechanical threshold of group III and group IV afferents were 11.5 mN (median = 30.5 kPa) and 22.0 mN (median = 57.9 kPa), respectively. Comparable thresholds have been reported by Taguchi el al. [38] and Paintal [31]. Although low- and high-threshold pressure-sensitive receptors have been reported to be present in group III and IV afferents [25], the current data did not suggest unambiguous segregation of mechanical threshold for either group III or IV afferents.
The present study demonstrated incision-induced mechanical sensitization of group IV afferents. It has been reported that acidosis sensitized cutaneous afferents to mechanical stimulation in vitro [37]. A similar mechanism is possibly present in muscle afferents. In agreement, intramuscular infusion of acidic phosphate buffer (pH 5.2) induced mechanical hyperalgesia and referred pain in human volunteers [9]. Future studies will examine mechanical responses and the influence of acid. In our behavioral study, skin incision was sufficient to induce the full extent of mechanical hyperalgesia; muscle incision did not further exacerbate withdrawal thresholds early after incision. Of note, monofilament testing has limited sensitivity for mechanical responses from the deep tissue [40; 39].
Comparison of injured muscle and skin afferents
Previously, we studied cutaneous Aδ- and C-fibers responding to pH 6.0 lactic acid; after incision[14]. A greater percentage of muscle afferents responded to pH 6.0 lactic acid than cutaneous afferents in both uninjured (20.8% vs. 8.3%) and injured (55.4% vs. 27.5%) conditions. Lactic acid produced a pH-dependent increase in response to pH 6.5, 6.0, and 5.5 in incised skin but not in incised muscle.
A study has also compared heat response properties of C-fibers in normal and incised skin [1]. The heat response threshold of muscle afferents (40.5 °C) is similar to that of cutaneous afferents (39.8 °C). In injured tissue, the average heat threshold of muscle afferents (38.0 °C) is slightly greater than that of skin (36.7 °C). In incised skin, afferents were had greater responses to heat; together, suggesting a greater heat sensitization of cutaneous afferents than muscle afferents after incision.
When mechanical responses of cutaneous afferent were examined, the mean mechanical threshold of fibers in injured skin tended to be lower than fibers in normal skin (9.6 mN vs. 16.5 mN for Aδ-fibers, 14.2 mN vs. 23.1 mN for C-fibers). In addition, C-fibers showed greater response magnitudes to 20 and 40 mN stimuli. More remarkable, mechanical sensitization was induced by injury in muscle afferents; group IV afferents had greater response magnitude to all levels of stimuli.
Possible involvement of group III and IV afferents in nonnociceptive functions
Group III and IV afferents respond to both noxious and innocuous stimulation (e.g. muscle contraction and innocuous mechanical pressure) [11; 20]. Attempts have been made to categorize these afferents into nociceptors and ergoreceptors, respectively [15]. Ergoreceptors mediate the reflex increase in cardiovascular and respiratory function during excise and physical work and may also signal fatigue [23; 17]. Lactate acid is one metabolic product that accumulates during static muscle contraction and has been suggested to contribute to metaboreception during exercise [34; 21]. In the present study, we can not determine that we are exclusively studying nociception and exaggerated pain responses after injury. Group III and IV afferents may also transmit metaboreception in post-injury states. Fatigue is a common complaint in patients after surgery [7]. Since lactic acid increases in injured muscle and injured muscle develops increases responsiveness to lactic acid, the contribution of group III and IV afferents to the development of postoperative fatigue can not be excluded.
Conclusion
The present study demonstrates that 1 day after incision, chemosensitivity of muscle afferents to lactic acid was enhanced. Injury increased the prevalence of afferent ongoing activity and also sensitized afferent response to heat and mechanical stimuli. Both increased heat and chemo-sensitivity could contribute to ongoing activity of nociceptors and unprovoked resting pain after deep muscle injury. Compared with skin afferents, greater sensitization of muscle afferents after injury suggests more effort should be made to understand deep tissue pain and hyperalgesia in the post-injury state.
Acknowledgments
The authors are grateful for the support by the Department of Anesthesia at the University of Iowa and by the National Institutes of Health, Bethesda, Maryland grant GM067762 to T.J.B.
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112:204–213. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain. 2009;141:41–51. doi: 10.1016/j.pain.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berberich P, Hoheisel U, Mense S. Effects of a carrageenan-induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J Neurophysiol. 1988;59(5):1395–1409. doi: 10.1152/jn.1988.59.5.1395. [DOI] [PubMed] [Google Scholar]
- 4.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 5.Brock LG, Eccles JC, Rall W. Experimental investigations on the afferent fibres in muscle nerves. Proc R Soc Lond B Biol Sci. 1951;138:453–475. doi: 10.1098/rspb.1951.0035. [DOI] [PubMed] [Google Scholar]
- 6.Cassar K. Intermittent claudication. BMJ. 2006;333:1002–1005. doi: 10.1136/bmj.39001.562813.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen T, Kehlet H. Postoperative fatigue. World J Surg. 1993;17:220–225. doi: 10.1007/BF01658930. [DOI] [PubMed] [Google Scholar]
- 8.Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89:1153–1160. doi: 10.2106/JBJS.F.00940. [DOI] [PubMed] [Google Scholar]
- 9.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge W, Khalsa PS. Encoding of compressive stress during indentation by group III and IV muscle mechano-nociceptors in rat gracilis muscle. J Neurophysiol. 2003;89:785–792. doi: 10.1152/jn.00624.2002. [DOI] [PubMed] [Google Scholar]
- 11.Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain. 2001;17(1):2–10. doi: 10.1097/00002508-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Hu-Tsai M, Winter J, Woolf CJ. Regional differences in the distribution of capsaicin-sensitive target-identified adult rat dorsal root ganglion neurons. Neurosci Lett. 1992;143:251–254. doi: 10.1016/0304-3940(92)90276-d. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Brennan TJ. Chemosensitivity and mechanosensitivity of nociceptors from incised rat hindpaw skin. Anesthesiology. 2009;111:155–164. doi: 10.1097/ALN.0b013e3181a16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao FF. An experimental study of the pathway involved in exercise hyperpnoea employing cross-circulation techniques. In: Cunningham DJC, Lloyd BB, editors. The Regulation of Human Respiration. Blackwell; Oxford: 1963. pp. 461–502. [Google Scholar]
- 16.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12(6):429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 18.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273:179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Light AR, Perl ER. Unmyelinated afferent fibers are not only for pain anymore. J Comp Neurol. 2003;461:137–139. doi: 10.1002/cne.10691. [DOI] [PubMed] [Google Scholar]
- 21.MacLean DA, LaNoue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitial metabolite responses in the cat. J Appl Physiol. 1998;85:1583–1592. doi: 10.1152/jappl.1998.85.4.1583. [DOI] [PubMed] [Google Scholar]
- 22.Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105:815–821. doi: 10.1213/01.ane.0000278091.29062.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mense S. Muscular nociceptors. J Physiol (Paris) 1977;73(3):233–240. [PubMed] [Google Scholar]
- 25.Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol. 1985;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meru AV, Mittra S, Thyagarajan B, Chugh A. Intermittent claudication: an overview. Atherosclerosis. 2006;187:221–237. doi: 10.1016/j.atherosclerosis.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Moiniche S, Dahl JB, Erichsen CJ, Jensen LM, Kehlet H. Time course of subjective pain ratings, and wound and leg tenderness after hysterectomy. Acta Anaesthesiol Scand. 1997;41(6):785–789. doi: 10.1111/j.1399-6576.1997.tb04784.x. [DOI] [PubMed] [Google Scholar]
- 28.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 30.Ogonda L, Wilson R, Archbold P, Lawlor M, Humphreys P, O’Brien S, Beverland D. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005;87:701–710. doi: 10.2106/JBJS.D.02645. [DOI] [PubMed] [Google Scholar]
- 31.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 33.Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- 34.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 35.Simone DA, Marchettini P, Caputi G, Ochoa JL. Identification of muscle afferents subserving sensation of deep pain in humans. J Neurophysiol. 1994;72(2):883–889. doi: 10.1152/jn.1994.72.2.883. [DOI] [PubMed] [Google Scholar]
- 36.Staud R. Future perspectives: pathogenesis of chronic muscle pain. Best Pract Res Clin Rheumatol. 2007;21:581–596. doi: 10.1016/j.berh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taguchi T, Sato J, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol. 2005;94:2822–2831. doi: 10.1152/jn.00470.2005. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Taguchi T, Itoh K, Okada K, Kawakita K, Mizumura K. Influence of surface anesthesia on the pressure pain threshold measured with different-sized probes. Somatosens Mot Res. 2005;22:299–305. doi: 10.1080/08990220500420475. [DOI] [PubMed] [Google Scholar]
- 40.Treede RD, Rolke R, Andrews K, Magerl W. Pain elicited by blunt pressure: neurobiological basis and clinical relevance. Pain. 2002;98:235–240. doi: 10.1016/S0304-3959(02)00203-8. [DOI] [PubMed] [Google Scholar]
- 41.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–475. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144(3):329–339. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112:153–164. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]