Abstract
Amyloid was extracted from the spleen of a patient with primary amyloidosis by homogenizing it at high speed with water after preliminary treatments, first to remove proteins soluble in saline, and then to remove salts. The extracts containing amyloid appeared to be clear at concentrations up to 6 mg/ml of protein. The material gave little sediment on being centrifuged up to 20,000 g for 1 hr, but the protein was sedimented at 100,000 g in 1 hr. The amyloid could be precipitated from the extracts by addition of NaCl to 0.0075 mole/liter or of CaCl2 to 0.0025 mole/liter. The protein-bound Congo red formed a red precipitate and this property was used to estimate recovery and purity of amyloid during extraction.
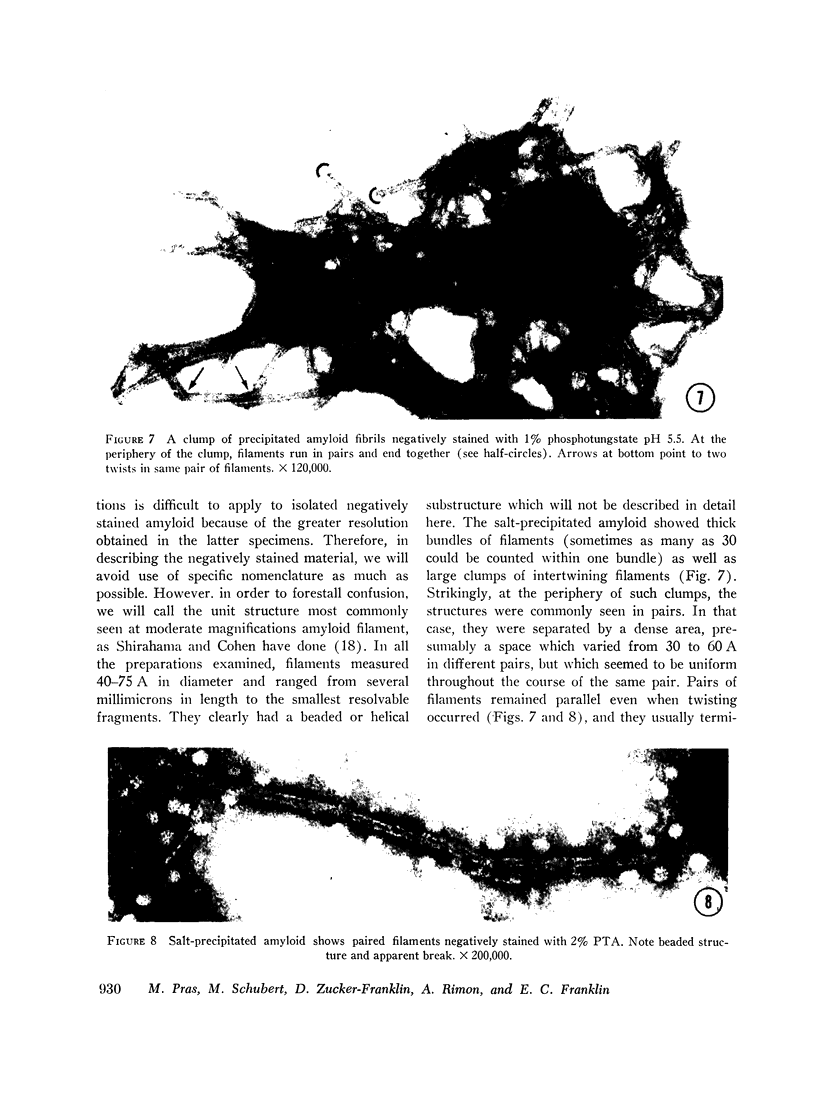
On electronmicroscopy the isolated amyloid proved to be morphologically pure. It existed either as single filaments measuring 60-80 Å in diameter or as large aggregates of these filaments.
Freshly isolated amyloid in water sedimented as a single homogeneous peak with an s°20,[unk] of about 45-50S. On standing, the solution became cloudy and more rapidly sedimenting components appeared. On electrophoresis the material migrated as a homogeneous peak towards the anode. The protein had an amino acid composition different from that of all known serum proteins. It was rich in acidic amino acids and had little cysteine and methionine and no hydroxyproline. The total content of carbohydrate was less than 2%.
Full text
PDF
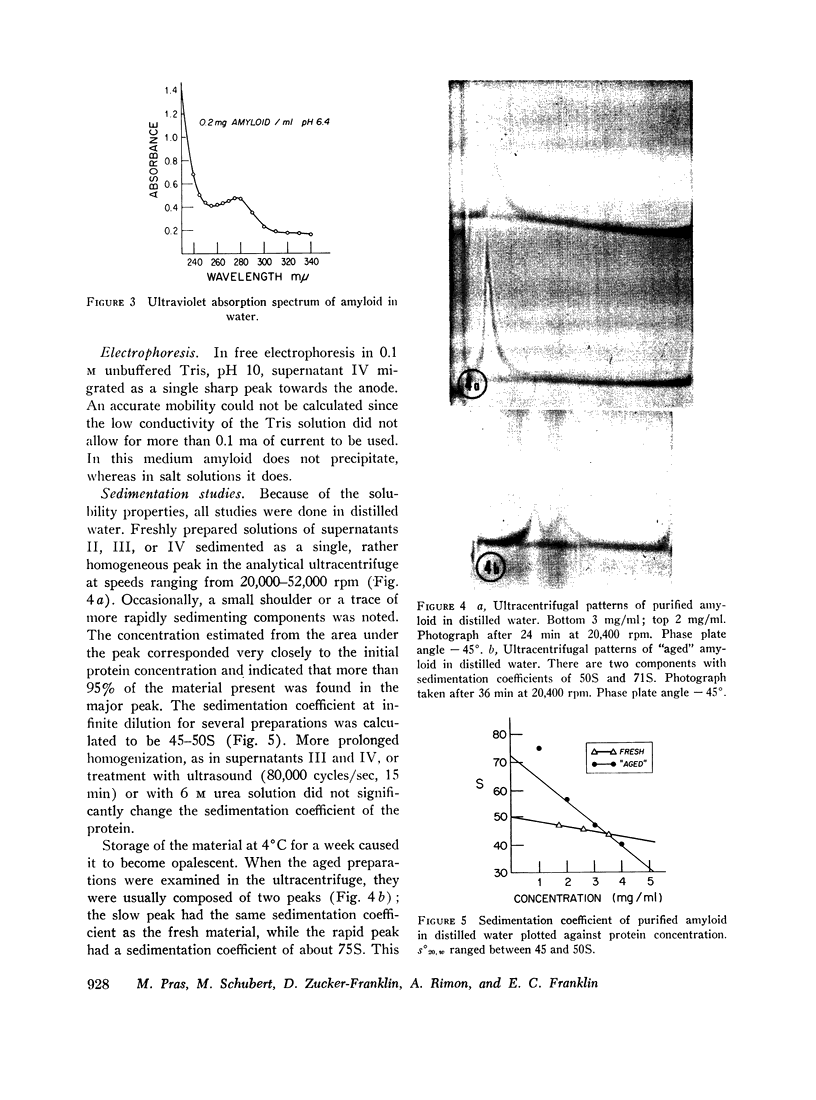




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi Y., Hersko C., Gafni J., Sohar E., Heller H. Isolation of highly purified amyloid. Isr J Med Sci. 1967 Jul-Aug;3(4):569–571. [PubMed] [Google Scholar]
- BENDITT E. P., LAGUNOFF D., ERIKSEN N., ISERI O. A. Amyloid. Extraction and preliminary characterization of some proteins. Arch Pathol. 1962 Oct;74:323–330. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid. 3. A protein related to the subunit structure of human amyloid fibrils. Proc Natl Acad Sci U S A. 1966 Feb;55(2):308–316. doi: 10.1073/pnas.55.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Nylen M. U., Glenner G. G. The ultrastructure of human amyloid as revealed by the negative staining technique. J Ultrastruct Res. 1966 Mar;14(5):449–459. doi: 10.1016/s0022-5320(66)80075-8. [DOI] [PubMed] [Google Scholar]
- CAESAR R. [The fine structure of the spleen and liver in experimental amyloidosis]. Z Zellforsch Mikrosk Anat. 1960;52:653–673. [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959 Apr 25;183(4669):1202–1203. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- COHEN A. S., FRENSDORFF A., LAMPRECHT S., CALKINS E. A study of the fine structure of the amyloid associated with familial Mediterranean fever. Am J Pathol. 1962 Nov;41:567–578. [PMC free article] [PubMed] [Google Scholar]
- GUEFT B., GHIDONI J. J. THE SITE OF FORMATION AND ULTRASTRUCTURE OF AMYLOID. Am J Pathol. 1963 Nov;43:837–854. [PMC free article] [PubMed] [Google Scholar]
- HEEFNER W. A., SORENSON G. D. Experimental amyloidosis. I. Light and electron microscopic observation of spleen and lymph nodes. Lab Invest. 1962 Aug;11:585–593. [PubMed] [Google Scholar]
- LETTERER E., GEROK W., SCHNEIDER G. Vergleichende Untersuchungen über den Aminosäurenbestand von Serum-Eiweiss, Lebereiweiss, Amyloid, Hyalin und Kollagen. Virchows Arch Pathol Anat Physiol Klin Med. 1955;327(3):327–342. doi: 10.1007/BF00955747. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe D. S., Cohen A. S. Solubility characteristics of isolated amyloid fibrils. Biochim Biophys Acta. 1965 Jul 8;104(2):480–486. doi: 10.1016/0304-4165(65)90353-3. [DOI] [PubMed] [Google Scholar]
- PERNIS B., SCHNEIDER G., WUNDERLY C. Quantitative Aminosäurenanalyse von Amyloidsubstanz, elektrophoretischen Serum-Eiweissfraktionen und Bindegewebsprotein. Arztl Forsch. 1953 Oct 10;7(10):I/454–I/458. [PubMed] [Google Scholar]
- SPIRO D. The structural basis of proteinuria in man; electron microscopic studies of renal biopsy specimens from patients with lipid nephrosis, amyloidosis, and subacute and chronic glomerulonephritis. Am J Pathol. 1959 Jan-Feb;35(1):47–73. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. High-resolution electron microscopic analysis of the amyloid fibril. J Cell Biol. 1967 Jun;33(3):679–708. doi: 10.1083/jcb.33.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]