Abstract
Following a myocardial infarction (MI), the homeostatic balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) is disrupted as part of the left ventricle (LV) response to injury. The full complement of responses to MI has been termed LV remodeling and includes changes in LV size, shape and function. The following events encompass the LV response to MI: 1) inflammation and LV wall thinning and dilation, 2) infarct expansion and necrotic myocyte resorption, 3) accumulation of fibroblasts and scar formation, and 4) endothelial cell activation and neovascularization.1, 2 In this review, we will summarize MMP and TIMP roles during these events, focusing on the spatiotemporal localization and MMP and TIMP effects on cellular and tissue-level responses. We will review MMP and TIMP structure and function, and discuss specific MMP roles during both the acute and chronic phases post-MI, which may provide insight into novel therapeutic targets to limit adverse remodeling in the MI setting.
Keywords: myocardial infarction, left ventricular remodeling, matrix metalloproteinases, tissue inhibitors of metalloproteinases, review
1. Introduction
A. MMP nomenclature, structure and activation
Matrix Metalloproteinases (MMPs), also known as matrixins, are zinc-dependent enzymes that can both cleave extracellular matrix (ECM) components as well as non-ECM substrates. Currently, the MMP family is composed of 25 proteinases that can be categorized into five groups based on their in vitro substrate preferences: collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs. The collagenases (MMP-1, -8, -13) can cleave fibrillar type collagens. The gelatinases (MMP-2, -9) can degrade gelatins. The stromelysins (MMP-3, -10) and matrilysins (MMP-7, -26) are broad-spectrum proteinases, and the membrane-type MMPs (MT-MMPs) are anchored to the plasma membrane.3, 4, 5, 6 These classifications are somewhat arbitrary and substrate preferences overlap for all of these groups. For example, MMP-14 is assigned to the membrane type subgroup but can also be classified as a collagenase.7 MMP-9 was first described as only being able to process collagen that was denatured or already cleaved by collagenases; however, recent literature has shown that MMP-9 can process full length interstitial collagens in addition to a broad array of other substrates.8, 9
Enzymes assigned to the MMP family share sequence homology to MMP-1, including a conserved cysteine switch motif heptapeptide PRCGXPD and the zinc-binding motif HEXGHXXGXXH found in the catalytic domain.10 The general MMP structure contains a prodomain of approximately 80 amino acids that maintains the enzyme in its zymogen latent form, a catalytic domain of 160 to 170 amino acids, and a hemopexin-like domain of approximately 210 amino acids that coordinate protein-protein interactions (Figure 1).11 Most MMPs also contain a signal sequence (17–20 amino acids) that targets the proteins for secretion into the extracellular space.11 All MMPs contain a 50 amino acid zinc-binding region within the catalytic domain that interacts with the prodomain to maintain the enzyme in its zymogen form.11 In addition, some MMPs contain specialized amino acid modules that support substrate recognition. MMP-2 and MMP-9, for example, contain three conserved fibronectin type II repeats in their catalytic domain that increase their affinity to gelatin, laminin and collagenous substrates.12 Unique to MMP-9 is an extended linker region located between the catalytic domain and the hemopexin domain, and this linker region contains a collagen V-like motif that is heavily glycosylated to enhance substrate affinity and selectivity.13, 14
Figure 1.
The general MMP structure contains a signal sequence (ss), a prodomain, a catalytic domain, and a hemopexin-like domain. The catalytic domain contains a zinc-binding region that interacts with the prodomain. For several MMPs (e.g., MMP-2 and MMP-9), the catalytic domain also contains multiple fibronectin type II repeats.
MMPs are preserved in a latent form through a bond between cysteine 73 in the prodomain and zinc in the catalytic domain that physically obstructs the catalytic site of the enzyme.11 The cysteine is the key amino acid in this mechanism; therefore, activation of MMPs is referred to as the cysteine switch mechanism of activation.15 MMP activation involves cleavage of approximately 10 kDa from the N-terminus that includes both signal sequence and prodomain. The exceptions to the rule that MMPs are extracellularly activated are MMP-11 and the membrane type MMPs (MMP-14, -15, -16, and -17), and these MMPs contain a furin cleavage sequence to allow intracellular activation.11 In vitro activators of MMPs include p-amino-phenol-mercuric acetate (APMA) and sodium dodecyl sulfate (SDS). SDS is able to activate MMPs without cleaving the 10 kDa prodomain, which explains why zymograms are able to detect distinct pro and active MMP forms. In vivo, MMP activators include other MMPs, serine proteases, trypsin, and tissue kallikrein.16 In addition, modifications such as S-glutathiolation produced by peroxinitrite and glutathione can activate pro-MMPs.17 MMPs can also be activated by MMP cascades. For example, MMP-3 is known as the universal activator that can autocatalyze itself or be activated by serine or cysteine proteases. In turn, active MMP-3 can cleave pro-MMP-9, and MMP-9 can also be activated by MMP-2.18–20 Activated MMP-14 can activate MMP-13, which in turn can activate MMP-9.20, 21 Similarly, pro-MMP-2 activation can be tightly regulated and localized to the cell membrane by MMP-14 in a spatial and temporal manner that requires binding of TIMP-2 to MMP-14.22 MMP-14 forms homodimers through the hemopexin domain as part of the activation of pro-MMP-2.23 Therefore, there are multiple examples of MMP interdependence.
B. TIMP structure, MMP inhibition, and non-MMP related functions
MMPs are endogenously inhibited by the tissue inhibitors of metalloproteinases (TIMPs), a family comprised of four members, TIMP-1, -2, -3, and -4. All four TIMPs have a secretory signal sequence, 12 conserved cysteines, a conserved hallmark sequence (VIRAK), and a similar molecular mass that ranges from 24 to 28 kDa.24, 25 Because of the close molecular weights among the TIMPs, immunoblotting or ELISA are the best ways to quantify TIMP levels. Some of the early reverse zymography experiments that report TIMP-1 levels cannot rule out that other TIMP family members were included in the quantitation, and interpretation of these data should take this into consideration. TIMPs have a high affinity for the catalytic domain of active MMPs, thereby blocking the MMP catalytic site in a 1:1 molar ratio. Moreover, some TIMPs can also form complexes with pro-MMPs to tightly regulate MMP function prior to activation.24 For example, TIMP-1 can bind pro-MMP-9 while TIMP-2 can bind pro-MMP-2.25 MMP/TIMP ratios are commonly used as a relative stoichiometric representation of MMP activity; however, this raises the interesting question as to whether MMPs require activation to be functionally relevant. Dufour and colleagues showed that pro-MMP-2 and pro-MMP-9 use a non-proteolytic mechanism that involves homodimerization to enhance cell migration, and the hemopexin domains of both MMPs facilitated homodimer formation.26 Therefore, it appears that pro-MMPs can have functional roles that do not require the MMP to be activated. In addition, reporting results as ratios (e.g., MMP-9 to TIMP-1 ratios) assumes that TIMP-1 binding to MMP-9 is the only relevant MMP/TIMP pairing in the system. Given that there are 25 MMPs and 4 TIMPs, and that all TIMPs can inhibit all MMPs, this rationale remains to be proven valid.
Independent of action on MMPs, TIMPs can elicit diverse biological responses. The Mann laboratory has shown that adenoviral-induced over-expression of all 4 TIMPs increases cardiac fibroblast cell proliferation and differentiation to myofibroblasts. Further, TIMP-2 expression increases cardiac fibroblast collagen synthesis and TIMP-3 influences the balance between cell survival and cell death increasing the incidence of apoptosis.27 Thus, TIMP functions are complex and not merely related to MMP inhibition.
C. MMP function post-MI: ECM and non-ECM substrates
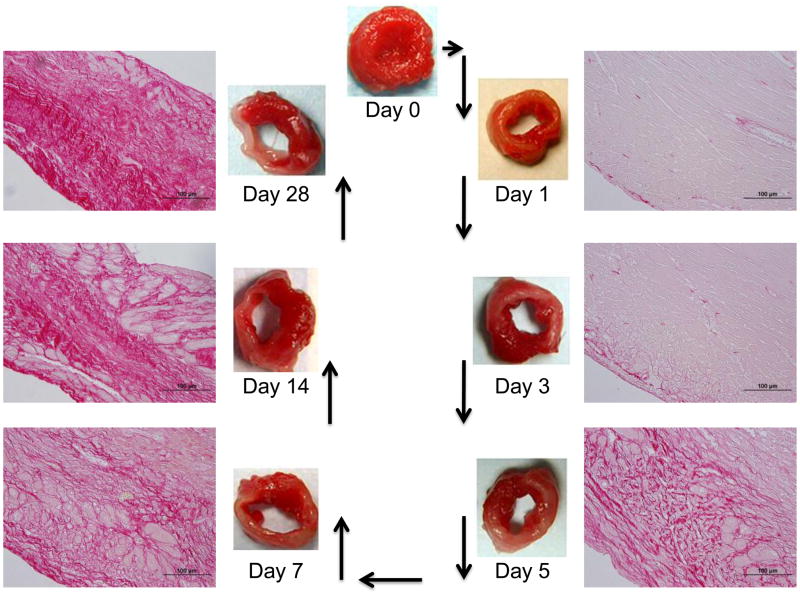
MMPs coordinate tissue development, repair, and regeneration by modulating the ECM network to regulate cell adhesion, migration, proliferation, and epithelial-mesenchymal transition as well as other cellular responses.22 Similar to a specific role during development, MMPs and TIMPs follow a spatio-temporal pattern during repair of the myocardium in the post-MI setting. 22, 28, 29 Figure 2 illustrates the temporal progression of LV wall thinning and collagen deposition through day 28 post-MI in the mouse. At each stage, MMPs regulate the tissue response to MI by acting on a myriad of substrates.
Figure 2.
The temporal progression of LV wall thinning and collagen deposition through day 28 post-MI in the mouse. The LV slices are stained with 1% 2,3,5-triphenyltetrazolium chloride to detect metabolically active tissue. Viable tissue stains red, while infarct tissue remains white. The histological sections are stained with 1% picrosirius red, which stains collagen red.
MMPs modulate multiple responses (e.g. inflammation and angiogenesis) post-MI by processing ECM and non-ECM substrates. For example, we have previously demonstrated that MMP-7 regulates arrythmogenesis post-MI by cleavage of the gap junction connexin-43. As a consequence, MMP-7 null mice had improved non-rupture survival rates and displayed favorable electrical conduction and increased levels of connexin-43 post-MI.30 In Table 1, we have listed a sampling of non-collagen ECM and non-ECM substrates regulated by MMPs.
Table 1.
Examples of Non-collagen ECM and non-ECM substrates regulated by MMPs 30, 81–98
| Substrate | MMP | Regulated Activity |
|---|---|---|
| Aggrecan | -3, -8, -14 | Inflammation |
| CD44 (hyaluronan receptor) | -14 | Cell Migration |
| Complement C1q | -1, -2, -3, -9 | Neutrophil superoxide production |
| Connexin 43 | -7 | Myocyte Electrical Conduction |
| Fibrinogen | -2, -3, -7, -9, -14 | Myocyte Contractility |
| Fibronectin | -7, -9 | Gene Expression |
| Fibroblast Growth Factor Receptor 1 | -2 | Mitogenesis and Angiogenesis |
| Galectin-3 | -2, -9 | Cell Proliferation and Phagocytosis |
| Interleukin-1β | -1, -2, -3, -9 | Inflammation |
| Insulin-like Growth Factor Binding Protein-3 | -1, -2, -3 | Cell Proliferation |
| Latent TGF-β1, -β2, -β3 | -2, -9 | Cell Differentiation and Angiogenesis |
| Laminin | -2, -3, -9, -14 | Cell Migration |
| Monocyte Chemotactic Proteins-1, -2, -4 | -1, -3 | Cell Chemotaxis |
| Monocyte Chemotactic Protein-3 | -2, -14 | Cell Chemotaxis |
| Myosin Light Chain 1 | -2 | Contractile Dysfunction |
| Osteopontin | -2, -3, -7, -9 | Cell Adhesion |
| Serpina 1d | -9 | Protease Inhibition |
| Tenascin-C | -7, -9 | MMP Expression |
| Thrombospondin-1 | -9 | Angiogenesis |
| Tumor Necrosis Factor-α | -1, -2, -3, -7, -9 | Inflammation |
| Troponin-I | -2 | Cardiac Mechanical Function |
2. MMPs and TIMPs post-MI
Cytokines, chemokines, growth factors and bioactive peptides can regulate MMP and TIMP expression and activation.31 For example, in an autocrine manner, brain natriuretic peptide produced by cardiac fibroblasts induces MMP-1, -2, -3, -14, and TIMP-2 expression.32 At the transcriptional level, pro-inflammatory cytokines, tumor necrosis factor alpha (TNFα) and interleukin 1β (IL-1β), present during remodeling induce MMP-1, -3, -7, -9, -13 and TIMPs -1, -2.31 In the MI setting, transforming growth factor beta (TGFβ1) inhibits MMP-1 expression and attenuates MMP-1-induced myocyte injury and death, while matricellular proteins such as thrombospondin-1 can regulate activation of latent MMP-2 and MMP-9.33, 34
After chronic permanent occlusion in humans and animal models, the levels of MMPs -1, -2, -3, -7, -8, -9, -12, -13, -14 as well as all 4 TIMPs have been reported to respond to cardiac tissue repair stimuli.35 A literature search reveals that MMP-2 and MMP-9 are the most highly studied MMPs in cardiovascular research. However, this is due to the technical fact that historically zymograms were the first way to observe MMP activity, and gelatin zymography was the easiest method to perform. Therefore, there is not necessarily a direct correlation between publication numbers and particular MMP biological importance. The roles of MMPs -10, -11, and -15 through -28 have not been evaluated post-MI (Table 2). Because most of these newer MMPs have commercially available antibodies, we should expect to see an increase in the publications focused on these additional MMPs.
Table 2.
MMP and TIMP expression in cell types involved in the post-MI LV response.
| Cell types involved post-MI | MMPs expressed | MMPs not yet studied |
|---|---|---|
| Cardiac myocytes | MMP-1, -2, -3, -7, -9, -1438, 48, 49, 30, 48, 64, 99 TIMP-1, -448, 67 | MMPs -10, -11, -15, -16, -17, -18, -19, -20, -21, -22, -23, -24, -26, -27 and -28 |
| Cardiac fibroblasts | MMP-1, -2, -3, -9, -13, -1439, 45, 49 TIMP-1, -239, 45 | |
| Myofibroblasts | MMP-2, -14100 | |
| Neutrophils | MMP-8, -9 59, 101, 102 | |
| Macrophages | MMP-1, -3, -7, -8, -9 -1230, 52, 58 | |
| Endothelial Cells | MMP-2, -949 | |
| VSMCS | MMP-2, -949 |
A. Cell specific expression of MMPs post-MI
MMP-1 (57/52 kDa latent and 49/37 kDa active forms) was first identified as fibroblast collagenase. MMP-1 is abundant in normal LV and is expressed by fibroblast-like cells and endothelial cells.36, 37 Karl Weber’s team was the first to report increased MMP-1 levels in the rat LV post-MI.38 MMP-1 activity within the infarct region begins at day 2, reaches a maximum at day 7 and begins to decline through day 14 when the activity returns control levels. Fibroblast-like cells express MMP-1 mRNA beginning at day 7 post-MI suggesting that MMP-1 transcription could be triggered by depletion of latent MMP-1.39, 40 This data was somewhat controversial at the time, as many groups had reported that adult rodents do not express MMP-1.41 Carlos Lopez-Otin’s group has since demonstrated that rodents express slightly different MMP-1 genes compared to humans. These two genes, termed McoI-a and Mcol-b, are homologous to human MMP-1 and are primarily expressed during murine embryogenesis.42 In humans, MMP-1 protein decreases in LV tissue extracts from patients with dilated cardiomyopathy.43
MMP-2 (72 kDa latent and 66/62 kDa active forms) was first named gelatinase a. MMP-2 is detected in normal murine LV extracts and is expressed by cardiomyocytes, endothelial cells, vascular smooth muscle cells, and fibroblasts.6, 44, 45 Immunolocalization of MMP-2 within the cell shows MMP-2 within the sarcolemmal membrane and nucleus of cardiomyocytes.46 In a transgenic mouse model where the MMP-2 promoter is fused to the β-galactosidase reporter, MMP-2 expression is present at day 1 post-MI and continues through day 7 in fibroblasts.47 Increased MMP-2 activity is detected at day 2, peaks at day 7 and declines to control levels by day 14 in the rat LV infarct 39 Similar to the MMP-2 profile during development, in the rabbit MI model, MMP-2 protein increases modestly in response to MI.48 MMP-2 is constitutively detected at high levels in normal and control sham LV, which suggests a homeostatic role for MMP-2 in the heart.37, 43 In 1 to 6-day old infarct tissue extracted from humans, MMP-2 is detected in myocytes, fibroblasts, endothelial, smooth muscle, and inflammatory cells.49 In human cardiomyopathy LV extracts, no differences in MMP-2 levels are detected when compared to normal extracts.43 While MMP-2 levels show a comparatively modest change in the post-MI setting, exciting work by Schulze and colleagues has revealed novel intracellular roles for MMP-2. This team has identified myosin light chain and troponin I as intracellular MMP-2 substrates.50 Therefore, quantitation of total levels may not be as informative as localization studies for MMP-2.
MMP-3 (59/57 kDa latent and 48 kDa active forms) was first named stromelysin-1. Cardiac fibroblasts, cardiomyocytes and macrophages express MMP-3, and in normal tissues including human and murine LV extracts MMP-3 (57 kDa and 48 kDa) expression is detected.44, 45, 51, 52 In the experimental rabbit MI model, MMP-3 enzymatic activity detected in cardiomyocytes begins at day 2, peaks at day 4, and declines by day 14.48 In human LV cardiomyopathic extracts, MMP-3 increases in dilated LV when compared to normal controls.43
MMP-7 (28 kDa latent and 19 kDa active forms) was first named matrilysin and is the smallest MMP member. MMP-7 is found in murine LV infarcts and is expressed by cardiomyocytes and macrophages.30, 44 The role of MMP-7 has been studied at 7 days and 8 weeks post-MI. In mouse studies of 7 day MI, expression and activity of MMP-7 increases in cardiomyocytes found in the remote tissue and within macrophages that have infiltrated the infarct region.30 In sheep, MMP-7 expression decreases in the remote and infarct regions 8 weeks after MI, and like the mouse MI model, MMP-7 expression is robust in remote regions.53
MMP-8 (64 kDa latent and 58 kDa active forms) was originally named neutrophil collagenase, leading to the misconception that MMP-8 was only expressed in neutrophils. Macrophages express MMP-8, and low levels of MMP-8 mRNA and protein (58 kDa) are found in control and remote murine and porcine myocardium.44, 52, 54, 55 Post-MI, an increase in MMP-8 mRNA is detected at 6 hours and peaks after 12 hours in the infarcted myocardium.55 MMP-8 protein expression increases at 2 weeks post-ligation and persists through week 16, suggesting that MMP-8 is actively participating in late remodeling events.56 In the sheep MI, MMP-8 expression is conserved in the remote areas but peaks in the infarct areas at 8 weeks post-MI.53 Interestingly, in humans, elevated MMP-8 mRNA levels are detected in dilated cardiomyopathy LV samples, and increased activity is found in 1–6 day post-MI ruptured ventricles indicating early activation of MMP-8 may contribute excessive collagenolysis that results in rupture.49, 57
MMP-9 (105 kDa in mouse and 92kDa in human latent and 95 kDa in mouse and 88 kDa in human active forms) was first known as neutrophil gelatinase or gelatinase b. While highly associated with neutrophil and macrophages, MMP-9 is also expressed in cardiac myocytes, fibroblasts, vascular smooth muscle cells and endothelial cells. The 92 kDa form of MMP-9 can be detected in normal LV septum and free wall extracts, albeit at much lower levels than post-MI.44, 45, 58, 59 In the rat MI model, MMP-9 mRNA increases as early as 6 hours after the ligation and peaks after 24 hours.55 MMP-9 promoter transcripts with β-galactosidase reporter show MMP-9 promoter activity at day 3 post-MI and peaks at day 7 within inflammatory cells.47 In the porcine MI model, MMP-9 activity increases 3 hours after coronary occlusion in the infarct region and remote-infarct border.60 In the rabbit model, increases in macrophage-derived MMP-9 are detected as early as 24 hours post-MI.48 Robust MMP-9 activity is found at days 1 through 4 in infarct and border infarct regions of murine LV that correspond with neutrophil and macrophage infiltration, respectively.40, 58 Moreover, in the rat, MMP-9 activity rises abruptly for the first week after the occlusion and declines by day 14 suggesting MMP-9 enzymatic activities predominate early responses post-MI.39, 56 In line with these findings, increases in MMP-9 are also detected in ruptured human ventricles, and MMP-9 null mice show attenuated remodeling at early time points.49, 61
MMP-12 (54 kDa latent and 45 kDa active forms) was originally found in conditioned medium of mouse peritoneal macrophages and was named macrophage elastase.62 In normal LV tissue extracts from young, middle age and adult mice, MMP-12 (54 kDa) protein is detected; however, in human LV samples of patients with dilated cardiomyopathy the levels of MMP-12 were below detection, suggesting that MMP-12 may serve a protective effect in the myocardium.44, 52
MMP-13 (60 kDa latent and 48 kDa active forms) was first identified as collagenase 3. Cardiac fibroblasts express MMP-13 and is found in normal LV tissue extracts (60 kDa and 48 kDa).44, 45 MMP-13 mRNA remains stable following MI through week 16 in the rat; however, a significant increase in MMP-13 enzyme activity is detected after week 1, peaks at week 2 and returns to baseline at week 8. 25 In the sheep model, MMP-13 levels are stable in the remote areas, but high MMP-13 activity is observed in the infarct 8 weeks after the occlusion.53, 56
MMP-14 (65 kDa latent and 54/45/40 kDa active forms) is also known as MT1-MMP. MMP-14 is expressed by cardiac myocytes and cardiac fibroblasts and all 4 forms have been identified in normal human and murine LV (65, 54, 45, and 40 kDa).44, 45, 63, 64 Like the proposed MMP-8 role in later remodeling events, a dramatic increase in MMP-14 (MT1-MMP) is detected in the rat during week 16 post-MI.56 In the sheep MI model, MMP-14 is found in remote and transition regions; however, noticeable increases are observed in infarct areas at 8 weeks post-MI.53 In humans, MMP-14 mRNA is present in dilated cardiomyopathy.57
B. Cell specific expression of TIMPs post-MI
TIMP-1 (29 kDa) co-localizes with MMP-1 in normal myocardium and is expressed by cardiac myocytes and fibroblasts.37, 44,48, 65 Unlike the increased expression and activity observed with MMPs, a general decrease in TIMP protein levels are observed acutely post-MI. In the rat infarct region, TIMP-1 mRNA increases within fibroblasts 6 hours following ligation and declines after 2 days with no detectable change in remote or sham areas.39 Interestingly, TIMP-1 protein is reduced for the first week after ischemia and returns to control levels by day 7 in the rabbit.48 In the sheep, TIMP-1 levels are lower in the transition areas compared to the controls, and the levels are below detection within the infarct.53 In agreement with these findings, in tissues from patients with ischemic cardiomyopathy TIMP-1 protein was also found reduced.66 Unexpectedly, in human 1–6 day-old ruptured ventricles, TIMP-1 protein levels are found elevated compared to control non-ruptured MI ventricles.49
Cardiac fibroblasts are a rich source of TIMP-2 (28 kDa) in normal myocadium.44, 45 TIMP-2 protein levels in the rat do not change in response to MI for the first week, but TIMP-2 protein shows dual peaks at weeks 2 and 16 after MI.56 In the sheep MI model, TIMP-2 protein levels remain similar to control in the transition areas; however, TIMP-2 levels in the infarct fall below detection levels.53 In humans, TIMP-2 mRNA and protein levels remain unchanged in the failing human ventricle.66
TIMP-3 (24 kDa) is detected in normal murine LV extracts.44 In sheep, TIMP-3 protein is found at significantly lower levels in the infarct regions at 8 weeks post-MI than in control or remote regions. 53 Similarly, in failing human hearts resulting from ischemic cardiomyopathy, TIMP-3 levels decrease in ventricular tissue.66 TIMP-3 deletion accelerates cardiac remodeling post-MI by promoting matrix degradation (MMP-9 levels were higher) and inflammatory cytokine expression (TNFα specifically).67 This suggests that TIMP-3 over-expression may prove beneficial in the LV remodeling process.
TIMP-4 (28 kDa glycosylated and 24 kDa forms) is expressed by cardiomyocytes and is abundant in normal, human and murine myocardium.44, 67 A common misconception is that TIMP-4 is a cardiac-specific TIMP. However, TIMP-4 is also highly expressed in brain, ovary, and skeletal muscle.68 After ischemic cardiomyopathy, TIMP-4 transcript levels remain stable in humans.66 In the rat MI model, TIMP-4 mRNA remains unchanged, but TIMP-4 protein levels decrease at one and eight weeks post-MI.56 Similarly in the sheep MI model, TIMP-4 protein is reduced at 8 weeks post-MI in the left ventricle infarct regions.53
3. Permanent occlusion MI model versus ischemia/reperfusion MI model
In this section, we will discuss factors in common and distinct between the permanent occlusion MI and reperfused MI models to provide mechanistic insight into the differential MMP and TIMP expression in these two settings. In Table 3 we have listed similarities and differences between permanent occlusion and ischemia/reperfusion MI models. Of note, the majority of studies use rodent models and the evaluation of MMPs and TIMPs in humans and large animal models remains a fertile field.
Table 3.
Similarities and differences between permanent occlusion and ischemia/reperfusion MI models. 56, 66, 69–71, 73, 75, 103
| Permanent Occlusion | Ischemia/Reperfusion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Infarct Size at 24 hours | ~30% | ~30% | ||||||||||
| (1) Ejection velocity and filling velocity | Continuous suppression | Returns to level of sham in 14 days | ||||||||||
| (1) Ejection Fraction at 14 days | 26. ± 3.1% | 24.1 ± 4.0% | ||||||||||
| (1) Mortality rate at 28 days | 35.6% | 5.8% | ||||||||||
| mRNA levels | 1h | 3h | d1 | d7 | 1h | 3h | d1 | d7 | ||||
| (2) IFN-γ | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ||||
| (2) IL-1β | ↑ | ↑ ↑ | ↓ | ↑ ↑ | ↑ | ↑ | ↓ | ↓ | ||||
| (2) IL-2 | ↑ ↑ | ↑ | ↑ ↑ | ↑ ↑ | ↑ | ↑ | ↓ | ↑ | ||||
| (2) IL-6 | ↑ | ↑ ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ||||
| (2) TGF-β1 | ↑↑ | ↑ | ↓ | ↑ ↑ | ↓ | ↑ | ↓ | ↑ | ||||
| (2) TNF-α | ↑ | ↑ | ↓ | ↑ ↑ | ↑ | ↑ | ↓ | ↑ | ||||
| Protein levels | d1 | d3 | d7 | d1 | d3 | d7 | ||||||
| (2) MMP-1 (54 kDa) | ↓ | ↑ | ↑ ↑ | ↓ | ↑ | ↑ | ||||||
| (2) MMP-2 (65 kDa) | ↓ | ↑ ↑ | ↑ ↑ | ↓ | ↑ | ↑ | ||||||
| (2) MMP-9 (92 kDa) | ↑ ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ||||||
| d7 | d14 | d35 | 3h | |||||||||
| (2) TIMP-1* | ↔ | ↑ | ↔ | (3) | nd | |||||||
| (2) TIMP-2* | ↔ | ↑ | ↑ | (3) | ↑ | |||||||
| TIMP-3 | nd | (4) | ↓ | |||||||||
| (2) TIMP-4* | ↔ | ↓ | ↔ | (3) | ↓ | |||||||
| (4) | ↔ | |||||||||||
(1) paired study in mouse; (2) rat; (3) pig; (4) dog; h-hours; d- days; w-week; ↑-increased expression; ↑↑ increased compared with ischemia/reperfusion; ↓-decreased expression; * independent studies; ↔-no change; nd- not detected
In ischemia/reperfusion MI models, MMP and TIMP profiles diverge from the profiles observed with permanent occlusion and these differences may help to explain the ultimate benefit of reperfusion on myocardial wound repair and LV function.69 Alterations in ECM expression and deposition as well as modifications to the inflammatory response kinetics are observed after reperfusion and contribute to the generation of a more organized scar within the infarct region.
A. MI size, mortality rate and LV remodeling
In comparing permanent occlusion to reperfusion, the Entman laboratory showed that for mice, when infarct sizes are similar (30% at 24 hours), permanent occlusion results in more infarct expansion.69 Mortality rate in the reperfused group was about 30% lower than total occlusion at 28-day post-MI. LV ejection and filling velocities returned to control levels after 2 weeks of reperfusion, while values remained suppressed with permanent occlusion.69, 70 Reperfusion reduces the degree of remodeling, as the gradual LV wall thinning observed with permanent ligation is attenuated with reperfusion, with a benefit seen beginning at day 4 post-MI and persisting through day 28.69, 70 Moreover, LV dilation significantly decreases in reperfusion models and the percent lumen surface is lower at days 4, 14 and 28 post-MI compared with permanent occlusion MI.70 In both models, the vasculature within the infarct region begins to regress 48 hours after ligation, but reperfusion stimulates more robust endothelial cell proliferation resulting in augmented angiogenesis compared to permanent occlusion.70
B. Inflammatory response and cellular infiltration
With respect to inflammation and cellular infiltration, similarities and differences are evident between permanent occlusion and reperfusion. The messenger RNA levels of IL-6 and TGFβ1 are reduced early after reperfusion, and although the levels return to baseline in both models after 24 hours, IL-1β, TNFα, and TGFβ1 increase again by day 7 in the permanent occlusion setting.71 Although cytokine levels are generally decreased with reperfusion, inflammatory cell influx is paradoxically increased. Reperfusion heralds in a much earlier inflammatory cell response, bringing in both neutrophils and macrophages simultaneously. In contrast, permanent occlusion results in an orchestrated influx of neutrophils followed by macrophages. Compared to permanent occlusion, reperfusion resulted in higher neutrophil and macrophages numbers, a prolonged presence of neutrophils, and increased myocyte remnant clearance.70 In line with this finding, in the canine MI model of reperfusion, neutrophil influx is 80% increased compared to the canine permanent occlusion model.72 In addition to differences in inflammatory cells, the percent of myofibroblasts in the permanent occlusion model was significantly higher at day 7 compared with the reperfused infarct.70
C. MMP, TIMP and ECM differences
Because reperfusion increases cell infiltration kinetics post-MI, MMP and TIMP levels are likely altered between the two models. Carlyle and colleagues used the coronary artery ligation model in the rat, with and without delayed reperfusion following 150 min occlusion, to quantify MMP activity up to 7 days post-MI.73 MMP-1, -2, and -9 were measured in the infarct region, and all 3 were elevated in the permanent occlusion model. Delayed reperfusion attenuated levels of each. In pig models of in vivo and ex-vivo ischemia/reperfusion, MMP-1 activity is markedly increased in the infarct regions compared to non-infarct controls.38, 54 In both permanent occlusion and reperfusion models, no apparent changes in MMP-2 activity are observed early after reperfusion in the porcine, rat or canine MI models.59, 74, 75 Beginning at day 7, MMP-2 activity is attenuated with reperfusion and is approximately 60% lower than levels in the permanent occlusion model. MMP-9 activity, which increases up to 100-fold with permanent occlusion, is reduced by 55% and 84% in the infarct region of reperfused myocardium at 24 and 48 hours, respectively, even though the neutrophil numbers increase with reperfusion.59, 73 In agreement with these findings, MMP-9 activity in a canine model of reperfusion increases in the infarct compared with remote non-infarct controls and sham LV.76 During moderate ischemia and reperfusion (90 min ischemia/90 min reperfusion) in pigs, MMP-9 increases 70% in the ischemic regions.74, 75
After 90 min ischemia followed by 120 min reperfusion in a canine model, Sawicki and colleagues found that TIMP-3 decreases in the infarct zone but TIMP-4 levels were unchanged.75 During moderate ischemia followed by reperfusion, TIMP-1 was not detected and TIMP-2 and TIMP-3 were present in both ischemic and non-ischemic tissue.74
In addition to the improved clearance of necrotic cardiomyocytes observed with reperfusion, earlier collagen deposition is also seen in the reperfused mouse MI model.70 In the rat infarct, reperfusion accelerates fibronectin expression compared with non-reperfused models.73 After 24 hours of reperfusion, fibronectin protein levels double in reperfused infarct. In ex vivo porcine hearts, reperfused 2 hours after occlusion, fibronectin and osteopontin levels both increase in the infarct region.54 Together, these data indicate that fibronectin expression responds before changes to collagen expression are detected, suggesting that fibronectin may be an earlier marker of remodeling.
4. Therapeutic treatments that regulate MMP and TIMP levels post-MI
We will focus on current therapeutic strategies and how pharmacologic agents such as angiotensin converting enzyme and β adrenergic receptor inhibitors alter MMP and TIMP levels.
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin II type I receptor blockers (ARBs), as well as β-adrenergic receptor (βAR) blockers, are common therapeutic strategies post-MI. Although the mechanisms of action are not entirely clear, each used as a therapeutic treatment can reduce MMP expression and activity. MMP-2 activity in human LV cardiomyopathy extracts is reduced in vitro by ACE inhibitors in a dose dependent manner.77 In the rat MI model, ACEi (ramipril) decreases MMP-2 activity to control levels, but increases MMP-1 activity.78 Similarly, in a rat congestive heart failure model, ACEi (trandolapril) reduced MMP-2 activity but induced TIMP-2 levels.79 Yamamoto and colleagues have shown, in a hamster MI model, that ACE inhibitors reduce MMP-9 activity post-MI by directly binding the MMP-9 active site.80 Ramipril also reduces TIMP-2 protein levels but increases TIMP-4 levels 80% above control.78 In a canine ischemia/reperfusion model, administration of valsartan, an ARB, had no significant effect on MMP activity. However, TIMP-3 protein significantly increased in the infarct region.75β-AR blockers also alter MMP expression and activity. In the rat MI model, propranolol significantly increased both MMP-8 and MMP-9 mRNA levels at 6, 12, 24, and 72 h. At the protein level, propanolol significantly reduced the enzymatic activity of MMP-2 after 6 hours and MMP-9 after 12 and 24 hours.55 Propanolol, therefore, may mimic some of the beneficial effects seen with reperfusion.
5. Future Directions & Conclusions
While a lot of information on MMP and TIMP roles in the post-MI setting has been gathered over the past 15 years, additional studies are needed to further clarify the role of MMPs and TIMPs in the post-MI setting (Table 4). For one, we still need to know the expression levels and roles of MMPs not yet studied. Of the 25 MMPs identified to date, the role of 16 MMPs post-MI remains to be investigated. Additionally, we need to know the full complement of MMPs and TIMPs expressed for each cell type in the post-MI LV. We need to distinguish between data that is absent and negative data indicating a lack of expression. A combination of in vivo, ex vivo, and in vitro tools will need to be utilized to reveal the MMP and TIMP profiles for each cell present during cardiac repair. We also need to know what the aggregate functions for each MMP and TIMP are. In addition, it will be important to know whether the MMP or TIMP is involved in protein processing or modulating cellular processes during remodeling.
Table 4.
Future Directions
We still need to know:
|
A better understanding of the diverse directions MMP function can take will help develop novel strategies to target MMP-regulated function by deviating from the traditional inhibition of the catalytic domain and focusing on other enzyme domains (e.g., the hemopexin or pro-domains). Along these lines, more details on the spatiotemporal expression are needed. As we have seen with multiple MMPs, it is important to measure levels at multiple times and places in order to confirm known biological mechanisms and uncover novel functions. As was illustrated by Rick Schulz and his team, MMPs can be found in surprising locations including the nuclei of cardiac myocytes. Targeting nuclear MMPs and substrates can potentially help us develop ways to correct DNA repair errors.46 Interestingly, more intracellular MMP substrates are being identified, and we are increasingly becoming aware of the potential for MMPs to process membrane proteins and other non-traditional substrates. We will need to incorporate these ideas into strategies to develop new inhibitors.
Finally, we need to catalogue the full list of MMP substrates. In order to develop a successful therapeutic treatment, an inclusive list of targets and potential pathways must be provided to understand the global effect of the treatment strategy. In summary, we reviewed the current literature on MMP and TIMP levels, localization, and roles in the post-MI setting. This evaluation reveals new directions that must be taken in future research in order to design more effective MMP inhibition strategies.
Acknowledgments
The authors acknowledge grant support from the American Heart Association POST2150178 and 0855119F (RZ and MLL) and from NIH HL75360, the Max and Minnie Tomerlin Voelcker Fund, and the Morrison Trust (MLL).
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 4.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 5.Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242(1):201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- 6.Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152(2):189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiki M, Koshikawa N, Yana I. Role of pericellular proteolysis by membrane-type 1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Metastasis Rev. 2003;22(2–3):129–143. doi: 10.1023/a:1023087113214. [DOI] [PubMed] [Google Scholar]
- 8.Lauer-Fields JL, Whitehead JK, Li S, Hammer RP, Brew K, Fields GB. Selective modulation of matrix metalloproteinase 9 (MMP-9) functions via exosite inhibition. J Biol Chem. 2008;283(29):20087–20095. doi: 10.1074/jbc.M801438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeblad M, Werb Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat Rev Cancer. 2002;2(3):163–176. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 10.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 11.Keeling J, Herrera GA. Human matrix metalloproteinases: characteristics and pathologic role in altering mesangial homeostasis. Microsc Res Tech. 2008;71(5):371–379. doi: 10.1002/jemt.20565. [DOI] [PubMed] [Google Scholar]
- 12.Tochowicz A, Maskos K, Huber R, Oltenfreiter R, Dive V, Yiotakis A, Zanda M, Pourmotabbed T, Bode W, Goettig P. Crystal structures of MMP-9 complexes with five inhibitors: contribution of the flexible Arg424 side-chain to selectivity. J Mol Biol. 2007;371(4):989–1006. doi: 10.1016/j.jmb.2007.05.068. [DOI] [PubMed] [Google Scholar]
- 13.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22(10):571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 14.Fry SA, Van den Steen PE, Royle L, Wormald MR, Leathem AJ, Opdenakker G, McDonnell JM, Dwek RA, Rudd PM. Cancer-associated glycoforms of gelatinase B exhibit a decreased level of binding to galectin-3. Biochemistry. 2006;45(51):15249–15258. doi: 10.1021/bi061254l. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy SM, Bove PF, Matthews DE, Akaike T, van der Vliet A. Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry. 2008;47(21):5832–5840. doi: 10.1021/bi702496v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corthorn J, Rey S, Chacon C, Valdes G. Spatio-temporal expression of MMP-2, MMP-9 and tissue kallikrein in uteroplacental units of the pregnant guinea-pig (Cavia porcellus) Reprod Biol Endocrinol. 2007;5:27. doi: 10.1186/1477-7827-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276(31):29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 18.Fridman R, Toth M, Pena D, Mobashery S. Activation of Progelatinase B (MMP-9) by Gelatinase A (MMP-2) Cancer research. 1995;55:2548–2555. [PubMed] [Google Scholar]
- 19.Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochemical and biophysical research communications. 2003;308(2):386–395. doi: 10.1016/s0006-291x(03)01405-0. [DOI] [PubMed] [Google Scholar]
- 20.Dreier R, Grassel S, Fuchs S, Schaumburger J, Bruckner P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Experimental cell research. 2004;297(2):303–312. doi: 10.1016/j.yexcr.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Knauper V, Smith B, Lopez-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur J Biochem. 1997;248:369–373. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 22.Alexander SM, Jackson KJ, Bushnell KM, McGuire PG. Spatial and temporal expression of the 72-kDa type IV collagenase (MMP-2) correlates with development and differentiation of valves in the embryonic avian heart. Dev Dyn. 1997;209(3):261–268. doi: 10.1002/(SICI)1097-0177(199707)209:3<261::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Itoh Y, Ito N, Nagase H, Evans RD, Bird SA, Seiki M. Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol Biol Cell. 2006;17(12):5390–5399. doi: 10.1091/mbc.E06-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbel M, Boichot E, Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res. 2000;33(7):749–754. doi: 10.1590/s0100-879x2000000700004. [DOI] [PubMed] [Google Scholar]
- 25.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 26.Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217(3):643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288(2):H461–468. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 28.Ratajska A, Cleutjens JP. Embryogenesis of the rat heart: the expression of collagenases. Basic Res Cardiol. 2002;97(3):189–197. doi: 10.1007/s003950200011. [DOI] [PubMed] [Google Scholar]
- 29.Brauer PR, Cai DH. Expression of tissue inhibitor of metalloproteinases (TIMPs) during early cardiac development. Mech Dev. 2002;113(2):175–179. doi: 10.1016/s0925-4773(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113(25):2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 31.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006;69(3):666–676. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC., Jr Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91(12):1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Li D, Saldeen T, Mehta JL. TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2003;284(5):H1612–1617. doi: 10.1152/ajpheart.00992.2002. [DOI] [PubMed] [Google Scholar]
- 34.Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 2007;5(1):21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 35.Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: Causes and consequences. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi SC, Cleutjens JP. Myocardial collagenase: purification and structural characterization. Can J Cardiol. 1996;12(2):165–171. [PubMed] [Google Scholar]
- 37.Tyagi SC, Kumar SG, Banks J, Fortson W. Co-expression of tissue inhibitor and matrix metalloproteinase in myocardium. J Mol Cell Cardiol. 1995;27(10):2177–2189. doi: 10.1016/s0022-2828(95)91443-9. [DOI] [PubMed] [Google Scholar]
- 38.Danielsen CC, Wiggers H, Andersen HR. Increased amounts of collagenase and gelatinase in porcine myocardium following ischemia and reperfusion. J Mol Cell Cardiol. 1998;30(7):1431–1442. doi: 10.1006/jmcc.1998.0711. [DOI] [PubMed] [Google Scholar]
- 39.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27(6):1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 40.Tao ZY, Cavasin MA, Yang F, Liu YH, Yang XP. Temporal changes in matrix metalloproteinase expression and inflammatory response associated with cardiac rupture after myocardial infarction in mice. Life Sci. 2004;74(12):1561–1572. doi: 10.1016/j.lfs.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 41.Tsubota M, Sasano Y, Takahashi I, Kagayama M, Shimauchi H. Expression of MMP-8 and MMP-13 mRNAs in rat periodontium during tooth eruption. J Dent Res. 2002;81(10):673–678. doi: 10.1177/154405910208101004. [DOI] [PubMed] [Google Scholar]
- 42.Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, Quesada V, Bordallo J, Murphy G, Lopez-Otin C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276(13):10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- 43.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97(17):1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 44.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66(2):410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 45.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee R, Mingoia JT, Bruce JA, Austin JS, Stroud RE, Escobar GP, McClister DM, Jr, Allen CM, Alfonso-Jaume MA, Fini ME, Lovett DH, Spinale FG. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291(5):H2216–2228. doi: 10.1152/ajpheart.01343.2005. [DOI] [PubMed] [Google Scholar]
- 48.Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68(7):799–814. doi: 10.1016/s0024-3205(00)00982-6. [DOI] [PubMed] [Google Scholar]
- 49.van den Borne SW, Cleutjens JP, Hanemaaijer R, Creemers EE, Smits JF, Daemen MJ, Blankesteijn WM. Increased matrix metalloproteinase-8 and -9 activity in patients with infarct rupture after myocardial infarction. Cardiovasc Pathol. 2009;18(1):37–43. doi: 10.1016/j.carpath.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of Myosin Light Chain in Isolated Rat Hearts Subjected to Ischemia-Reperfusion Injury: A New Intracellular Target for Matrix Metalloproteinase-2. Circulation. 2005;112(4):544–552. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 51.Johnson LL, Bornemeier DA, Janowicz JA, Chen J, Pavlovsky AG, Ortwine DF. Effect of species differences on stromelysin-1 (MMP-3) inhibitor potency. An explanation of inhibitor selectivity using homology modeling and chimeric proteins. J Biol Chem. 1999;274(35):24881–24887. doi: 10.1074/jbc.274.35.24881. [DOI] [PubMed] [Google Scholar]
- 52.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130(2):147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107(22):2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 54.Kossmehl P, Schonberger J, Shakibaei M, Faramarzi S, Kurth E, Habighorst B, von Bauer R, Wehland M, Kreutz R, Infanger M, Schulze-Tanzil G, Paul M, Grimm D. Increase of fibronectin and osteopontin in porcine hearts following ischemia and reperfusion. J Mol Med. 2005;83(8):626–637. doi: 10.1007/s00109-005-0642-8. [DOI] [PubMed] [Google Scholar]
- 55.Deten A, Volz HC, Holzl A, Briest W, Zimmer HG. Effect of propranolol on cardiac cytokine expression after myocardial infarction in rats. Mol Cell Biochem. 2003;251(1–2):127–137. [PubMed] [Google Scholar]
- 56.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000;46(2):307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 57.Felkin LE, Birks EJ, George R, Wong S, Khaghani A, Yacoub MH, Barton PJ. A quantitative gene expression profile of matrix metalloproteinases (MMPS) and their inhibitors (TIMPS) in the myocardium of patients with deteriorating heart failure requiring left ventricular assist device support. J Heart Lung Transplant. 2006;25(12):1413–1419. doi: 10.1016/j.healun.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5(10):1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 59.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103(17):2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 60.Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, Baicu S, Mukherjee R, Manhaini M, Spinale FG. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol. 2001;281(3):H987–994. doi: 10.1152/ajpheart.2001.281.3.H987. [DOI] [PubMed] [Google Scholar]
- 61.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. American journal of physiology. 2006;290(1):H232–239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 62.Belaaouaj A, Shipley JM, Kobayashi DK, Zimonjic DB, Popescu N, Silverman GA, Shapiro SD. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem. 1995;270(24):14568–14575. doi: 10.1074/jbc.270.24.14568. [DOI] [PubMed] [Google Scholar]
- 63.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90(5):520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 64.Coker ML, Jolly JR, Joffs C, Etoh T, Holder JR, Bond BR, Spinale FG. Matrix metalloproteinase expression and activity in isolated myocytes after neurohormonal stimulation. Am J Physiol Heart Circ Physiol. 2001;281(2):H543–551. doi: 10.1152/ajpheart.2001.281.2.H543. [DOI] [PubMed] [Google Scholar]
- 65.Tyagi SC, Kumar S, Glover G. Induction of tissue inhibitor and matrix metalloproteinase by serum in human heart-derived fibroblast and endomyocardial endothelial cells. J Cell Biochem. 1995;58(3):360–371. doi: 10.1002/jcb.240580309. [DOI] [PubMed] [Google Scholar]
- 66.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98(17):1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 67.Tian H, Cimini M, Fedak PW, Altamentova S, Fazel S, Huang ML, Weisel RD, Li RK. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. Journal of molecular and cellular cardiology. 2007;43(6):733–743. doi: 10.1016/j.yjmcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Leco KJ, Apte SS, Taniguchi GT, Hawkes SP, Khokha R, Schultz GA, Edwards DR. Murine tissue inhibitor of metalloproteinase-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS letters. 1997;401:213–217. doi: 10.1016/s0014-5793(96)01474-3. [DOI] [PubMed] [Google Scholar]
- 69.Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Entman ML. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol. 1999;277(2 Pt 2):H660–668. doi: 10.1152/ajpheart.1999.277.2.H660. [DOI] [PubMed] [Google Scholar]
- 70.Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. non-reperfused murine myocardial infarction. Cardiovasc Pathol. 2006;15(2):83–90. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146(2):419–428. [PMC free article] [PubMed] [Google Scholar]
- 72.Chatelain P, Latour JG, Tran D, de Lorgeril M, Dupras G, Bourassa M. Neutrophil accumulation in experimental myocardial infarcts: relation with extent of injury and effect of reperfusion. Circulation. 1987;75(5):1083–1090. doi: 10.1161/01.cir.75.5.1083. [DOI] [PubMed] [Google Scholar]
- 73.Carlyle WC, Jacobson AW, Judd DL, Tian B, Chu C, Hauer KM, Hartman MM, McDonald KM. Delayed reperfusion alters matrix metalloproteinase activity and fibronectin mRNA expression in the infarct zone of the ligated rat heart. J Mol Cell Cardiol. 1997;29(9):2451–2463. doi: 10.1006/jmcc.1997.0482. [DOI] [PubMed] [Google Scholar]
- 74.Lu L, Gunja-Smith Z, Woessner JF, Ursell PC, Nissen T, Galardy RE, Xu Y, Zhu P, Schwartz GG. Matrix metalloproteinases and collagen ultrastructure in moderate myocardial ischemia and reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2000;279(2):H601–609. doi: 10.1152/ajpheart.2000.279.2.H601. [DOI] [PubMed] [Google Scholar]
- 75.Sawicki G, Menon V, Jugdutt BI. Improved balance between TIMP-3 and MMP-9 after regional myocardial ischemia-reperfusion during AT1 receptor blockade. J Card Fail. 2004;10(5):442–449. doi: 10.1016/j.cardfail.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-Dependent Mechanism of Neutrophil-Mediated Release and Activation of Matrix Metalloproteinase 9 in Myocardial Ischemia/Reperfusion. Circulation. 2001;103:2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 77.Reinhardt D, Sigusch HH, Hensse J, Tyagi SC, Korfer R, Figulla HR. Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart. 2002;88(5):525–530. doi: 10.1136/heart.88.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seeland U, Kouchi I, Zolk O, Itter G, Linz W, Bohm M. Effect of ramipril and furosemide treatment on interstitial remodeling in post-infarction heart failure rat hearts. J Mol Cell Cardiol. 2002;34(2):151–163. doi: 10.1006/jmcc.2001.1497. [DOI] [PubMed] [Google Scholar]
- 79.Fraccarollo D, Bauersachs J, Kellner M, Galuppo P, Ertl G. Cardioprotection by long-term ET(A) receptor blockade and ACE inhibition in rats with congestive heart failure: mono- versus combination therapy. Cardiovasc Res. 2002;54(1):85–94. doi: 10.1016/s0008-6363(01)00553-3. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto D, Takai S, Jin D, Inagaki S, Tanaka K, Miyazaki M. Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol. 2007;43(6):670–676. doi: 10.1016/j.yjmcc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Buttner FH, Hughes CE, Margerie D, Lichte A, Tschesche H, Caterson B, Bartnik E. Membrane type 1 matrix metalloproteinase (MT1-MMP) cleaves the recombinant aggrecan substrate rAgg1mut at the ‘aggrecanase’ and the MMP sites. Characterization of MT1-MMP catabolic activities on the interglobular domain of aggrecan. Biochem J. 1998;333 ( Pt 1):159–165. doi: 10.1042/bj3330159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bini A, Wu D, Schnuer J, Kudryk BJ. Characterization of stromelysin 1 (MMP-3), matrilysin (MMP-7), and membrane type 1 matrix metalloproteinase (MT1-MMP) derived fibrin(ogen) fragments D-dimer and D-like monomer: NH2-terminal sequences of late-stage digest fragments. Biochemistry. 1999;38(42):13928–13936. doi: 10.1021/bi991096g. [DOI] [PubMed] [Google Scholar]
- 83.Boyd JH, Chau EH, Tokunanga C, Bateman RM, Haljan G, Davani EY, Wang Y, Walley KR. Fibrinogen decreases cardiomyocyte contractility through an ICAM-1-dependent mechanism. Crit Care. 2008;12(1):R2. doi: 10.1186/cc6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. Proteomic Analysis Identifies In vivo Candidate Matrix Metalloproteinase-9 Substrates in the Left Ventricle Post-Myocardial Infarction. Proteomics. doi: 10.1002/pmic.200900587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiao YA, Zamilpa R, Lopez EF, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. In vivo Matrix Metalloproteinase-7 Substrates Identified in the Left Ventricle Post-Myocardial Infarction Using Proteomics. J Proteome Res. doi: 10.1021/pr100147r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161(7):3340–3346. [PubMed] [Google Scholar]
- 87.Rajah R, Nunn SE, Herrick DJ, Grunstein MM, Cohen P. Leukotriene D4 induces MMP-1, which functions as an IGFBP protease in human airway smooth muscle cells. Am J Physiol. 1996;271(6 Pt 1):L1014–1022. doi: 10.1152/ajplung.1996.271.6.L1014. [DOI] [PubMed] [Google Scholar]
- 88.Fowlkes JL, Enghild JJ, Suzuki K, Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269(41):25742–25746. [PubMed] [Google Scholar]
- 89.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- 90.Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63(9):2292–2299. [PubMed] [Google Scholar]
- 91.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100(4):1160–1167. [PubMed] [Google Scholar]
- 92.Christensen B, Schack L, Klaning E, Sorensen ES. Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D. J Biol Chem. 285(11):7929–7937. doi: 10.1074/jbc.M109.075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370(6490):555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 94.Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation. 2005;112(4):544–552. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 95.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106(12):1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- 96.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93(14):7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruiz S, Henschen-Edman AH, Nagase H, Tenner AJ. Digestion of C1q collagen-like domain with MMPs-1,-2,-3, and -9 further defines the sequence involved in the stimulation of neutrophil superoxide production. Journal of leukocyte biology. 1999;66(3):416–422. doi: 10.1002/jlb.66.3.416. [DOI] [PubMed] [Google Scholar]
- 99.Nie R, Xie S, Du B, Liu X, Deng B, Wang J. Extracellular matrix metalloproteinase inducer (EMMPRIN) is increased in human left ventricle after acute myocardial infarction. Arch Med Res. 2009;40(7):605–611. doi: 10.1016/j.arcmed.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 100.Riches K, Morley ME, Turner NA, O’Regan DJ, Ball SG, Peers C, Porter KE. Chronic hypoxia inhibits MMP-2 activation and cellular invasion in human cardiac myofibroblasts. J Mol Cell Cardiol. 2009;47(3):391–399. doi: 10.1016/j.yjmcc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawakami R, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Nakagawa Y, Nakanishi M, Tanimoto K, Usami S, Yasuno S, Kinoshita H, Chusho H, Tamura N, Ogawa Y, Nakao K. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation. 2004;110(21):3306–3312. doi: 10.1161/01.CIR.0000147829.78357.C5. [DOI] [PubMed] [Google Scholar]
- 102.Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, Grau E, Mao J, Sathe GM, Ohlstein EH, Yue TL. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res. 2002;54(3):549–558. doi: 10.1016/s0008-6363(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 103.Lalu MM, Pasini E, Schulze CJ, Ferrari-Vivaldi M, Ferrari-Vivaldi G, Bachetti T, Schulz R. Ischaemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J. 2005;26(1):27–35. doi: 10.1093/eurheartj/ehi007. [DOI] [PubMed] [Google Scholar]