Abstract
Age-related macular degeneration (AMD) can be classified into two main categories: the atrophic, dry form and the exudative, wet form. The crucial difference between dry and wet AMD is the development of choroidal neovascularization in wet AMD. One fundamental cause of the neovascularization is the increased expression of VEGF (vascular endothelial growth factor) in retinal pigment epithelial cells. Progression of AMD is linked to augmentation of cellular stress, for example, oxidative stress, proteotoxic stress, inflammation and hypoxia. All these conditions can trigger stress in endoplasmic reticulum (ER), which maintains protein quality control in cells. ER stress induces the unfolded protein response (UPR) via IRE1 (inositol-requiring protein-1), PERK (protein kinase RNA-like ER kinase) and ATF6 (activating transcription factor-6) transducers. UPR signaling is a double-edged sword, that is, it can restore cellular homeostasis as far as possible, but ultimately may lead to chronic, overwhelming stress that can cause apoptotic cell death. Interestingly, ER stress is a well-known inducer of angiogenesis in cancer. Moreover, stress conditions associated with the progress of AMD can induce the expression of VEGF. We discuss the role of ER stress in the regulation of neovascularization and the conversion of dry AMD to its wet, detrimental counterpart.
INTRODUCTION
Age-related macular degeneration (AMD) can cause a progressive loss of central vision in elderly individuals. The macula region at the retina contains a dense layer of photoreceptors that are metabolically supplied by retinal pigment epithelial cells (RPE). RPE cells are crucial nursing cells of photoreceptors; for example, they phagocytose photoreceptor outer segments and guarantee both nutrient availability and ionic equilibrium. Correspondingly, RPE cells are supplied by the choroidal capillaries, which are located posterior to the RPE layer, behind the Bruch membrane. The pathogenesis of AMD involves the accumulation of lipofuscin in RPE cells and of extracellular deposits, called drusens, between the RPE and the Bruch membrane (1–3). The accumulation of drusen deposits is one of the first clinical signs of AMD, and these deposits disturb the metabolic function of RPE cells and may even damage the RPE layer. Clinically, AMD can be classified into two main categories: the atrophic, dry form of AMD and the exudative, wet form of AMD (3). These two stages display certain similarities, for example, inflammatory signs involving cytokine secretion and pathological changes in the Bruch membrane, but the degree of pathology progresses as the disease worsens. The crucial difference between the dry and wet AMD is the development of choroidal neovascularization in wet AMD. Choroidal capillaries can grow through the breaks in the Bruch membrane and evoke fluid exudation, lipid deposition, fibrotic scars and subretinal hemorrhages which ultimately damage the RPE cells and subsequently also damage the photoreceptors, causing the loss of central vision (3).
AMD is clearly a multifactorial disease that has several risk factors, including aging, genetic characteristics, smoking, obesity and hypertension (3,4). Results of pathological studies have highlighted the role of oxidative stress and inflammatory changes in the pathogenesis of AMD (1,2). The role of inflammation has also been confirmed by results of genetic studies, which have demonstrated that polymorphism of complement factor H (CFH) is a major risk factor for AMD (4). We have recently reviewed the mechanisms leading to the activation of the innate immunity system in AMD (5). One fundamental cause of the neovascularization is the expression of vascular endothelial growth factor (VEGF) by RPE cells. Recently, several observations have indicated that elevated cellular stress in RPE cells during the progression of AMD can induce prolonged stress in endoplasmic reticulum (ER). ER stress may trigger an excessive expression and secretion of VEGF and subsequently the neovascularization and conversion from the dry form of AMD to its more serious wet counterpart (see below).
ER STRESS: A DOUBLE-EDGED SWORD
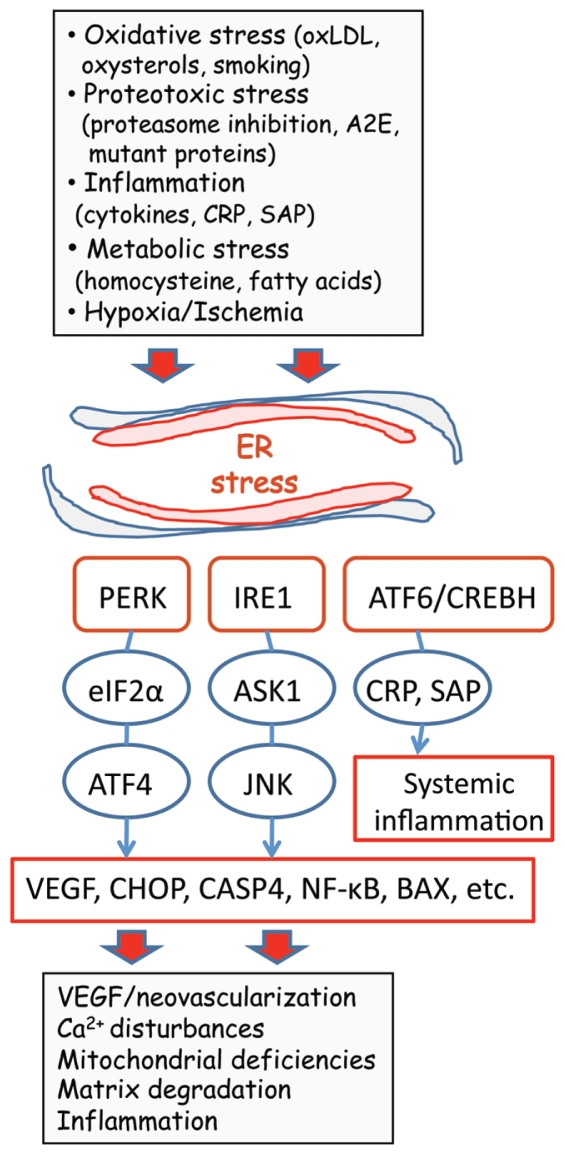
The capacity to maintain homeostasis is a crucial characteristic of cells if they are to combat environmental insults and intrinsic stress. The host defense system consists of different sensor mechanisms that recognize distinct harmful signals and trigger adaptive responses to restore cellular function. The capability of this defense system to monitor protein quality maintains a condition called proteostasis, which is a prerequisite for the survival of cells (6). The ER is a membrane-enclosed reticular network where newly synthesized proteins are matured by undergoing folding and posttranslational modifications. The ER tubular system also maintains cellular calcium homeostasis via a complex set of calcium-dependent molecular chaperones required for protein folding (7). Many different cellular stresses, for example, deficiencies in proteasome function and impaired redox and calcium balances, can induce protein misfolding in ER and release luminal calcium into the cytoplasm. This ER stress disturbs the protein quality control and this triggers the activation of ER-located transducer receptors, which generate an adaptive response called the unfolded protein response (UPR) (8–10) (Figure 1).
Figure 1.
Schematic presentation depicting the role of ER stress in the pathogenesis of neovascular AMD. Several AMD risk factors trigger ER stress and activate UPR signaling via IRE1, PERK and ATF6/CREBH transducers. UPR induces the expression of stress resistance components, but excessive and prolonged insults can trigger the expression of VEGF, CHOP, CASP4 and NF-κB, evoking neovascularization and pathological changes in the macula region. Only the pathways known to be present in human RPE cells have been included.
There are three branches of transducer proteins which recognize ER stress, inositol-requiring protein-1 (IRE1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor-6 (ATF6) (see Figure 1). The function of these transducers has recently been reviewed in detail (8–11). The activation of these transducer proteins triggers signaling cascades, which induce downstream an adaptive UPR via protein kinases and transcription factors. The most important pathways with respect to AMD (see below) are the PERK/eIF2α/ATF4 and IRE1/ASK1/JNK cascades (11,12). These pathways can elicit several AMD-related pathological changes via the induction of VEGF, C/EBP homologous protein (CHOP), caspase-4 (CASP4), and nuclear factor-κB (NF-κB) (see Figure 1).
The principal function of UPR signaling is to restore cellular function, especially proteostasis. UPR signaling can stimulate the expression of molecular chaperones as well as antioxidants and other guardian molecules. In addition, UPR signaling can reduce the level of protein synthesis and in that way mitigate the folding stress in the ER (13). For instance, PERK activates nuclear factor erythroid-2 related factor 2 (NRF2) to induce defense against oxidative stress (14). However, ER stress is a double-edged sword, that is, it can act to achieve cellular homeostasis as far as possible but ultimately it will trigger apoptotic cell death (15). This response is crucial for the preservation of tissue function, because apoptosis sacrifices one cell to preserve the tissue from being subjected to necrotic damage. The mechanisms that regulate the apoptogenic switch are still largely unknown, but it seems that proapoptotic members of the BCL-2 family, BAX, BAK and BIM, control the release of calcium from the ER (16). Briefly, ER and mitochondria construct a structural and functional interaction network called the mitochondria-associated membranes (16,17). The mitochondria-associated membranes mediate the ER/mitochondrial crosstalk via calcium release from the ER and its uptake into mitochondria. Proapoptotic BCL-2 members BAX and BAK can oligomerize and enhance the efflux of calcium from the ER. Mitochondrial calcium overload can subsequently trigger the efflux of apoptogenic factors, for example, cytochrome c and apoptosis-inducing factor, from mitochondria to cytoplasm, where they activate caspase cascades (16,18). In addition, the activation of ER stress transducers can stimulate the expression of CHOP, which also enhances apoptosis (19) (see Figure 1). In conclusion, the UPR induced by ER stress is an evolutionarily conserved host defense system that primarily can restore cellular homeostasis but also can initiate the cellular death program.
ER STRESS AND AMD PATHOGENESIS
ER stress plays a fundamental role in the pathogenesis of several diseases, for example, diabetes, cancer and neurodegenerative diseases (20,21). In ophthalmology, few studies have examined the presence of ER stress in eye diseases. Doh et al. (22) demonstrated in a rat glaucoma model that chronic ocular hypertension could trigger ER stress and subsequently apoptotic cell death in retinal ganglion cells. The expression of GRP78/BiP, p-PERK, p-eIF2α and CHOP was observed to significantly increase in the early phase of elevated intraocular pressure. In rat retina, the early stage of diabetic retinopathy also was demonstrated to induce changes indicative of the presence of ER stress, that is, the activation of IRE1α and eIF2α and subsequently the upregulation of GRP78/BiP in the retina of a rat model of diabetes and oxygen-induced retinopathy (23). Gorbatyuk et al. (24) demonstrated that the overexpression of GRP78/BiP can alleviate ER stress in a rat model of autosomal-dominant retinitis pigmentosa and prevent photoreceptor degeneration. Inokuchi et al. (25) observed that intravit-real injection of BIX (BiP inducer X) clearly stimulated the expression of GRP78/BiP in mouse retina and subsequently reduced the ER stress and retinal cell death induced by tunicamycin injection. Moreover, Dong et al. (26) demonstrated that the genetic reduction of GRP78/BiP expression inhibits tumor growth and angiogenesis in mice. This finding implies that chronic overexpression of GRP78/BiP can enhance neovascularization, especially in cancer.
The definitive demonstration of the incidence of ER stress in retinal pigment epithelium in human AMD is still lacking, but several parameters involved in the pathogenesis of AMD are potent inducers of ER stress both in RPE cells as well as in many other cell types. Libby and Gould (27) have recently proposed that ER stress could be an important mechanism in the pathogenesis of AMD. Sauer et al. (28) proposed that therapy with chemical chaperones, for example, TMAO (trimethylamide-N-oxide), PBA (4-phenylbutyric acid) and TUDCA (tauroursodeoxycholic acid) might be a way to alleviate protein aggregation in AMD.
Oxidative Stress
Results of several studies have indicated that oxidative stress has a crucial role in the pathogenesis of AMD (29,30). High oxygen consumption, constant phagocytosis, high levels of polyunsaturated fatty acids, and exposure to light irradiation provide an ideal environment for oxidative stress in the macula region. Moreover, inflammatory responses evoke reactive oxygen species (ROS) and oxidative molecular damage in RPE. Prolonged oxidative stress can also promote ocular neovascularization in the macula (31). Extensive data have been reported that demonstrate that oxidative stress is a potent inducer of stress in ER and also in RPE cells. On the other hand, ER stress itself can sustain oxidative stress and aggravate pathogenesis (32–34). Oxidized phospholipids and oxidized low-density lipoproteins (oxLDLs) potently elicit ER stress in endothelial cells (35–37). Oxidized lipoproteins inhibit the degradation of photoreceptor outer segments in RPE cells (38) and induce AMD-type pathological changes in cultured human RPE cells (39). AMD lesions, for example, drusens, contain a high level of oxLDLs and oxysterols, in particular 7-ketocholesterol (40) (see Figure 1). In human RPE cells, 7-ketocholesterol activates caspase-8 and caspase-12, evidence of the activation of ER stress (41). 7-Ketocholesterol induces the expression of CHOP and NADPH oxidase via IRE-1/JNK signaling (42) (see Figure 1). In particular, the activation of NADPH oxidase enhances choroidal neovascularization (43). OxLDLs can also activate VEGF expression via PERK/ATF4 signaling and in that way trigger neovascularization (36) (see below). Furthermore, oxLDLs are efficient inducers of inflammatory genes via the ATF4 and XBP1 branches of the UPR in endothelial cells (35).
Epidemiological studies have revealed that smoking is a strong risk factor for AMD (44). Cigarette smoke extract (CSE), which contains benzopyrene, is a potent inducer of ER stress and via the PERK pathway it can inhibit protein synthesis and provoke tissue atrophy (45) (see Figure 1). In human RPE cells, CSE stimulates oxidative stress, which induces the expression of VEGF and complement components and increases mitochondrial DNA damage and exocytotic activity (46,47). Benzopyrene can increase the activity of multiple caspase pathways, for example, caspase-3, caspase-9 and caspase-12 in human RPE cells (48). These effects of CSE on RPE cells may well explain why cigarette smoking is a risk factor for AMD.
Proteotoxic Stress
In addition to lipids, ROS can also oxidize proteins and cause dysfunctions in the maintenance of proteostasis. Oxidized, misfolded proteins are generally degraded in proteasomes (49). However, proteasomal peptidases themselves are sensitive targets of ROS and they are clearly inactivated in oxidative stress, which means that the efficiency of proteasomal degradation is impaired and misfolded proteins aggregate into cytosol (49). Moreover, proteasomal inhibition triggers ER stress, which acts through UPR signaling to stimulate the synthesis of the chaperone proteins (50) (see Figure 1). In human RPE cells, oxidative stress inhibits proteasomal activity, although it stimulates ubiquitin conjugation activity (51). Li et al. (52) demonstrated that aging of human RPE cells clearly decreased the activity of 20S proteasomes. We have observed that proteasome inhibition leads to aggresome formation of ubiquitinated proteins in human RPE cells (53). Suppression of proteasomal function also inhibits the ER-associated degradation pathway, which enhances the protein misfolding in the ER and triggers ER stress (54). Activation of PERK inhibits protein synthesis via eIF2α, and in that way it can alleviate ER stress. In human RPE cells, excessive proteasomal inhibition generates ER stress and stimulates the expression of hypoxia-inducible factor (HIF)-1α, VEGF and angiopoietin-2, factors that can initiate the neovascularization in AMD (55) (see below).
Accumulation of lipofuscin into lysosomes in RPE cells is a hallmark of aging, and in particular, AMD pathogenesis (2,56). A2E, a toxic lipofuscin fluorophore, is produced as a byproduct of the retinoid visual cycle (57). Deposition of A2E and lipofuscin into RPE cells can switch on the generation of ROS, perturb cholesterol metabolism and even induce the expression of VEGF and in this way support neovascularization (58,59). Inhibition of proteasomal degradation can also enhance lipofuscin accumulation (60). Several studies have indicated that lysosomal storage disorders can induce ER stress, and the experimental disruption of lysosomal homeostasis can also trigger ER stress (61). Moreover, accumulation of lysosomal inclusions into rat retinal pigment epithelium can cause a prominent loss of smooth ER structures (62). This loss impairs the ability of ER to respond to cellular stress through the induction of molecular chaperone expression.
As a proof-of-principle, there are some inherited AMD-like diseases in which the retention of mutated proteins into the ER can trigger ER stress in RPE cells. Roybal et al. (63) demonstrated that the missense mutation in R345W of fibulin-3 protein leads to the accumulation of misfolded protein within the ER compartment. Retention of mutant fibulin-3 into the ER provokes ER stress and induces the expression of VEGF in RPE cells. This disease, known as malattia leventinese, and Doyne honeycomb retinal dystrophy, are early-onset autosomal dominant maculopathies (63). Another similar disorder is a rare, late-onset retinal macular degeneration involving drusenlike deposits, macular degeneration and in the late stage, choroidal neovascularization (64). Wild-type C1QTNF5 (CTRP5) protein is secreted, whereas the mutated version is misfolded and retained within ER (64). The mutant protein is degraded by proteasomes, probably via ER-associated degradation pathway. Currently, it is not known whether the late-onset retinal macular degeneration syndrome is caused by ER stress.
Inflammation and Metabolic Stress
Inflammation is a crucial player in the pathogenesis of AMD. We have recently reviewed the role of innate immunity receptors and danger signals, which evoke and sustain inflammation in AMD (5). Recently, a large body of literature has reported data indicating that ER stress can induce inflammatory responses (11,65), although UPR signaling can also support the development and survival of some immune cells and affect the appearance of autoimmunity (66). ER stress stimulates inflammatory responses mainly via the IRE1-TRAF2 and IRE1-XBP1 pathways or via the ER-located inflammatory caspases, that is, CASP4 and caspase-12 (see 11). In human RPE cells, proinflammatory cytokines and lipopolysaccharide induce the expression of CASP4, whereas dexamethasone and interleukin (IL)-10 block the induction (67). CASP4 activation can enhance inflammatory responses and trigger apoptotic cell death. In some cell types, chronic ER stress can switch on negative feedback and impede inflammatory insults (68). Bian et al. (69) observed that caspase-12S, a short form of caspase-12, is a predominant form of caspase-12 in human RPE cells. Caspase-12S contains only the CARD (caspase activation recruitment domain) and thus it can inhibit caspase signaling pathways. Interestingly, Bian et al. (69) demonstrated that the ER stress inducers tunicamycin and thapsigargin clearly increase the expression of caspase-12S mRNA in human RPE cells. IL-1β, tumor necrosis factor-α and lipopolysaccharide downregulated the expression of caspase-12S, whereas IL-10 upregulated the expression of caspase-12S. These results indicate that caspase-12S could be a potent negative regulator of apoptosis and inflammatory responses. However, these results must be confirmed at the protein level, because Bian et al. (69) used the reverse transcription–polymerase chain reaction technique in their expression studies.
ER stress is also a well-known inducer of the systemic inflammatory response, which can propagate AMD pathogenesis, for example, via the effects of C-reactive protein (CRP) and serum amyloid P (SAP) proteins. ER stress in liver activates the CREBH transducer, a member of the ATF6 family, which triggers the expression and secretion of CRP and SAP (70) (see Figure 1). In human RPE cells, CRP can stimulate IL-8 and VEGF expression (71). Seddon et al. (72) demonstrated that an elevated serum CRP concentration is a clear risk factor for AMD, among both smokers and nonsmokers. The function of XBP1 has not been studied in human RPE cells and needs to be clarified in the case of inflammatory responses.
The ER is a metabolic sensor organelle, that is, fatty-acid and free-cholesterol overload and hypo/hyperglycemia trigger ER stress, and subsequently the UPR signaling attempts to adjust the metabolism to meet the environmental challenges (73). Chronic metabolic overload can also elicit inflammatory responses (74). During aging and especially in AMD, the pathological changes in the Bruch membrane can disturb the diffusion of oxygen and macromolecules (75) and in that way trigger hypoxia (see below) and metabolic disorders.
Epidemiological studies have demonstrated that an elevated level of homocysteine is associated with exudative, neovascular AMD but not with dry AMD (76). Homocysteine is a well-known risk factor for cardiovascular diseases. Homocysteine is involved in the function of the methyl acceptor/donor cycle, which transfers methyl groups to a wide variety of acceptors. Homocysteine itself is a toxic metabolite that triggers ER stress (see Figure 1), for example, in the liver after high alcohol consumption (77). Interestingly, alcohol abuse is considered to be a risk factor for AMD (78). In human RPE cells, homocysteine activates the PERK-ATF4 pathway, which stimulates the expression of VEGF (79). This finding seems to confirm the role of homocysteine in neovascularization in wet AMD.
Hypoxia/Ischemia
Pathological studies and noninvasive perfusion measurements have indicated that reduced choroidal circulation and subsequent hypoxia/ischemia can aggravate the progress of AMD pathogenesis, in particular choroidal neovascularization (80–82). Hypoxia and ischemia are potent inducers of ER stress in different tissues (83,84). Hata et al. (85) demonstrated that ischemia-reperfusion in the rat eye clearly triggered ER stress in retina, which involved increased expression of IRE1α, ASK1 and p-JNK (see Figure 1). The ganglion cell layer was most affected after ischemia. The ER stress generated by hypoxia is normally beneficial, because mild, chronic hypoxia can induce hypoxia resistance via the PERK/eIF2α/ATF4 pathway, but in the case of cancer, it can support tumor growth (86). Hypoxia is also an efficient inducer of VEGF expression and secretion, which support angiogenesis in growing tumors (87). HIF-1α is a well-known inducer of VEGF secretion and neovascularization that is also associated with AMD (81). However, ER stress is an HIF-1α–independent inducer of VEGF and angiogenesis (88,89), although there is some evidence that ER stress can up-regulate HIF-1α expression in normoxic conditions (90).
ER Stress: Inducer of VEGF Expression and Angiogenesis
Angiogenesis, that is, the process by which new capillary blood vessels grow out of preexisting vessels, is a vital event during development but also in adult life (91). Angiogenesis is normally a beneficial process that maintains tissue homeostasis, for example, during increased aerobic exercise or in wound healing and cardiovascular diseases in which blood flow is compromised. However, excessive angiogenesis can have detrimental pathological effects on tumor growth and macular degeneration. VEGF-A, also called VEGF, is the major vascular growth factor in blood vessel maturation, although many other angiogenic factors, for example, fibroblast growth factor, transforming growth factor β and angiopoietins, can enhance capillary growth (91,92). The expression of VEGF-A is regulated mainly at the transcriptional level, although its expression and activity can be controlled by posttranscriptional, translational and posttranslational mechanisms (92). Furthermore, alternative splicing can generate an anti-angiogenic isoform, VEGF-A165b (93). Magnussen et al. (93) demonstrated that the injection of this splice variant can inhibit angiogenesis in a mouse model of retinal neovascularization. The presence of alternative splicing of VEGF-A in association with ER stress has not been demonstrated. There is a long list of transcription factors that can transactivate the VEGF expression, for example, HIF-1α, NF-κB, Sp1 and ERα (92,94). A plethora of stimulating signals can activate VEGF expression, for example, hypoxia/ischemia, cytokines, growth factors and some hormones.
Recently, several studies have demonstrated that ER stress is a potent inducer of VEGF expression (36,79,89,95). Studies in which different ER stress models were used revealed that the expression of VEGF is activated via the ATF transcription factor in both human RPE cells (79,95) and human umbilical vein endothelial cells (36) (see Figure 1). Roybal et al. (95) demonstrated that oxidative stress induced via arsenite exposure increased eIF2α phosphorylation and ATF4 expression in human RPE cells. These investigators also established that the ATF4 complex binds to the intronic AARE (amino-acid response element) site within the VEGF gene and transactivates gene expression. Ghosh et al. (89) demonstrated that in cancer cells, all three ER stress transducers are involved in the induction of VEGF and angiogenesis. The induction of VEGF expression is mediated via the PERK/ATF4, IRE1α/XBP-1 and ATF6α pathways, although HIF-1α is not involved in ER stress–mediated VEGF expression (89). It seems that different stimuli trigger distinct UPR pathways, for example, hypoxia/ischemia and glucose deprivation activate VEGF via the IRE1α pathway (96).
ER STRESS: TRIGGER OF VEGF-DRIVEN NEOVASCULARIZATION IN AMD?
The best evidence supporting the role of VEGF in the neovascularization that occurs in wet AMD is the effectiveness of the therapy with anti-VEGF antibodies (bevacizumab and ranibizumab) or VEGF-receptor inhibitors (for example, VEGF Trap) (97,98). In addition, there are promising inhibitors of tyrosine kinases downstream from the VEGF receptor (for example, Vatalanib and Pazopanib) (98). However, RPE-derived VEGF is the growth factor for the maintenance of the choriocapillaris in the adult macula (99). In addition, RPE cells also secrete pigment epithelium-derived factor (PEDF), which is a natural inhibitor of angiogenesis because it can reduce vascular endothelial cell proliferation and inhibit VEGF-dependent signaling (100). Moreover, PEDF exerts several antiapoptotic and neuroprotective effects (101). Interestingly, ER stress decreases the expression of PEDF in human RPE cells (102). In the macula, it seems that the well-being of RPE cells has a profound effect on the stability of the entire choriocapillary network, that is, insults that jeopardize the homeostasis of RPE cells trigger capillary growth, probably to restore the metabolic balance.
As described above, a wide variety of insults can induce ER stress in human RPE cells, and all are direct or indirect risk factors for AMD pathogenesis. The UPR responses induced by ER stress follow hormetic regulation, which means that mild stress induces stress tolerance whereas excessive stress can cause harmful effects and lead to apoptotic cell death (103,104). VEGF is a perfect example of this kind of regulation and it seems to be the crucial factor that triggers the conversion from dry AMD to its wet form. The complex and integrated regulation of VEGF expression highlights the significance of VEGF in neovascularization. There is an extensive cross-talk between the mediators of oxidative stress (NRF2), oxygen deficiency (HIF-1α) and unfolded protein stress (for example, PERK/ATF4) in the regulation of VEGF (89,92,105). However, the role of UPR seems to be crucial, because the dry form of AMD involves several disturbances in protein quality control (56), and aggregated proteins stimulate the adaptive UPR response involving molecular chaperones and antioxidants to maintain homeostasis in the RPE. Overwhelming stress, however, seems to activate a set of stress-related transcription factors and trigger excessive VEGF expression, which subsequently causes neovascularization in the macula.
ACKNOWLEDGMENTS
This study was financially supported by grants from the Academy of Finland and the University of Eastern Finland, Kuopio, Finland. The authors thank E MacDonald for checking the language of the manuscript.
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the discussion reported in this review.
Online address: http://www.molmed.org
REFERENCES
- 1.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 2.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–63. [PubMed] [Google Scholar]
- 3.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 4.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retinal Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaarniranta K, Salminen A. Age-related macular degeneration: activation of innate immunity system via pattern recognition receptors. J Mol Med. 2009;87:117–23. doi: 10.1007/s00109-008-0418-z. [DOI] [PubMed] [Google Scholar]
- 6.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–91. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 7.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 8.Schroder M. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 9.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 10.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 11.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflamm. 2009;6:e41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine Y, Takeda K, Ichijo H. The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases. Curr Mol Med. 2006;6:87–97. doi: 10.2174/156652406775574541. [DOI] [PubMed] [Google Scholar]
- 13.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 14.Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–32. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetz C, Glimcher L. The daily job of night killers: alternative roles of the BCL-2 family in organelle physiology. Trends Cell Biol. 2007;18:38–44. doi: 10.1016/j.tcb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Giorgi C, et al. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–27. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyadomari S, Mori M. Roles of CHOP/DAGG153 in endoplasmic stress. Cell Death Differ. 2004;11:381–89. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–58. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin JH, Walter P, Yen TSB. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol Mech Dis. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doh SH, Kim JH, Lee KM, Park HY, Park CK. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010;1308:158–66. doi: 10.1016/j.brainres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–7. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorbatyuk MS, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A. 2010;107:5961–6. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inokuchi Y, et al. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest Ophthalmol Vis Sci. 2009;50:334–44. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- 26.Dong D, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 27.Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv Exp Med Biol. 2010;664:403–9. doi: 10.1007/978-1-4419-1399-9_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer T, Patel M, Chan CC, Tuo J. Unfolding the therapeutic potential of chemical chaperones for age-related macular degeneration. Expert Rev Ophthalmol. 2008;3:29–42. doi: 10.1586/17469899.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty S, Koh HH, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 30.Shen JK, et al. Oxidative damage in age- related macular degeneration. Histol Histopathol. 2007;22:1301–8. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 31.Dong A, et al. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219:544–52. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword. Antioxidant Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 33.He S, et al. Endoplasmic reticulum stress induced by oxidative stress in retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2008;246:677–83. doi: 10.1007/s00417-008-0770-2. [DOI] [PubMed] [Google Scholar]
- 34.Santos CXC, Tanaka LY, Wosniak J, Jr, Laurindo FRM. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: Roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxidant Redox Signal. 2009;11:2409–27. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 35.Gargalovic PS, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–6. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 36.Oskolkova OV, et al. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 2008;112:330–9. doi: 10.1182/blood-2007-09-112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanson M, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells. Prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–36. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 38.Hoppe G, Marmorstein AD, Pennock EA, Hoff HF. Oxidized low density lipoprotein- induced inhibition of processing of photoreceptor outer segments by RPE. Invest Ophthalmol Vis Sci. 2001;42:2714–20. [PubMed] [Google Scholar]
- 39.Yamada Y, et al. Oxidized low density lipoproteins induce a pathological response by retinal pigmented epithelial cells. J Neurochem. 2008;105:1187–97. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- 40.Javitt NB, Javitt JC. The retinal oxysterol pathway: a unifying hypothesis for the cause of age-related macular degeneration. Curr Opin Ophthalmol. 2009;20:151–7. doi: 10.1097/ICU.0b013e32832af468. [DOI] [PubMed] [Google Scholar]
- 41.Luthra S, et al. Activation of caspase-8 and caspase-12 pathways by 7-ketocholesterol in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5569–75. doi: 10.1167/iovs.06-0333. [DOI] [PubMed] [Google Scholar]
- 42.Pedruzzi E, et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–17. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, et al. Downregulation of p22phox in retinal pigment epithelial cells inhibits choroidal neovascularization in mice. Mol Ther. 2008;16:1688–94. doi: 10.1038/mt.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 45.Hengstermann A, Muller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Rad Biol Med. 2008;44:1097–107. doi: 10.1016/j.freeradbiomed.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Bertram KM, Baglole CJ, Phipps RP, Libby RT. Molecular regulation of cigarette smoke induced oxidative stress in human retinal pigment epithelial cells: implications for age-related macular degeneration. Am J Physiol Cell Physiol. 2009;297:C1200–10. doi: 10.1152/ajpcell.00126.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang AL, et al. Changes in retinal pigment epithelium related to cigarette smoke: possible relevance to smoking as a risk factor for age- related macular degeneration. PLoS One. 2009;4:e5304. doi: 10.1371/journal.pone.0005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma A, et al. Effects of benzo(e)pyrene, a toxic component of cigarette smoke, on human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 2008;49:5111–7. doi: 10.1167/iovs.08-2060. [DOI] [PubMed] [Google Scholar]
- 49.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389:203–9. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 50.Fribley A, Wang CY. Proteasome inhibitor induces apoptosis through induction of endoplasmic reticulum stress. Cancer Biol Ther. 2006;5:745–8. doi: 10.4161/cbt.5.7.2971. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, et al. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:3622–30. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, et al. Alterations of activity and intracellular distribution of the 20 S proteasome in ageing retinal pigment epithelial cells. Exp Gerontol. 2008;43:1114–22. doi: 10.1016/j.exger.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 53.Ryhänen T, et al. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J Cell Mol Med. 2009;13:3616–31. doi: 10.1111/j.1582-4934.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–57. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez AF, et al. Proteasome-dependent regulation of signal transduction in retinal pigment epithelial cells. Exp Eye Res. 2006;83:1472–81. doi: 10.1016/j.exer.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaarniranta K, Salminen A, Eskelinen EL, Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways: implications for age-related macular degeneration (AMD) Ageing Res Rev. 2009;8:128–39. doi: 10.1016/j.arr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:11026–31. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iriyama A, et al. A2E, a component of lipofuscin, is pro-angiogenic in vivo. J Cell Physiol. 2009;220:469–75. doi: 10.1002/jcp.21792. [DOI] [PubMed] [Google Scholar]
- 60.Terman A, Sandberg S. Proteasome inhibition enhances lipofuscin formation. Ann N Y Acad Sci. 2002;973:309–12. doi: 10.1111/j.1749-6632.2002.tb04657.x. [DOI] [PubMed] [Google Scholar]
- 61.Wei H, et al. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–77. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 62.Okubo A, Sameshima M, Unoki K, Uehara F, Bird AC. Ultrastructural changes associated with accumulation of inclusion bodies in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2000;41:4305–12. [PubMed] [Google Scholar]
- 63.Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 2005;46:3973–9. doi: 10.1167/iovs.05-0070. [DOI] [PubMed] [Google Scholar]
- 64.Shu X, et al. Disease mechanisms in late-onset retinal macular degeneration associated with mutation in C1QTNF5. Hum Mol Genet. 2006;15:1680–9. doi: 10.1093/hmg/ddl091. [DOI] [PubMed] [Google Scholar]
- 65.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–74. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 67.Bian ZM, Elner SG, Elner VM. Dual involvement of caspase-4 in inflammatory and ER stress-induced apoptotic responses in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:6006–14. doi: 10.1167/iovs.09-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayakawa K, et al. Acquisition of anergy to proinflammatory cytokines in nonimmune cells through endoplasmic reticulum stress response: A mechanism for subsidence of inflammation. J Immunol. 2009;182:1182–91. doi: 10.4049/jimmunol.182.2.1182. [DOI] [PubMed] [Google Scholar]
- 69.Bian ZM, Elner SG, Elner VM. Regulated expression of caspase-12 gene in human retinal pigment epithelial cells suggests its immunomodulating role. Invest Ophthalmol Vis Sci. 2008;49:5593–5601. doi: 10.1167/iovs.08-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–99. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, et al. Induction of interleukin-8 gene expression and protein secretion by C-reactive protein in ARPE-19 cells. Exp Eye Res. 2010;91:135–42. doi: 10.1016/j.exer.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291:704–10. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 73.Mandl J, Meszaros T, Banhegyi G, Hunyady L, Csala M. Endoplasmic reticulum: nutrient sensor in physiology and pathology. Trends Endocrinol Metab. 2009;20:194–201. doi: 10.1016/j.tem.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–34. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Booij JC, Baas DC, Beisekeeva J, Gorgels TGMF, Bergen AAB. The dynamic nature of Bruch’s membrane. Prog Retinal Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Axer-Siegel R, et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol. 2004;137:84–9. doi: 10.1016/s0002-9394(03)00864-x. [DOI] [PubMed] [Google Scholar]
- 77.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23:S16–24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2008;145:707–15. doi: 10.1016/j.ajo.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Roybal CN, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–52. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- 80.Metelitsina TI, et al. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:358–63. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K. Regulatory role of HIF-1α in the pathogenesis of age-related macular degeneration (AMD) Ageing Res Rev. 2009;8:349–58. doi: 10.1016/j.arr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Feigl B. Age-related maculopathy – linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Retinal Eye Res. 2009;28:63–86. doi: 10.1016/j.preteyeres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–45. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Truettner JS, Hu K, Liu CL, Dietrich WD, Hu B. Subcellular stress response and induction of molecular chaperones and folding proteins after transient global ischemia in rats. Brain Res. 2009;1249:9–18. doi: 10.1016/j.brainres.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hata N, Oshitari T, Yokoyama A, Mitamura Y, Yamamoto S. Increased expression of IRE1α and stress-related signal transduction proteins in ischemia-reperfusion injured retina. Clin Ophthalmol. 2008;2:743–52. doi: 10.2147/opth.s3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fels DR, Koumenis C. The PERK/eIF2α/ATF4 module of UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–8. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 87.Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67:10631–4. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- 88.Ameri K, et al. Anoxic induction of ATF-4 through HIF-1 independent pathways of protein stabilization in human cancer cells. Blood. 2004;103:1876–82. doi: 10.1182/blood-2003-06-1859. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh R, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Werno C, Zhou J, Brune B. A23187, ionomycin and thapsigargin upregulate mRNA of HIF-1α via endoplasmic reticulum stress rather than a rise in intracellular calcium. J Cell Physiol. 2008;215:708–14. doi: 10.1002/jcp.21351. [DOI] [PubMed] [Google Scholar]
- 91.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 92.Loureiro RMB, D’Amore PA. Transcriptional regulation of vascular endothelial growth factor in cancer. Cytok Growth Fact Rev. 2005;16:77–89. doi: 10.1016/j.cytogfr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Magnussen AL, et al. VEGF-A165B is cytoprotective and anti-angiogenic in the retina. Invest Ophthalmol Vis Sci. 2010;51:4273–81. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pages G, Pouyssegur J. Transcriptional regulation of the vascular endothelial growth factor gene: a concert of activating factors. Cardiovasc Res. 2005;65:564–73. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 95.Roybal CN, et al. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280:20331–9. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 96.Drogat B, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 2007;67:6700–7. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 97.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502–8. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 98.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68:1029–36. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 99.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaries. Proc Natl Acad Sci U S A. 2009;106:18751–6. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem. 2009;106:769–75. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 101.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retinal Eye Res. 2004;23:561–77. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Koyama Y, et al. Induction of amyloid β accumulation by ER calcium disruption and resultant upregulation of angiogenic factors in ARPE19 cells. Invest Ophthalmol Vis Sci. 2008;49:2376–83. doi: 10.1167/iovs.07-1067. [DOI] [PubMed] [Google Scholar]
- 103.Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattson MP. Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol. 2008;27:155–62. doi: 10.1177/0960327107083417. [DOI] [PubMed] [Google Scholar]
- 105.Afonyushkin T, et al. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–13. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]