Abstract
OBJECTIVE
Our objective was to assess whether sex hormone levels are associated with subsequent development of prostate cancer.
METHODS
A case-cohort study was conducted within the ongoing Osteoporotic Fractures in Men cohort study of community-dwelling men ≥65 years old recruited at 6 US clinical sites. After a mean follow-up of 4.7 years, all men with incident confirmed prostate cancer and a random sample of the full cohort (subcohort) were selected for analysis: after excluding men with a history of prostate cancer and those who reported androgen or antiandrogen therapy at baseline, the resulting analytic sample comprised 275 cases and 1652 non-cases with complete sex hormone measurements. Serum testosterone, estradiol, estrone, and sex hormone-binding globulin were assayed at baseline (pre-diagnosis) by gas chromatography combined with mass spectrometry. Associations between incident prostate cancer and each sex hormone were evaluated using Cox proportional hazards regression models adjusted for age, race, study site, body mass index, and person-time.
RESULTS
In the subcohort, the mean age was 73 years. Higher serum estrone was strongly related to an increased risk of prostate cancer: compared to men in the lower quartile, the risk of prostate cancer among those in the highest three quartiles (>24.9 pg/dl) was nearly four-fold higher (adjusted HR=3.93, CI: 1.61–9.57). Other sex hormones were not associated with the risk of prostate cancer.
CONCLUSIONS
In this cohort of older men, higher estrone levels were strongly associated with an increased risk of incident prostate cancer. This association between estrone and prostate cancer risk needs to be clarified by further study.
Keywords: prostate cancer, sex hormones, estrone, risk
Prostate cancer is the leading cause of cancer in United States men. Well established risk factors for prostate cancer are African American race, older age, and having a first degree relative with a history of prostate cancer [1]. Prospective cohort studies suggest that other potential risk factors for prostate cancer include high consumption of dairy food, red meat, and saturated fat [1]. A history of prostatitis or sexually transmitted diseases and other undefined risk factors also may contribute to the development of prostate cancer [2–3]. Although sex steroids, particularly androgens, have been implicated in the pathogenesis of prostate cancer, most epidemiologic studies have found no association, though a small number of studies have shown assoiciations both positive and negative [4–8].
The influence of sex hormones on prostate cancer risk remains poorly understood. Epidemiological studies that have evaluated sex hormones and prostate cancer risk have primarily been individual case-control studies, and meta-analyses of prospective and nested case-control studies. Studies have traditionally measured serum androgens and estrogens using radioimmunoassays (RIA). Recent studies, which have inconsistently shown a positive relationship between sex hormones and prostate cancer, illustrate a need to re-examine the possible link between sex hormones and prostate cancer [4–8]. Therefore, in order to clarify the association between sex hormone levels and incident prostate cancer in older men, we analyzed data from the Osteoporotic Fractures in Men (MrOS) study, a longitudinal study of community-dwelling older men [9–10]. In addition, the use of gas chromatography combined with mass spectrometry, used in MrOS to measure sex hormones, provide more precise and reliable data on specific sex hormone levels compared to radioimmunoassays used in previous studies [11]. These measurements used in our study are now considered the gold standard for measuring sex steroids.
MATERIALS AND METHODS
Study Population
We conducted a case-cohort study within the MrOS study. MrOS is a community-based study of 5,995 men from six geographic regions of the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. To be a MrOS participant, men had to be at least 65 years old, able to walk without the assistance of another person, not have had bilateral hip replacements, and have completed a minimum set of study measures (self-administered questionnaire, height, weight, bone density scan and vertebral radiograph).Body composition measures were obtained using dual energy x-ray absorptiometry (DXA) (Hologic, Inc., MA). Participants were recruited from their communities, using population-based listings, such as motor vehicle registrations, targeted community presentations, and community and senior newspaper advertisements. Responses to mass mailings at some sites surpassed 10% to 15%, and appointment show rates averaged above 85%, reflecting a high interest and commitment to this study. The Institutional Review Board at each clinical site approved the study protocol, and written informed consent was obtained from all study participants.
Prostate Cancer Adjudication
Prostate cancer cases were identified through self-report using a mailed triannual follow-up questionnaire. For study participants who did not return the questionnaire, information about prostate cancer diagnosis was elicited through in-person or telephone interviews. For each prostate cancer report, medical records were requested from the hospital or clinic including the following: pathology reports, Prostate Specific Antigen (PSA) laboratory reports, clinical notes, reason for prostate biopsy, staging studies, and treatment notes. All medical records were reviewed and prostate cancer diagnosis adjudicated centrally at the MrOS coordinating center (University of California, San Francisco) without knowledge of sex hormone levels.
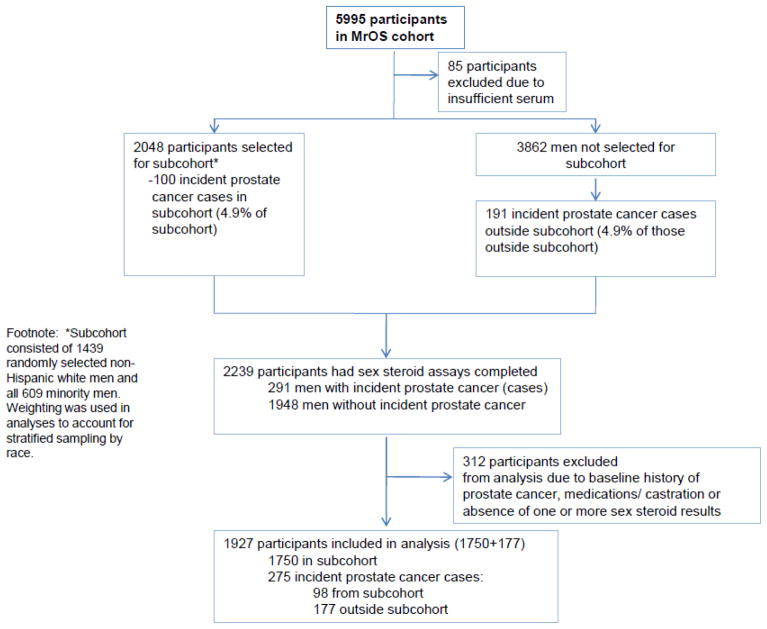
Subjects completed questionnaires about demographic, clinical and lifestyle factors and submitted serum samples for analysis of testosterone, estradiol, estrone, and sex hormone-binding globulin (SHBG). Figure 1 shows the case-cohort design for the MrOS sex steroids and prostate cancer study. All participants who had at least five 1-ml aliquots of stored serum available for sex steroid assays were eligible for inclusion in the baseline sample (N=5910 [including 5301 white and 609 minority]). The subcohort consisted of 1439 randomly selected non-Hispanic white men and all 609 minority men from the total 5910 eligible. Serum was stored at −70 degrees. Sex hormones were assayed by gas chromatography combined with mass spectrometry (Taylor Technology; Princeton, New Jersey) and free and bioavailable testosterone and estradiol were calculated using the method of Södergard, et al [12]. Sex steroids assays were completed by Taylor labs (Princeton, New Jersey) and included total estradiol, total testosterone, and estrone. SHBG was analyzed by the Oregon Health & Science Center OCTRI labs.
Figure 1.
Case-cohort design for the MrOS sex steroids and incident prostate cancer study
Data Analysis
A case-cohort study was conducted within the ongoing MrOS cohort study to examine time-to-event outcomes (incident prostate cancer diagnosis) [13]. The case-cohort design involved stratified random sampling of participants with sufficient serum available from the baseline visit (March 2000–April 2002). Strata were 1) non-Hispanic white and 2) all Hispanic and non-white participants. These two groups comprised the subcohort (N=1750) and were followed from enrollment (which began in March 2000) through July 2006 for incident prostate cancer. In July 2006, all incident prostate cancer cases (including those not in the subcohort) that had occurred from March 2000 through July 2006 made up the case group for this case-cohort study. Men with a history of prostate cancer at baseline (N=229) and those who reported androgen or anti-androgen therapy at baseline (N=96) were excluded (total N=325). The resulting analytic sample comprised 275 cases and 1652 non-cases with complete sex hormone measurements.
Descriptive analyses included examination of distributions for continuous variables and frequencies for categorical variables. Spearman correlation coefficients (r) were calculated for several variables, including sex hormones, SHBG, body mass index (BMI), and American Urological Association (AUA) symptom score [14]. Associations between time to incident prostate cancer and each sex hormone were evaluated using Cox proportional hazards regression models. Person-time was weighted to account for stratified sampling and the case-cohort design [15]. Hazard ratios (HR) and 95% confidence intervals (CI) were examined per standard deviation (SD) change and by quartile of each sex hormone. For each sex steroid variable, the unadjusted HR and the age- and race-adjusted HR were calculated. In addition, the percent change in the HR was calculated between the unadjusted model and after adjustment for potential confounders, including study site, family history of prostate cancer, smoking, alcohol use, physical activity scale for the elderly (PASE) score [16], history of hypertension or diabetes, BMI, and weight. If the inclusion of a potential confounder altered the unadjusted HR of any of the sex steroid variables by 5% or more, it was retained in the multivariate model. We assessed the linearity of relationships using restricted cubic spline models and plots based on Cox proportional hazard regression models [17]. All statistical analyses were performed with SAS software, version 9.1.3 Service Pack 4 (SAS Institute Inc., Cary, NC).
RESULTS
In the randomly selected subcohort, the mean age was 73 years (Table 1). The average duration of follow-up for the whole cohort and for the subcohort after exclusions was 4.7 ± 0.9 years. Two-hundred seventy-five men were diagnosis-confirmed with prostate cancer during the follow-up period. The median time between sex hormone blood draw and prostate cancer diagnosis was 2.4 years (range: 15 days to 3.6 years). Men who did not develop prostate cancer (the majority of the analytic sample) were followed until the end of the study period, resulting in an overall mean follow-up time greater than that for cases alone. Sixty percent of the cases had a T1 stage, and approximately 50% had a Gleason summary score of ≧7 and a poorly differentiated histological stage.
Table 1.
Baseline characteristics of men in the subcohort and of men with incident prostate cancer (not mutually exclusive).
| Subcohort (N=1750) Mean ± SD, Median (IQR), or % | Prostate cancer cases (N=275) Mean ± SD, Median (IQR), or % | |
|---|---|---|
| Demographics | ||
| Age | 73.2 ± 5.7 | 72.6 ± 5.1 |
| Race | ||
| White, non-Hispanic | 70.5 | 90.6 |
| African-American | 11.0 | 4.0 |
| Asian | 8.7 | 2.6 |
| Hispanic | 6.2 | 1.8 |
| Other | 3.5 | 1.1 |
| College graduate | ||
| No | 48.7 | 47.3 |
| Yes | 51.3 | 52.7 |
| Study Site | ||
| Birmingham | 16.9 | 10.6 |
| Minneapolis | 14.5 | 23.6 |
| Palo Alto | 20.2 | 20.0 |
| Pittsburgh | 14.2 | 16.0 |
| Portland | 17.1 | 13.8 |
| San Diego | 17.1 | 16.0 |
| History of prostate cancer | ||
| Any relative | 12.6 | 18.2 |
| First-degree relative | 10.8 | 16.4 |
| Lifestyle/diet | ||
| Smoking | ||
| Never | 37.1 | 42.2 |
| Ever | 62.9 | 57.8 |
| Alcohol consumption in past year | ||
| None | 36.0 | 29.1 |
| 0–7drinks/week | 38.6 | 44.7 |
| ≥ 7 drinks/week | 25.3 | 25.5 |
| Physical activity score (PASE) | 146.7 ± 69.3 | 157.7 ± 70.8 |
| Anthropometric measures | ||
| BMI (kg/m2) | 27.3 ± 3.7 | 27.3 ± 3.6 |
| Weight (kg) | 82.6 ± 13.4 | 82.5 ± 12.5 |
| Height (cm) | 173.6 ± 7.1 | 173.8 ± 6.6 |
| Glucose level (mg/dl) | 100.0 (17.0) | 100.0 (16.0) |
| Insulin level (ƒÊIU/ml) | 7.7 (6.3) | 7.6 (5.3) |
| Medical history | ||
| History of hypertension | 45.8 | 42.9 |
| History of diabetes | 12.6 | 8.7 |
| AUA prostate symptom score | 7.0 (9.0) | 6.0 (9.0) |
| Body Composition Measures | ||
| Total body fat mass (kg) | 21.5 ± 7.0 | 21.2 ± 6.6 |
| Total body lean mass (kg) | 57.8 ± 7.7 | 57.8 ± 7.1 |
| Trunk fat mass (kg) | 12.1 ± 4.2 | 12.1 ± 4.1 |
| Sex Steroid Measures | ||
| Total testosterone (ng/dl) | 411 ± 156 | 415 ± 153 |
| Bioavailable testosterone (ng/dl) | 207 ± 62 | 214 ± 57 |
| Free testosterone (ng/dl) | 7.9 ± 2.3 | 8.1 ± 2.1 |
| Total estradiol (pg/ml) | 22.9 ± 7.7 | 22.6 ± 6.9 |
| Bioavailable estradiol (pg/ml) | 14.6 ± 4.7 | 14.7 ± 4.4 |
| Free estradiol (pg/ml) | 0.54 ± 0.17 | 0.54 ± 0.16 |
| Estrone (pg/ml) | 33.8± 13.7 | 33.4± 12.2 |
| SHBG (nM) | 49.4 ± 19.8 | 47.4 ± 19.2 |
| Total testosterone:estradiol ratio | 18.8 ± 6.8 | 19.2 ± 6.7 |
In the subcohort, mean total testosterone was 411 ng/dl; mean bioavailable testosterone was 207 ng/dl; mean free testosterone was 7.9 ng/dl; mean estradiol was 23 pg/ml; mean estrone was 33.8 pg/dl, and mean SHBG was 49.4 nM.
Spearnan correlation coefficients for continuous variables, including sex steroids variables, were calculated among men in the subcohort. Weak correlations were detected between all sex hormones and age, BMI, height, weight, and AUA symptom scores (Table 2). As expected, there was a strong correlation between testosterone and SHBG (r=.68) and estrone and estradiol (r=.65); and weaker but significant correlations between free testosterone and free estradiol (r =.33) and free testosterone and SHBG (r =.31).
Table 2.
Spearman correlation coefficients (p value) for continuous variables among Subcohort)
| T | Free T | Bio T | E2 | Free E2 | Bio E2 | SHBG | T:E2 ratio | |
|---|---|---|---|---|---|---|---|---|
| Age | −0.03 (0.26) | −0.15 (<0.0001) | −0.17 (<0.0001) | 0.04 (0.12) | −0.04 0.14) | −0.06 (0.01) | 0.21 (<0.0001) | −0.06 (0.01) |
| BMI | −0.31 (<0.0001) | −0.25 (<0.0001) | −0.26 (<0.0001) | 0.02 (0.40) | 0.15 (<0.0001) | 0.14 (<0.0001) | −0.29 (<0.0001) | −0.34 (<0.0001) |
| Height | −0.05 (0.03) | −0.05 (0.07) | −0.04 (0.09) | −0.01 (0.54) | −0.02 (0.51) | −0.006 (0.81) | −0.04 (0.10) | − 0.03 (0.27) |
| Weight | −0.28 (<0.0001) | −0.23 (<0.0001) | −0.24 (<0.0001) | 0.01 (0.58) | 0.12 (<0.0001) | 0.12 (<0.0001) | −0.26 (<0.0001) | −0.30 (<0.0001) |
| AUA score | 0.0008 (0.97) | −0.004 (0.89) | −0.02 (0.48) | −0.04 (0.09) | −0.05 (0.06) | −0.06 (0.02) | 0.02 (0.37) | 0.04 (0.09) |
| PASE score | 0.04 (0.08) | 0.09 (0.0007) | 0.09 (0.0002) | −0.05 (0.06) | −0.03 (0.17) | −0.03 (0.28) | −0.03 (0.21) | 0.08 (0.0006) |
Quartile data for sex hormones clearly illustrate a strong association between incident prostate cancer risk and increasing estrone levels, but not for other sex hormones. After adjusting for age, race, study site, BMI, and person-time weighted to account for stratified sampling and the case-cohort design, the adjusted HR for prostate cancer was 3.93 (CI: 1.61–9.57) for the upper three quartiles of estrone compared to the lowest quartile (Table 3). The adjusted HR for men in the upper three quartiles compared to the lower quartile of free testosterone and free estradiol were 1.23 (CI: 0.88–1.72) and 1.27 (CI: 0.94–1.71), respectively.
Table 3.
Hazard ratios (95% CI) for selected sex steroid measures and incident prostate cancer.
| Multivariate* adjusted HR (95% CI) | |
|---|---|
| Total testosterone (per SD increase) | 1.02 (0.90–1.16) |
| Quartile 1 (< 301 ng/dl) | ref |
| Quartile 2 (301–391 ng/dl) | 1.01 (0.71–1.42) |
| Quartile 3 (392–494 ng/dl) | 1.07 (0.76–1.50) |
| Quartile 4 (> 494 ng/dl) | 1.00 (0.70–1.43) |
| Free testosterone (per SD increase) | 1.09 (0.96–1.23) |
| Quartile 1 (<6.34 ng/dl) | ref |
| Quartile 2 (6.34–7.77 ng/dl) | 1.23 (0.86–1.76) |
| Quartile 3 (7.78–9.21 ng/dl) | 1.42 (1.00–2.01) |
| Quartile 4 (>9.21 ng/dl) | 1.27 (0.88–1.83) |
| Total estradiol (per SD increase) | 1.03 (0.91–1.16) |
| Quartile 1 (< 17.5 pg/ml) | ref |
| Quartile 2 (17.5–21.9 pg/ml) | 0.94 (0.67–1.31) |
| Quartile 3 (22.0–27.2 pg/ml) | 1.12 (0.81–1.56) |
| Quartile 4 (>27.2 pg/ml) | 0.95 (0.67–1.34) |
| Free estradiol (per SD increase) | 1.09 (0.96–1.23) |
| Quartile 1 (< 0.42 pg/ml) | ref |
| Quartile 2 (0.42–0.51 pg/ml) | 1.24 (0.88–1.74) |
| Quartile 3 (0.52–0.63 pg/ml) | 1.44 (1.04–2.00) |
| Quartile 4 (>0.63 pg/ml) | 1.26 (0.89–1.79) |
| Estrone (per SD increase) | 1.04 (0.91–1.19) |
| Quartile 1 (< 24.9 pg/ml) | ref |
| Quartile 2 (24.9–31.7 pg/ml) | 3.56 (1.47–8.61) |
| Quartile 3 (31.8–40.4 pg/ml) | 4.16 (1.79–9.69) |
| Quartile 4 (>40.4 pg/ml) | 3.46 (1.52–7.85) |
| SHBG (per SD decrease) | 1.10 (0.96–1.27) |
| Quartile 4 (> 59.1 nM) | ref |
| Quartile 3 (45.9–59.1 nM) | 0.95 (0.66–1.35) |
| Quartile 2 (35.5–46.0 nM) | 1.16 (0.82–1.64) |
| Quartile 1 (<35.5 nM) | 1.24 (0.86–1.77) |
| T:E2 ratio (per SD increase) | 0.98 (0.86–1.11) |
| Quartile 1 (< 2.64) | ref |
| Quartile 2 (2.64–2.87) | 1.10 (0.77–1.57) |
| Quartile 3 (2.88–3.11) | 1.16 (0.81–1.65) |
| Quartile 4 (>3.11) | 1.01 (0.70–1.46) |
Age, race, study site, body mass index. Person-time was weighted to account for stratified sampling and the case-cohort design. Ref: reference group.
T (Testosterone), E2 (Estradiol), SHBG (Sex Hormone-Binding Globulin)
Results from Cox proportional hazards regression adjusted models conducted using only white participants were similar to those reported here for all participants. There were no significant interactions by race (white vs. non-white) with E1, E2, T or SHBG (all p>0.3).
Adjusted hazard ratios were not greater for Gleason score >=7 (2.71, 95% CI: 1.04–7.07) than for Gleason score <7 (8.95, 95% CI: 1.20–66.6) for estrone. The linearity of relationships using restricted cubic spline models and plots based on Cox proportional hazard regression models showed that estrone was nonlinear (p for linearity from cubic spline model: 0.007) and the quartile analysis indicated that there may be a threshold near 17 pg/ml.
COMMENT
In this large cohort of older men, higher estrone levels were strongly associated with an increased risk of incident prostate cancer. Few studies have explored the role of estrone in prostate cancer pathogenesis because of the difficulty in measuring low circulating levels in men using older assays. Estrone sulfate has been shown to be a prognostic marker for tumor aggressiveness in prostate cancer [18]. Estrone sulfate is converted to estrone by the enzyme steroid sulfatase (STS). STS is important in the formation, regulation, and conversion of inactive sex steroids into biologically active sex steroids, which are known to stimulate tumor growth in breast cancer, as well as prostate cancers [19]. Research into STS inhibitors has shown promise, similar to aromatase inhibitors for breast cancer, and these inhibitors may become increasingly important in future prostate cancer management [20].
Although androgens are known to stimulate prostate cell proliferation, results of previous studies examining increased androgen levels as a risk factor for prostate cancer have been inconclusive. Most of the case-control studies have found no association between sex hormones and prostate cancer [4–6]. A meta-analysis in 1998, which systematically reviewed 8 quantitative studies, also found no association between sex hormones and prostate cancer, except for androstanediol-glucoronide (A-diol g) which had an overall odds ratio (OR) of 1.05; 95% CI 1.00–1.11) for all of the studies combined [7]. However, a more recent meta-analysis, published in 2000, found approximately a 2-fold increased risk of prostate cancer in men with either serum total testosterone and insulin-like growth factor 1 (IGF-1) in the upper quartile of the study population [21]. Furthermore, the most significant recent analyses of this relationship was reported by the Baltimore Longitudinal Study of Aging (2005), which showed a positive relationship between elevated serum testosterone and an increased risk of prostate cancer [22]. In a European prospective study of Scandinavian men, high levels of circulating androgens were not associated with an increased prostate cancer risk [6, 23]. Our study results extend previous findings and are concordant with those from systematic reviews and other published data, which have found no association between endogenous androgens and prostate cancer risk [5, 7–8]. From mouse and human studies, including in vivo and in vitro studies, it is clear that androgens positively influence prostate gland growth; however, little is unknown how hormone levels interact with each other and whether or how they influence malignant growth in humans [24]. On the other hand, we also know clinically that androgen deprivation, often used as a form of prostate cancer treatment, can induce prostate cancer remission or retardation.
Our study has several limitations. Our follow-up was approximately 5 years and it is unclear whether extended follow-up would change the association. In the Massachusetts Male Aging Study (MMAS), which had 8 years of follow-up, researchers found no association between sex hormones measured by radioimmunoassay, including estrone, and prostate cancer risk [5]. Our study, in contrast to the MMAS, had a larger number of prostate cancer (MrOS=275 prostate cancer cases vs. MMAS=70 prostate cancer cases) which may have increased our ability to detect differences between cases and subcohort [5, 25–26]. Furthermore, in the MrOS study, we centrally adjudicated all prostate cancer diagnoses, while the MMAS identified prostate cancer cases through the Massachusetts cancer registry, medical records, self-report (confirmed by registry and/or medical records), death certificates, and medical records. We measured hormone levels in a single serum sample at one point in time, with a median of 2.4 years prior to prostate cancer diagnosis. Whether serial measurements of sex hormones over time are a better index of hormone status is unknown. In the Baltimore Longitudinal Study of Aging study, which evaluated serial hormone level measurements over nearly 40 years (median follow-up of 18.5 years for all study participants), higher levels of calculated serum free testosterone were associated with increased risk of prostate cancer [22]. Unfortunately, this study used less reliable sex hormone concentration measurements by using mass action equations for estimating free testosterone in serum [22, 27].
Our study also had several important strengths, including its sample size, volunteer participants from the general community, all study participants were closely followed, all prostate cancer cases were centrally adjudicated, and sex hormones were assayed by gas chromatography combined with mass spectrometry.
CONCLUSIONS
In conclusion, in this large prospective study we found that higher levels of estrone, but not androgens and other estrogens, were associated with a substantially higher risk of prostate cancer. A recent meta-analysis which examined endogenous sex hormones and prostate cancer did not find an association between androgens and estradiol and prostate cancer risk, although this collaborative analysis did not specifically report results for estrone and prostate cancer risk likely in part due to the fact that few studies have studied this relationship [8]. It is unclear from our study and other similar studies whether a single pre-diagnosis serum sample for sex hormones is useful to predict prostate cancer risk, although estrone was strongly associated with prostate cancer risk in our study. It may be that multiple or serial sex hormone measurements using highly accurate assays are needed to further define the influence of sex hormones on prostate cancer risk. It is possible that serum sex hormone levels do play a significant in prostate cancer, and perhaps, the specific interaction at the sex hormone receptor level in the prostate gland may play a significant role. Some studies have found that high levels of androgen receptors are associated with increased prostate cell proliferation, markers of aggressive prostate cancer, and are predictive of decreased recurrence-free survival [28]; these findings suggest an important role of the androgen receptor on prostate cancer development and progression, and the potential role of highly-selective androgen receptor inhibitors in the prevention and treatment of prostate cancer.
The role of endogenous and dietary estrogens, changes in the androgen:estrogen ratios as a man ages, and the influence of estrogen receptors on the prostate in the development of prostate cancer have also been suggested as important mediators of prostate cancer pathogenesis [29]. The prostate gland contains estrogen receptors and studies in rodents have shown that the prostate gland is sensitive to increased levels of estrogens, suggesting that it is plausible that there may be direct receptor-mediated effects of estrone on the prostate gland [29]. Rodents exposed to estrogens early during life have shown an increase prostate gland proliferation, inflammation, and dysplastic prostate epithelial changes later in life [29]. In addition, adult rodents with exposed to exogenous estrogens along with androgens have been shown to lead to prostate epithelial metaplasia, prostatic intraepithelial neoplasia-type lesions and adenocarcinoma of the prostate. Furthermore, antiestrogen inhibition in transgenic mouse models has lead to a reduction in prostate cancer development beyond prostatic intraepithelial neoplasia-type lesions. Epidemiologic data show that African-American men, who have the highest risk for developing prostate cancer, have significantly higher estrone plasma levels compared to European-Americans [30]. Therefore, further study is needed to determine the influence of the estrone-specific receptor activity on prostate cell malignant growth. Further inquiries, into the role of estrone as a biomarker for incident prostate cancer risk, may help to determine whether steroid sulfatase inhibitors will be effective in the management of prostate cancer.
Acknowledgments
The Osteoporotic Fractures inq Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. Presented in part: Annual meeting of the American Urological Association, Chicago, 26 April 2009 (podium session presentation).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gann PH. Risk factors for prostate cancer. Rev Urol. 2002;4 (Suppl 5):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes RB, Pottern LM, Strickler H, et al. Sexual behavior, STDs and risks for prostate cancer. Brit J Cancer. 2000;82:718–725. doi: 10.1054/bjoc.1999.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenblatt KA, Wicklund KG, Stanford JL. Sexual factors and the risk of prostate cancer. Amer J Epidemiol. 2000;153:1151–1158. doi: 10.1093/aje/153.12.1152. [DOI] [PubMed] [Google Scholar]
- 4.Gann PH, Hennekens CH, Ma J, et al. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–26. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 5.Mohr BA, Feldman HA, Kalish LA, et al. Are serum hormones associated with the risk of prostate cancer? Prospective results from the Massachusetts Male Aging Study. Urology. 2001;57:930–5. doi: 10.1016/s0090-4295(00)01116-x. [DOI] [PubMed] [Google Scholar]
- 6.Stattin P, Lumme S, Tenkanen L, et al. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004;108:418–24. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- 7.Eaton NE, Reeves GK, Appleby PN, et al. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br J Cancer. 1999;80:930–4. doi: 10.1038/sj.bjc.6690445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endogenous Hormones and Prostate Cancer Collaborative Group. Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study - A large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Hsing AW, Stanczyk FZ, Bélanger A, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007;16:1004–8. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- 12.Södergård R, Bäckström T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL. A Case-Cohort Design for Epidemiologic Cohort Studies and Disease Prevention Trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 14.Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. Journal of Urology. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 15.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 16.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and Evaluation. Journal of Clinical Epidemiology. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 17.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–8. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 18.Giton F, de la Taille A, Allory Y, et al. Estrone sulfate (E1S), a prognosis marker for tumor aggressiveness in prostate cancer (PCa) J Steroid Biochem Mol Biol. 2008;109:158–67. doi: 10.1016/j.jsbmb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Reed MJ, Purohit A, Woo LW, et al. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005;26:171–202. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Suzuki T, Fukuda T, et al. Steroid sulfatase and estrogen sulfotransferase in human prostate cancer. Prostate. 2006;66:1005–12. doi: 10.1002/pros.20426. [DOI] [PubMed] [Google Scholar]
- 21.Shaneyfelt T, Husein R, Bubley G, et al. Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol. 2000;18:847–53. doi: 10.1200/JCO.2000.18.4.847. [DOI] [PubMed] [Google Scholar]
- 22.Parsons JK, Carter HB, Platz EA, et al. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–60. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 23.Dorgan JF, Albanes D, Virtamo J, et al. Relationships of serum androgens and estrogens to prostate cancer risk: results from a prospective study in Finland. Cancer Epidemiol Biomarkers Prev. 1998;7:1069–74. [PubMed] [Google Scholar]
- 24.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 25.Fonda SJ, Bertrand R, O’Donnell A, et al. Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:385–90. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Wheeler T, Dai H, et al. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–34. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Härkönen PL, Mäkelä SI. Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:297–305. doi: 10.1016/j.jsbmb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]