Abstract
Premature delivery occurs in 12% of all births, accounts for nearly half of neonatal morbidity and is increasing in frequency. Current therapeutic approaches to preterm delivery are ineffective and present serious risks to both the mother and fetus. Although there are multiple factors that contribute to the etiology of preterm birth, the single most common cause is infection. Recently, using cDNA microarray analysis of human placental tissue, we demonstrated that human placental matrix metalloproteinase-1 (MMP-1) is upregulated during labor. In a separate line of investigation, we have shown that blockade of endothelin-1 (ET-1) action through the use of an endothelin-converting enzyme-1 (ECE-1) inhibitor, an established commercially available endothelin receptor antagonist or a novel quinolone-derived endothelin receptor antagonist synthesized by our group also prevents preterm labor and delivery in a mouse model. We have now shown that induction of preterm labor with lipopolysaccharide in our mouse model is associated with increased levels of MMP-1. Furthermore, we showed that silencing the ECE-1/ET-1 pathway by using ECE-1 RNA interference prevents both the onset of preterm labor and upregulation of MMP-1. The data indicate that ET-1 and MMP-1 act in the same molecular pathway in preterm labor.
INTRODUCTION
Premature delivery, defined as delivery occurring before 37 weeks’ gestation, occurs in 12% of all births, and accounts for nearly half of long-term neonatal neurological morbidity and 60%–80% of perinatal mortality, excluding infants with congenital anomalies. Despite the advances that have been made in obstetrics and neonatalogy, the rate of premature delivery has not decreased over the past 20–30 years. In fact, the National Center for Health Statistics (Centers for Disease Control and Prevention) has reported a 20% increase (from 10.6% to 12.7%) in the percentage of premature deliveries during the years 1990 to 2005. Sadly, the existing therapies for prevention of preterm labor are ineffective and introduce risks to the mother and fetus (1).
Intrauterine infection is often associated with preterm labor. It has been suggested that at least 40% of all premature deliveries occur to mothers with intrauterine infection, and that gestational age is inversely related to the frequency of intrauterine infections (2). Considerable evidence suggests that the proinflammatory cytokine–prostaglandin cascade plays a central role in the pathogenesis of infection-associated premature delivery (3,4).
Previous in vitro investigations have shown that endothelin-1 (ET-1), an extremely potent vasoconstrictor peptide (5), increases myometrial smooth muscle tone (6–8). It has also been shown that infection and inflammatory cytokines stimulate ET-1 production (9) and that ET-1, in turn, stimulates an inflammatory cytokine pathway through activation of the ETB receptor (10). In previous work, we have shown that treating lipopolysaccharide (LPS)-stimulated pregnant mice with the endothelin-converting enzyme-1 (ECE-1) inhibitor phosphoramidon decreases the incidence of premature delivery in a mouse model (11). In addition, we have recently found that treating LPS-stimulated pregnant mice with 6-alkoxy substituted-3-carboxybenzyl-N-benzyl-quinol-4-ones, a novel endothelin receptor antagonists synthesized by our group, results in decreased incidence of premature delivery (12).
The matrix metalloproteinases (MMPs) are a family of enzymes (with more than 20 members identified) that use zinc-dependent catalysis to break down the components of the extracellular matrix (ECM), allowing for cell movement and tissue reorganization to support the growing fetus. Several MMPs are constitutively generated by reproductive tissues, and a fluctuation in the gene expression level of certain MMPs has been observed during the various stages of parturition (13). The ECMs of the cervix, fetal membranes, placenta and uterus are comprised mainly of collagen types I and III. Investigators have shown that a remodeling of these collagens, mediated by the MMPs, may play a role in the pathway leading to birth (14). Using cDNA microarray analysis of human placental tissue, we recently showed that human placental matrix metalloproteinase-1 (MMP-1) is upregulated in labor (15).
The work we present here provided biochemical, pharmacologic and molecular evidence that ET-1 and MMP-1 both play critical roles in the pathogenesis of inflammation-associated preterm birth. Furthermore, the results of our investigation showed that silencing the ECE-1/ET-1 pathway by using ECE-1 RNA interference (RNAi) prevents both MMP-1 upregulation and the onset of preterm labor. The data suggest that ET-1 and MMP-1 act in the same molecular pathway in infection-associated preterm labor.
METHODS
Mouse Models of Premature Delivery
C57B1/6 mice from Taconic laboratory were used for all experiments. Animals were housed in plastic cages in a temperature-controlled animal facility with alternating 12:12 h light-dark cycles, with ad libitum access to food and water. All experimental protocols used were approved by the St. John’s University Animal Care and Utilization Committee of the College of Pharmacy and Allied Health Professions, and the research was conducted according to the requirements of the NIH Guide for the Care and Use of Laboratory Animals (revised 1996). A total of 28 timed pregnant mice weighing 22–28 g were used for the first series of experiments. Mice were randomly assigned to four groups: infection and labor and delivery (n = 8), infection and no labor or delivery (n = 7), no infection and no labor or delivery (n = 9) and labor and delivery without infection (n = 4). We have found that the potency of commercially prepared LPS (serotype 026:B6; Sigma, St Louis, MO, USA) varies several-fold from batch to batch. This phenomenon results from the variability in purity of the endotoxin preparation. The best approach is to conduct an entire study with a single preparation of LPS. In our case, different experiments were conducted at different points in time and several distinct LPS preparations were used. For each experiment in which we used a new batch of LPS, the LPS dose was re-titrated. For example, a dose of 6.6 mg/kg of one batch of commercially prepared LPS may induce premature delivery in 50% of mice, whereas a dose of 3.3 mg/kg of another batch of LPS may induce premature delivery in 90%, and a dose of 9.9 mg/kg of yet a third batch of the endotoxin may result in premature delivery in 85%. In studies of inhibition of labor and delivery, known as tocolysis, in test animals treated with tocolytic agents and controls treated with vehicle, care is taken to use not only the same dose of LPS but also the same commercial preparation of the endotoxin in these two groups as well. To establish our models of preterm labor, mice in the first two groups received an intraperitoneal (ip) injection of 6.6 mg/kg LPS dissolved in 0.5 mL phosphate-buffered saline (PBS) at embryonic day (E)15.5. Mice progressing to delivery within 24 h of receiving this intermediate dose of LPS were placed in the first group, and mice failing to develop labor as a result of the LPS injection were placed in the second group. Mice in the third group (reference controls) received ip injections of 0.5 mL PBS at E15.5. Mice in the fourth group received an ip injection of 6.7 mg/kg prostaglandin F2α (PGF-2α) (Sigma) dissolved in 0.5 mL PBS administered every 3 h, beginning at E16.0, and delivered within 13 h of the first injection of PGF-2α.
Tissue Collection and Preparation
Placentas were collected at E16.0–E16.5, immediately after delivery, at hysterectomy 24 h after LPS or PBS injection or at hysterectomy 12 h after the first injection of PGF-2α. Uteri were collected at E16.5, at hysterectomy, either 24 h after LPS or PBS injection or 12 h after the first injection of PGF-2α. Mice were killed by CO2 asphyxiation, as per the requirements of our animal care and use committee, and rapidly hysterectomized. Because all mice were euthanized by the same method, any potential effects on tissue oxygenation or progesterone levels were equivalent in test animals versus controls. All tissues were collected rapidly, frozen immediately and stored at −80°C.
Western Blotting
Placental and uterine tissues were homogenized in 10 volumes of ice cold homogenization buffer (20 mmol/L Tris/HCL, pH 7.5, containing 5 mmol/L MgCl2), by using a polytron homogenizer, for 90 s at 4°C. An equal volume of loading buffer was added to each sample. Samples were denatured by incubation for 7 min at 95°C. Proteins were separated by 16% Tricine gels (Invitrogen, Carlsbad, CA, USA) and transferred to polyvinylidene difluoride membranes (Invitrogen). The membranes were saturated with TBS-T (Tris-buffered saline with 0.1% Tween-20, pH 7.8) for 10 min and blocked with Western blocker solution (Sigma) for 120 min. Then, the membranes were hybridized with primary antibody diluted in the blocker solution for 120 min at room temperature. Anti–ECE-1 (United States Biological, Swampscott, MA, USA) was diluted to 0.5 μg/mL, anti MMP-1 antibody (Sigma) was diluted to 5 μg/mL and anti–β-actin (Sigma) and anti–glyceraldehyde-3- phosphate dehydrogenase (GAPDH) (Sigma) were both diluted to 20 μg/mL dilution. The membranes were washed with TBS-T several times and incubated with secondary antibody, anti–rabbit IgG, horseradish peroxidase–linked whole antibody (Amersham Biosciences, GE Healthcare, Little Chalfont, Buckinghamshire, UK), at a 1:2500 dilution in Western blocker solution at 4°C for 120 min. The membranes were washed again and treated with an ECL Plus Western blotting detection system (Amersham Biosciences) and chemifluorescence was detected by exposure to a phosphor screen (GE Healthcare). Densitometric quantification of the Western blot signal intensity of the bands was performed by a densitometric analysis program. The density values of GAPDH or actin were used for normalization. At least four different mice from each group were included in these experiments.
Tocolysis with GM6001
For mouse models of inhibition of labor and delivery (tocolysis) achieved through MMP-1 inhibition, 19 mice at E15.5 were injected with 3.3 mg/kg LPS. Mice receiving the MMP inhibitor were injected ip 12 h after the LPS injection with 300 μg of GM6001 (Chemicon, Temecula, CA, USA), also known as Ilosastat or N-[(2R)-2-(hydroxamidocar-bonylmethyl)-4-methylpentanoyl]-L-tryptophan methylamide, in 500 μL of a 12% ethanol solution. GM6001 inhibits MMP-1 with a Ki (dissociation constant for inhibitor binding) of 0.4 nmol/L (16). Control mice were injected ip 12 h after the LPS injection with 500 μL of a 12% ethanol solution, which contained no inhibitor.
RNA Knockdown
The target sequence of ECE-1 small interfering RNA (siRNA) (Dharmacon RNA Technologies, Lafayette, CO, USA) used was NNCUUCCACAGCCCC CGGAGU. The sense and antisense sequences of siRNAs were, respectively, CUUCC ACAGCCCCCGGAGUdTdT and ACUCCGGGGGCUGUGGAAGdTdT. Thirteen mice were randomly assigned to two groups: control group (n = 7) and RNA knockdown group (n = 6). All animals received an ip injection of LPS (9.9 mg/kg) diluted in 0.5 mL PBS at E15.5. Mice were anaesthetized with Cetacaine topical anesthetic spray (Cetylite Industries, Pennshauken, NJ, USA) through the tail skin. All mice in the RNA knockdown group were rapidly injected in the tail vein with 500 μg of ECE-1 siRNA dissolved in 0.5 mL PBS, by use of a hydrodynamic transfection method, three times, at scheduled intervals: 30, 12 and 3 h before LPS injection. All control mice received 0.5 mL PBS injected into the tail vein three times, by use of the same method and at the same scheduled times as the RNA knockdown group. All mice were observed for preterm delivery within 24 h of LPS injection. Placental tissue was collected either immediately after delivery or at hysterectomy, 24 h after LPS injection. Uteri were collected at hysterectomy 24 h after LPS injection. All animals were sacrificed to confirm pregnancy and count retained pups. The calculation of the percent of pups delivered was based on the total number of pups produced by the pregnant mice in the study, that is, the sum of the number of pups delivered plus the number of pups retained and found in utero at autopsy. We knew from experience that LPS-treated mice are sedentary and do not develop preterm labor and delivery for at least 6 to 8 h after injection. Beginning 8 h after the LPS injection, at the latest, an investigator remained with the injected mice and observed them, recording delivery times and number of fetuses delivered. The continual direct observation ensured that delivered pups were not ingested.
Statistical Analysis
Statistical significance of changes in protein expression detected by Western blotting analysis was evaluated with the Student t test. Statistical significance of effects of GM6001 and ECE-1 RNAi on LPS-induced preterm labor and numbers of pups retained in utero were evaluated with the Fisher exact test and the Mann–Whitney test.
RESULTS
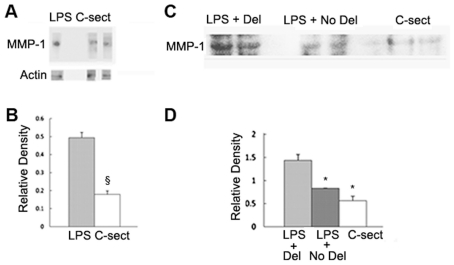
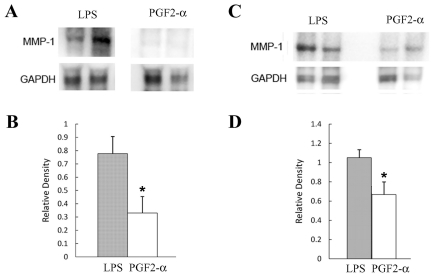
We tested for changes in expression of MMP-1 in placental and uterine tissues in the following four groups of C57Bl/6 E15.5/16.0 mice: mice that received LPS and developed premature delivery, mice that received the same dose of LPS as in the first group but did not develop labor or delivery, control mice that received vehicle and underwent Cesarean hysterectomy and mice that received PGF2α and developed premature delivery. Western blot analysis clearly showed elevation in MMP-1 expression in placentas of the first group of mice when compared with Cesarean hysterectomized controls (P < 0.00005) (Figure 1A, B). MMP-1 was also upregulated in the uterus in LPS-treated mice induced to deliver compared with either LPS-treated mice that did not progress to delivery (P < 0.05) or the Cesarean hysterectomized controls (P < 0.05) (Figure 1C, D). MMP-1 was also elevated in placental tissue in mice induced to deliver with LPS compared with the non-infectious mouse models induced with PGF2α (P < 0.05) (Figure 2A, B). Finally, MMP-1 was upregulated in the uterus in mice that developed preterm labor and delivery as a result of LPS injection compared with mice induced with PGF2α (P < 0.05) (Figure 2C, D).
Figure 1.
Elevated MMP-1 expression in gestational tissues from mice induced to deliver with LPS compared with nondelivering mice. Immunoblots were prepared using homogenates of placentas (A and B) collected from C57Bl/6 E16 mice induced to labor and delivery with 6.6 mg/kg LPS (n = 8) and E16 mice that underwent Cesarean hysterectomy (n = 9) and from homogenates of uteri (C and D) collected from E16 mice induced to labor and delivery with LPS (n = 8), E16 mice that received identical doses of LPS but did not progress to delivery (n = 7) and E16 mice that underwent Cesarean hysterectomy (n = 9). Animal experiments were performed with approximately three animals a time and were carried out on at least 10 different days over the course of several weeks. Blots were incubated with rabbit anti–MMP-1 antibody and developed with horseradish peroxidase–linked goat antirabbit antibody followed by treatment with an ECL Plus Western blotting detection system. Blots were stripped and reprobed with either anti-β actin or anti-GAPDH antibody as gel loading controls, and the ratio of the density of the MMP-1 band divided by the density of the gel loading control band was determined for each sample. All gel electrophoresis and Western blot experiments were run at least three times. Representative results are shown in A and C. Results including all samples are shown in B and D. §, P < 0.00005; *, P < 0.05.
Figure 2.
Elevated MMP-1 expression in gestational tissues from mice induced to deliver with LPS compared with mice induced with PGF-2α. Immunoblots were prepared by using homogenates of placentas (A and B) and uteri (C and D) collected from C57Bl/6 E16 mice induced to labor and deliver with 6.6 mg/kg LPS (n = 8) and E16 mice induced to deliver with PGF-2α (n = 4). Animal experiments were performed with approximately three animals at a time and were carried out on at least 10 different days over the course of several weeks. Blots were incubated with rabbit anti–MMP-1 antibody and developed with horseradish peroxidase–linked goat antirabbit antibody followed by treatment with an ECL Plus Western blotting detection system. Blots were stripped and reprobed with anti-GAPDH antibody as gel loading control, and the ratio of the density of the MMP-1 band divided by the density of the gel-loading control band was determined for each sample. All gel electrophoresis and Western blot experiments were run at least three times. (A and C) representative results; (B and D) results including all samples. *, P < 0.05.
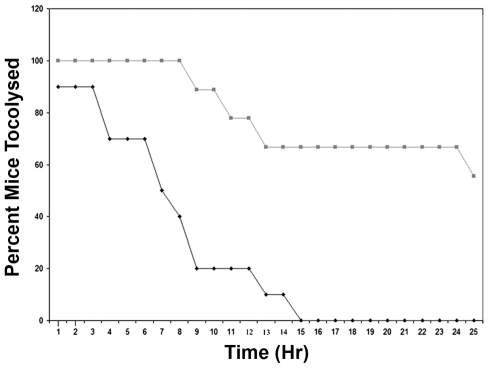
Upregulation of MMP-1 in the LPS-induced mouse models suggested that this enzyme played a critical role in the pathogenesis of infection-associated preterm labor. To test this hypothesis, we attempted to tocolyse LPS-treated E15.5 mice with the MMP inhibitor GM6001. Of the nine LPS-induced mice treated with the MMP inhibitor, four were successfully tocolyzed. In contrast, of the 10 LPS-induced mice that received only vehicle, none were successfully tocolyzed; within 20 h of receiving the LPS injection, nine mice delivered and one died (Figure 3, Table 1). The tocolytic effect of the MMP inhibitor was significant (P < 0.01). Furthermore, even among the mice that ultimately developed preterm labor, delivery was significantly delayed by GM6001 (P < 0.005) (Figure 3).
Figure 3.
Tocolysis of LPS-treated E.15.5 mice with GM6001. Nineteen C57Bl/6 mice were injected with 3.3 mg/kg LPS at E15.5. Nine were injected ip with GM6001 at E16 and 10 control mice were injected with vehicle at E16. Mice were checked every 15 min, and the number of mice successfully tocolyzed versus the number of mice having delivered was recorded. Animal experiments were performed with approximately three animals at a time and were carried out on at least 10 different days over the course of several weeks. Squares, mice treated with GM6001; diamonds, control mice injected with vehicle.
Table 1.
Effect of tocolytic methods in a murine model of inflammation-associated preterm birth.
| Tocolytic method | n | Number tocolyzed | Percent tocolyzed |
|---|---|---|---|
| Tocolysis experiment 1 | |||
| GM6001 | 9 | 5 | 56 |
| None | 10 | 0 | 0 |
| Tocolysis experiment 2 | |||
| ECE-1 RNAi | 6 | 5 | 83 |
| None | 7 | 2 | 29 |
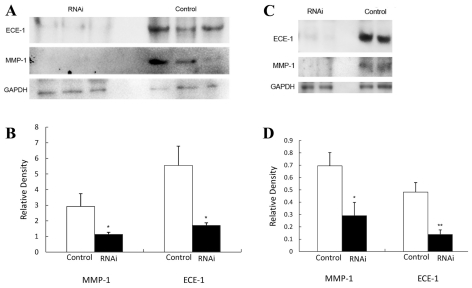
We have previously found that treating LPS-induced E15.5 mice with a commercially available ECE-1 inhibitor, a commercially available ET-1 receptor or our own synthetic ET-1–receptor antagonist results in tocolysis. (11,12,17). We hypothesized that activation of the ECE-1/ET-1 pathway triggers, in turn, upregulation of MMP-1. To test this hypothesis we knocked down the ECE-1/ET-1 pathway in six LPS-induced mice with ECE-1 siRNA and “treated” another seven control mice with only vehicle. Knockdown of ECE-1 was confirmed by Western blotting and in fact resulted in the elimination of the LPS-triggered rise in MMP-1 both in the placenta (P < 0.05) and the uterus (P < 0.05) (Figure 4).
Figure 4.
Elimination of the MMP-1 response in LPS-induced mice by ECE-1 knockdown. Thirteen C57Bl/6 E15.5 mice received 9.9 mg/kg ip LPS. Six of the mice underwent hydrodynamic transfection of ECE-1 RNAi. The target sequence of ECE-1 siRNA was NNCUUCCACAGCCCCCGGAGU. The sense and antisense sequences of siRNAs were, respectively, CUUCCACAGCCCCCGGAGUdTdT and ACUCCGGGGGCUGUGGAAGdTdT. The remaining seven mice underwent sham hydrodynamic transfection of vehicle. Animal experiments were performed with approximately two animals at a time and were carried out on at least 10 different days over the course of several weeks. Immunoblots were prepared by using homogenates of placentas (A and B) and uteri (C and D). Blots were probed with rabbit anti–ECE-1 antibody, to confirm success of mRNA knockdown, and then with anti–MMP-1 antibody. Blots were developed with horseradish peroxidase–linked goat antirabbit antibody followed by treatment with an ECL Plus Western blotting detection system. Blots were stripped and reprobed with anti-GAPDH antibody as gel loading control, and the ratio of the density of the MMP-1 band divided by the density of the gel loading control band was determined for each sample. All gel electrophoresis and Western blot experiments were run at least three times. (A and C) representative results; (B and D) results including all samples. *, P < 0.05; **, P < 0.01.
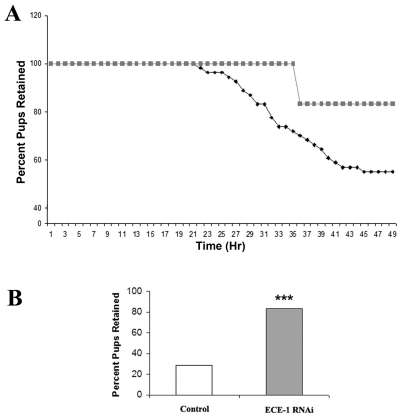
In the next experiment, we tested whether suppression of MMP-1 expression through ECE-1/ET-1 knockdown results in successful tocolysis. Two outcomes were tracked in this study: percentage of mice delivering over time and percentage of total pups dropped over time. In the control group, 71.4% of mice delivered their first pups within 24 h of LPS injection; 29% failed to deliver. In the MMP-1 suppressed group, only one (16.7%) of six mice delivered its first pups prematurely; 83% were successfully tocolyzed (P = 0.08) (Table 1). Furthermore, all mice in the control group that delivered prematurely delivered their first pup within 14 h of LPS injection, whereas the premature delivery time of the mouse in the MMP-suppressed group was at 17.4 h. No maternal death was observed in either group. MMP-1 suppression also decreased the percentage of prematurely delivered pups within 24 h of LPS injection: for the control group, 44% of pups were delivered prematurely, compared to only 2.4% for the ECE-1 knockdown/MMP suppression group (P < 0.005) (Figure 5).
Figure 5.
Effect of elimination of the MMP-1 response through ECE-1 knockdown on number of prematurely delivered pups. Thirteen C57Bl/6 mice were injected with 9.9 mg/kg LPS ip at E15.5. Six underwent hydrodynamic transfection of ECE-1 RNAi and seven underwent sham hydrodynamic transfection as described in the Figure 4 legend. At the end of the experiment the number of pups delivered and the number of pups retained in utero, determined by necropsy, was recorded for each mouse. (B) The proportion of total pups retained in utero in mice with elimination of MMP-1 response compared with control mice. Squares, mice treated with ECE-1 RNAi; diamonds, control mice treated with vehicle. ***, P < 0.005.
DISCUSSION
In this study we have shown that MMP-1 levels are increased in gestational tissues in pregnant mice induced to labor with LPS. We have also shown that tocolysis can be achieved in the LPS-stimulated mice with the MMP inhibitor GM6001, suggesting that MMP function is critical in the pathogenesis of preterm birth in this model. Because we have previously pursued several lines of investigation that demonstrated the role of the endothelin system in this same model (11,12,17) we were interested in investigating whether endothelin and MMP-1 acted in the same molecular pathway. Therefore we compared levels of MMP-1 in LPS-induced mice that were treated with RNAi directed at ECE-1 to levels of the protein in positive control LPS-induced mice with unaltered ET-1 systems. We observed that knocking down ET-1 synthesis knocked down MMP-1 upregulation as well. Furthermore, those mice in which ECE-1, and consequently MMP-1, levels were reduced were largely prevented from developing preterm delivery.
In the one mouse that did deliver in spite of receiving ECE-1 RNAi, the delivery was slightly delayed. Although the most successful type of tocolysis would prevent preterm delivery all together, any delay in delivery time in the setting of preterm labor reduces the risk of perinatal morbidity. In fact, transient extensions of the gestational period that are currently achieved in the clinic afford an opportunity for medical interventions that anticipate the early birth, such as steroid treatment to promote closure of the patent duct arteriosus and lung maturation. Pups in tocolyzed mice were identical to pups in nontocolyzed mice. There were no stillborn pups, pups with evidence of altered growth or pups with congenital anomalies found in the tocolyzed mice.
Several lines of investigation in other biologic systems have established that ET-1 affects the synthesis of MMPs (18). Koyama and Tanaka (19) have recently shown that ET-1 stimulates the production of MMP-3 in cultured rat astrocytes. Similarly, Manacu et al. (20) found that in primary cultures of human osteoarthritic chondrocytes the presence of ET-1 in the medium triggered increased production of MMP-1 and MMP-13. Felx et al. (21) found that ET-1 promotes induction of MMP-2 and MMP-9 in human osteosarcoma cells via the transcription factor nuclear factor–κβ. Furthermore, treatment of these malignant cells with ET- receptor antagonists decreases cell invasion. In ET-1–activated human optic nerve head astrocytes, levels of MMP-1, tissue inhibitors of MP (TIMP)-1 and TIMP-2 are all increased. These changes are mediated through the ERK map kinases and protein kinase C (22). ET-1 promotes cerebrovascular remodeling in type 2 diabetes through differential regulation of MMPs (23). Murray et al. (24) produced a marked increase in MMP-2 activity and a significant decrease in collagen volume fraction in rat hearts by administering ET-1. More recently, Murray et al. have shown that rats that underwent the surgical introduction of an aortocaval fistula had much less of a change in MMP-2 levels and in the collagen volume fraction of the heart if they were treated with bosentan, an endothelin receptor antagonist (25). Deschamps et al. (26) showed that the increase in MMP-1 levels in porcine hearts associated with ischemia and reperfusion injury did not occur if the animals were treated with the ET-1–receptor A antagonist BQ-123.
In addition to causing increased smooth muscle tone by binding the EA receptor, ET-1 triggers an inflammatory cytokine cascade via the endothelin B receptor–signaling pathway (10). Several inflammatory cytokines have been implicated in preterm birth, in particular, interleukin-1 and tumor necrosis factor-α (27–29). Because ET-1 is known to trigger upregulation of inflammatory mediators, and these cytokines in turn can induce MMPs, we hypothesize that knockdown of the endothelin pathway decreases MMP expression through attenuation of the inflammatory cascade.
Although we have previously reported that preventing the action of ET-1 (17), as well of MMPs (30), controls preterm birth individually, the data presented here are the first to show that ET-1 leads to production of MMPs in stimulated gestational tissue just as it does in injured myocardium and other pathologic settings (31). Although the role of endothelin in cardiovascular and other diseases has been investigated, its function in reproductive disorders is not as well understood. ET-1 does not serve as a therapeutic target for obstetrical disorders, because we know from work with both ET-1 knockout and ECE-1 knockout mice (32) that blockade of endothelin early in gestation leads to marked craniofacial anomalies and mortality in the perinatal period. Therefore the identification of another molecule in the same pathway, at which novel therapeutic approaches can be directed, is of potential clinical significance.
The results shown indicate that MMP expression is regulated by ET-1 in yet another system, distinct from any of those previously reported. This finding links an extremely potent vasoconstrictor and inflammatory mediator, ET-1, with a family of enzymes responsible for digestion of the extracellular matrix and tissue remodeling, the MMPs. Because of the important role played by the MMPs in remodeling of gestational tissues in normal parturition, we hypothesize that these enzymes act to weaken the tensile strength of the uterine cervix and extraplacental membranes in infection- associated preterm birth. A possible role of MMP-1 in luteolysis cannot be ruled out, however, and deserves further investigation in future studies. Moreover, studies are warranted to identify which particular MMPs besides MMP-1, if any, play a role in preterm birth mediated by ET-1. Taken together, these data, and the results of the work of other investigators discussed above, suggest that the ET-MMP connection is a general pathway in inflammation and tissue remodeling.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant 1K08HD1209 and by St. John’s University. We are grateful to H Scaramell and our animal facility staff for their assistance with our mouse colony and to Z Shi for his help with RNA knockdown.
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Vu T-D, et al. Placental matrix metalloproteinase-1 is up-regulated in labor. Reprod Sci. 2008;15:420–24. doi: 10.1177/1933719108314625. [DOI] [PubMed] [Google Scholar]
- 2.Koscica K, et al. The Effect of phosphoramidon on inflammation-mediated preterm delivery in a mouse model. Am J Obstet Gynecol. 2004;190:528–31. doi: 10.1016/j.ajog.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, et al. The endothelin converting enzyme-1 (ECE-1)/endothelin-1 (ET-1) pathway plays a critical role in infection-associated premature delivery in a mouse model. Am J Path. 2008;173:1–8. doi: 10.2353/ajpath.2008.080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olgun N, et al. The role of a 1,3,6-trisubstituted-2-carboxy-quinol-4-one, a novel putative selective ETA antagonist, in controlling preterm labor in a mouse model. Can J Physiol Pharmacol. 2008;86:571–75. doi: 10.1139/Y08-057. [DOI] [PubMed] [Google Scholar]
- 5.Simhan HN, Caritis SN. Prevention of preterm delivery. New Engl J Med. 2007;357:477–87. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, et al. Intrauterine infection and preterm delivery. New Engl J Med. 2000;342:1500–07. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 7.Lappas M, et al. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–73. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 8.Belt AR, et al. The nuclear transcription factor NF-kappaB mediates interleukin-1 beta induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–66. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- 9.Yanagisawa M, et al. A novel potent vasoconstrictor peptide produced by vascular en-dothelial cells. Nature. 1988;332:411–15. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 10.Kaya T, et al. Effects of endothelin-1 and calcium channel blockers on contractions in human myometrium. a study on myometrial strips from normal and diabetic pregnant women. J Reprod Med. 1999;44:155–21. [PubMed] [Google Scholar]
- 11.Yallampalli C, Garfield RE. Uterine contractile responses to endothelin-1 and endothelin receptors are elevated during labor. Biol Reprod. 1994;51:640–45. doi: 10.1095/biolreprod51.4.640. [DOI] [PubMed] [Google Scholar]
- 12.Wolff K, et al. Contractile effects of en-dothelin 1 and endothelin 3 on myometrium and small intramyometrial arteries of pregnant women at term. Gynecol Obstet Invest. 1993;36:166–71. doi: 10.1159/000292619. [DOI] [PubMed] [Google Scholar]
- 13.Woods M, et al. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999;55:902–09. [PubMed] [Google Scholar]
- 14.Motte S, et al. Endothelin receptor antagonists. Pharmacol Ther. 2006;110:386–414. doi: 10.1016/j.pharmthera.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Choi S, et al. Cervicovaginal matrix metal-loproteinase 9 and cervical ripening in human term parturtion. Eur J Obstet Gynecol Reprod Biol. 2009;142:43–47. doi: 10.1016/j.ejogrb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Vadillo-Ortega FG, et al. 92-kd Type iv collagenase (matrix-metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1999;116:43–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Galardy RE, et al. Low molecular weight inhibitors in corneal ulceration. Ann NY Acad Sci. 1994;732:315–23. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 18.Abraham D, et al. Connective tissue remodeling: cross-talk between endothelins and matrix metalloproteinases. Curr Vasc Pharmacol. 2005;3:369–79. doi: 10.2174/157016105774329480. [DOI] [PubMed] [Google Scholar]
- 19.Koyama Y, Tanaka K. Endothelins stimulate the production of stromelysin-1 in cultured rat astrocytes. Biochem Biophys Res Commun. 2008;371:659–63. doi: 10.1016/j.bbrc.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Manacu CA, et al. Endothelin-1 in osteoarthritic chondrocytes triggers nitric oxide production and upregulates collagenase production. Arthritis Res Ther. 2005;7:323–32. doi: 10.1186/ar1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felx M, et al. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci. 2006;110:645–54. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 22.He S, et al. Endothelin-1-mediated signaling in the expression of matrix metalloproteinases and tissue inhibitors of metallopro-teinases in astrocytes. Invest Ophthal Visual Sci. 2007;48:3737–45. doi: 10.1167/iovs.06-1138. [DOI] [PubMed] [Google Scholar]
- 23.Harris AK, et al. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–44. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 24.Murray DB, et al. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2295–99. doi: 10.1152/ajpheart.00048.2004. [DOI] [PubMed] [Google Scholar]
- 25.Murray DB, et al. Effects of nonselective endothelin-1 receptor antagonism on cardiac mast cell-mediated ventricular remodeling in rats. Am J Physiol Heart Circ Physiol. 2008;294:H1251–57. doi: 10.1152/ajpheart.00622.2007. [DOI] [PubMed] [Google Scholar]
- 26.Deschamps AM, et al. Interruption of en-dothelin signaling modifies membrane type 1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H875–83. doi: 10.1152/ajpheart.00918.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 29.Dudley DJ, et al. Inflammatory cytokine mRNA in human gestational tissue: implications for term and preterm labor. J Soc Gynecol Investig. 1996;3:328–335. [PubMed] [Google Scholar]
- 30.Koscica K, et al. The effect of a matrix metalloproteinase inhibitor on inflammation-mediated preterm delivery. Am. J. Obstet. Gynecol. 2007;196:551.e1–551.e3. doi: 10.1016/j.ajog.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Olgun NS, Reznik SE. The matrix metalloproteases and endothelin-1 in preterm birth. Obstet Gynecol Int. 2010;2010;657039 doi: 10.1155/2010/657039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagisawa H, et al. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–36. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]