Abstract
This investigation determined whether activation of the kappa opioid receptor (KOR) in the spinal cord produces estrogen-dependent, sex-specific modulation of acute and inflammation-induced persistent nociception. We demonstrate for the first time that KOR antinociception and gene expression are enhanced by exogenous or endogenous estrogen in the female. The lack of KOR antinociception and KOR gene expression are not altered by hormonal status (testosterone or estrogen) in males. Cannulae were implanted intrathecally in male, gonadectomized male (GDX), intact and ovariectomized female (OVX) Sprague-Dawley rats. Estradiol was injected subcutaneously, 48 h before testing (GDX+E and OVX+E). Intrathecal injection of U50, 488H, a selective KOR agonist, dose dependently increased heat-evoked tail flick latencies (TFLs) in proestrous and OVX+E groups, but not in male, GDX, GDX+E, OVX, and diestrous groups. Further, estrogen dose-dependently enhanced the effect of U50,488H in OVX rats. KOR selective antagonist, nor-binaltorphimine (Nor-BNI), blocked the antinociceptive effect of U50,488H. U50,488H reversed the carrageenan-induced thermal hyperalgesia in OVX+E rats, but not in male or OVX rats. However, U50,488H treatment did not alter mechanical thresholds in any group, with or without inflammation. KOR gene expression was enhanced in proestrous and OVX+E groups as compared to any other group. We conclude that selective activation of KOR in the spinal cord produces sex-specific, stimulus- and estrogen-dependent attenuation of acute and inflammatory pain in the rat via estrogen-induced upregulation of the KOR gene expression in the spinal cord. These findings may further implicate estrogen dependence of KOR effects in learning, epilepsy, stress response, addiction etc.
Selective activation of the kappa opioid receptor by intrathecal U50,488H produces antinociception and antihyperalgesia which are sex-specific, stimulus dependent and require the presence of estrogen.
Keywords: KOR; pain; sex differences; U50,488H; analgesia; dorsal horn
INTRODUCTION
Many pain syndromes such as migraine, trigeminal neuralgia, and irritable bowel syndrome have a higher prevalence in women [4,17]. We [11,36,43] and others [13,16,33,48] have shown that the administration of agonists selective for mu opioid (MOR), opioid receptor like 1 receptor (ORL1), and other G-protein coupled receptors (GPCRs) such as the α2-adrenoceptor produces greater antinociception in males. Estrogen attenuates antinociceptive effects ORL1 and α2 - adrenoreceptor activation in the female whereas testosterone is required for the expression of antinociception produced by intrathecal application of selective agonists for their respective receptors in the male [11,18,36,43].
Although controversial, the role of the kappa opioid receptor (KOR) in analgesia, including pregnancy-induced analgesia [23] and visceral antinociception through peripheral [15,39,42] and central KORs [40], has been shown. Mixed-action KOR agonists have been shown to be effective in relieving labor pain [44] and are more effective in women than men in a model of post-operative dental pain [19,20,21,22]. However, Fillingim et al. [17] reported no sex differences using experimentally induced pain in humans. Further, Mogil et al. [35] did not observe any sex-related differences in mixed action kappa opioid-induced analgesia in humans. Both men and women displayed comparable analgesic response to pentazocine; however, they reported a significant influence of melanocortin-1 receptor (M C1R) genotype on analgesia in women only. In animals, selective KOR agonists have been reported to produce greater antinociception in females in the tail withdrawal and hot plate tests [2]; and against mechanical but not thermal stimuli [6]. In contrast, greater antinociception in males in response to KOR-agonists has also been reported [31,35,37,41]. Similarly, conflicting findings have emerged from studies in chronic pain models [5,7,8,45,46,47]. These contradictory findings may result from several variables including dose, route of drug delivery, nociceptive assay, stimulus intensity, estrous cycle stage, and species and strain of animals tested.
Most importantly, previous studies with kappa opioids conducted in humans or animals have failed to separate females based on the level of estrogen present. Human studies either disregard menstrual cycle or were conducted between days 1–10 of the follicular phase when estrogen levels barely rise above the low levels during menstruation. Systemic application of kappa opioids used in previous studies is likely to produce global activation of KORs and would not be able to distinguish between the effects of peripheral or central KORs. Similarly, it would not differentiate between the effects of central KORs located in the spinal cord or the brain. Further, it remains unknown whether the lack of KOR-mediated analgesia in males is dependent on hormonal status.
We therefore investigated whether (i) selective activation of KOR in the spinal cord produces sex-specific modulation of acute spinal nociception as well as inflammation induced thermal hyperalgesia and tactile allodynia, (ii) KOR-mediated analgesia is estradiol dose-dependent or affected by fluctuating endogenous estrogen levels in females, and (iii) estrogen alters the expression of the KOR gene in the female.
EXPERIMENTAL PROCEDURES
Animals
The experimental groups consisted of male, gonadectomized (GDX) male, female, and ovariectomized (OVX) female Sprague-Dawley rats (Harlan, Indianapolis, IN, USA). They were housed in the animal care facility at Meharry Medical College certified by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) under a 12-hour light/dark cycle (lights on: 7 am; lights off: 7 pm). Food and water were available ad libitum. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Meharry Medical College and abided by the established guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pain (IASP). All efforts were made to minimize stress to the animals and the number of animals used.
Vaginal Cytology
Estrous cycle stages were determined by the standard vaginal smear method and as demonstrated previously by us [11], correlated with estradiol levels measured in normally cycling females at the proestrous (ProE) and diestrous (DiE) stages. Female rats were cannulated in the diestrous phase and underwent nociceptive testing after having established two regular estrous cycles.
Implantation of Intrathecal Cannulae
Gonadectomized (GDX) and ovariectomized (OVX) animals were given a 2 week recovery period prior to surgical implantation of cannulae. As described before [11,43], cannulae were implanted using aseptic surgical procedure in animals under ketamine and xylazine anesthesia (72 and 4 mg/kg i.p., respectively). The head of the rat was shaved and secured in a stereotaxic frame (David Kopf, Tujunga, CA). An incision was made above the atlanto-occipital membrane which was cleared to expose the dura. A stretched PE-10 cannula (Intramedic, Clay Adams, Parsippany, NJ; dead volume of 10 μl) was inserted into the subarachnoid space through a small slit in the dura. The cannula was gently passed to a length of 8.5 cm to reach the lumbosacral enlargement and was secured with dental cement to the base of the skull. The wound was sutured and the animal was placed on a heating blanket until it regained consciousness. Animals were allowed to recover for 5–7 days before undergoing nociceptive testing. Animals showing any signs of neurological impairment were killed immediately with 150 mg/kg (i.p.) of sodium pentobarbital. The position of the cannula was confirmed, at the end of testing, by administering 15 μl of 2% lidocaine (i.t.) which temporarily paralyzed the animal’s hind limbs.
Estradiol replacement
A single dose of estradiol benzoate (1 ng, 0.1, 1, 10, or 100 μg/100 μl of sesame oil; s.c.) was administered to OVX and GDX animals 48 hours prior to nociceptive testing. This method (single, s.c. injection) has been used previously in neuroendocrinology [3,39] and in the pain field [11,18,28,29,36,43]. Control groups received vehicle injections (sesame oil, 100 μl; s.c.). We have previously measured serum estradiol concentrations in OVX rats treated with varying doses of estradiol and at different time points after injection [36] as well as these levels in intact females at the proestrous and diestrous stages [11]. Although higher after 8 hr, estradiol levels 48 hr (time point for behavior testing) after 1, 10, and 100 μg estradiol injection were 25.24±0.54, 32.74±5.43 and 115.17±27.52 pg/ml respectively. These levels closely approximate normal physiological range of estradiol levels in diestrous (32.59±3.09 pg/ml) and proestrous (63.28±4.32 pg/ml) females. Further, estradiol levels 48 hours after 100 μg dose of estradiol were similar to that reported elsewhere in normally cycling females at the proestrous stage (about 75–100 pg/ml) [9,36]. Estradiol levels measured at the proestrous or the diestrous stage correlated well with the estrous cycle stage as determined by vaginal cytology [11]. In addition to ascertain a diestrous-like vaginal cytology in OVX rats, OVX and GDX animals were randomly picked and inspected for complete removal of gonads at the end of experiments.
Drugs
A highly selective KOR agonist U50,488H [trans-3,4-dichloro-N-methyl-N-{2-(1-pyrrolidinyl)-cyclohexyl}-benzeneacetamide] was intrathecally administered (25, 50, 100, or 250 nmol/5 μl) and the effects were examined on the thermal or mechanical stimulus-induced nociception. Nor-binaltorphimine (nor-BNI; 87 nmol/5 μl) [14], a selective KOR antagonist, was intrathecally administered 5 min prior to the administration of U50, 488H in proestrous and OVX + E (100 μg/100 μl, s.c) female rats. Both drugs were obtained from Sigma (St. Louis, MO).
Tail-flick assay
Tail flick assay [12] was conducted as previously described [11,41] and tail-flick latency (TFL) was measured automatically using a tail-flick analgesia meter (Model 33T, IITC Life Science, Woodland Hills, CA). Animals were loosely restrained in a plexiglas cylinder with the dorsal surface of the tail exposed to a radiant heat source mounted 8 cm above the tail, and radiant heat was applied 3–7 cm from the tip of the rat’s tail at three separate spots (~ 1.5 cm apart) to prevent sensitization as a result of heating the same spot in succession. An automatic cut off latency of 15 sec was set to prevent tissue damage. Prior to testing, the animals were allowed to acclimate to the restraint cylinder for 15 min. The heat intensity was adjusted to produce a baseline TFL of between 4 and 6 sec. Three baseline readings were taken at 10-min intervals prior to U50,488H administration and were subsequently recorded every 10 min for 90 min.
Carrageenan-induced inflammation
Inflammation was induced with subcutaneous injection of 0.1 ml of 2% lambda carrageenan (Sigma Chemical, St. Louis, MO) into the plantar surface of the right hind paw under isoflurane anesthesia. Maximum hyperalgesia occurs ~2 hours after carrageenan injection [27], therefore nociceptive testing was conducted ~ 2 hours subsequent to carrageenan injection.
Paw withdrawal assays
Thermal hyperalgesia was measured using a plantar analgesia meter (IITC model 390, Woodland Hills, CA), as described originally by Hargreaves [24]. Animals were placed in a clear plastic cage (27 × 8.625 × 5 in) placed on an elevated floor of clear glass (2 mm thick) and allowed to habituate for 30 min. A radiant heat source (halogen lamp) contained in a movable holder was placed beneath the glass floor and was positioned so as to focus on the plantar surface of one hind paw. The heat intensity was set to produce baseline latency between 10 and 12 sec. Nociceptive testing was conducted 2 hours post-carrageenan administration. For each animal, three baseline paw withdrawal latencies on each hind paw were recorded at 10-min intervals prior to U50, 488H administration and were subsequently recorded every 10 min for 90 min. The trial was terminated in the absence of a response within 20 sec.
Tactile allodynia was assessed using an automated dynamic plantar aesthesiometer (37400; Ugo Basile, Comerio, Italy). Subjects were placed in a plastic cage with a wire mesh floor and allowed to acclimate for 30 min before testing. The unit applied a metal filament (0.5 mm diameter) to the plantar surface of the right hindpaw and exerted an upwards force until the paw was withdrawn or the preset cutoff reached (50 g). The force applied was initially below the detection threshold, and then increased at a rate of 2.5 g/s. The force required to elicit withdrawal was recorded automatically. Three baseline mechanical thresholds on each paw were recorded at 10-min intervals, 2 hours post carrageenan injection and were recorded for another 90 min post-drug injection.
Tissue collection, reverse transcriptase-polymerase chain reaction (RT-PCR) and Taqman real-time quantitative PCR (qPCR)
The lumbosacral enlargement of the spinal cord was collected in RNAlater (0.5 ml; Ambion, Austin, TX, USA) from separate groups of animals under deep pentobarbital anesthesia (100 mg/kg; i.p.) and stored at −80 °C until further processing. RNA was isolated using the Rneasy lipid tissue mini kit (Qiagen, Inc., Valencia, CA, USA) and purified with Dnase I. cDNA was synthesized using the Reverse-transcriptase cDNA archive kit (Applied Biosystems, Foster City, CA, USA). KOR expression was measured by real-time PCR (Icycler, Biorad Laboratories, Hercules, CA, USA) using a Taqman gene expression kit (assay RN00567737_m1) from Applied Biosystems. A two-step PCR protocol was used: 50 °C, 2 min; 95 °C, 10 min; 95 °C for 15 s and 50 °C for 1 min for 40 cycles. Instructions from the manufacturers were followed through each step. Data were collected and the Starting Quantity (SQ) was utilized to calculate KOR gene expression in all groups. GAPDH gene expression was measured similarly and was used to normalize KOR expression.
Data analysis
The data from tail flick experiments were converted to the percentage of the maximum possible analgesic effect (% MPE), according to the formula % MPE = [(postdrug latency − baseline latency)/(cutoff latency − baseline latency)] × 100. The average of the three baseline readings was calculated to determine the baseline value. Data were analyzed using SPSS (SPSS Inc., Chicago, IL). All behavioral measures were submitted to ANOVA corrected for repeated measures with appropriate between- (sex, drugs) and within- (time course) group factors, and dependent variables (TFL or PWL). A post-hoc (Fisher’s LSD) test was performed where necessary for intergroup comparisons. A P-value of <0.05 was considered significant. Area under the curve (AUC) was calculated by trapezoid method using Prism (Graphpad Software, Inc., San Diego, CA, USA) for time course plots to obtain a single measure of the overall response. Data obtained from real-time PCR studies were submitted to one-way ANOVA. A post-hoc test (Fisher’s LSD) was conducted to compare multiple groups only where ANOVA yielded a significant main effect. A P-value of <0.05 was considered significant. The mean and the SEM were used for illustration.
RESULTS
Fluctuations in endogenous estrogen levels during the estrous cycle affect U50,488H-induced antinociception
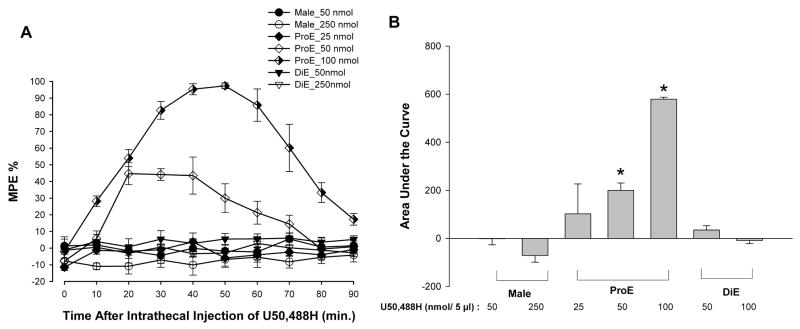
To assess whether activation of KOR results in sex-specific and estrogen-dependent modulation of spinal nociception, the effects of U50,488H on the heat stimulus evoked tail-flick reflex were tested in males and intact females during phases of high (proestrous) or low (diestrous) levels of circulating estrogen(Fig. 1A). Intrathecal administration of U50,488H significantly and dose dependently increased the MPE only in ProE females. ANOVA yielded significant main effects of time [F(9, 171) = 19.50; p < 0.01], group [F(6, 19) = 54.15; p < 0.01], and an interaction between the two [F(54, 171) = 9.50; p < 0.01]. U50,488H significantly increased MPEs at the 50 and 100 nmol doses but had no effect at the 25 nmol dose. The effects of U50,488H appeared peaked at 30–50 min after injection and lasted for 60–80 min. The 50- and 250 nmol doses produced no effect in male or DiE animals. The area under the curve (AUC), indicating an overall effect of U50,488H, was significantly increased, in a dose-dependent manner, in the ProE females as compared to male and DiE groups (Fig. 1B). ANOVA of the area under the curve yielded a significant main effect of group (F(6,25) = 60.01; p < 0.01).
Figure 1.
Fluctuations in endogenous estrogen levels in females modulate U50,488H antinociception. A, Antinociceptive effects of intrathecally administered U50,488H in male and normally cycling female rats. U50,488H demonstrated significant antinociceptive activity in proestrous rats (ProE; high estrogen level) in comparison to male and diestous (DiE; low estrogen level) rats. Values are given as percentage of maximum possible effect. B, U50,488H significantly and dose-dependently increased the AUC of the ProE group as compared to all other groups. * p < 0.01.
Estradiol-dependent modulation of acute thermal nociception by U50,488H
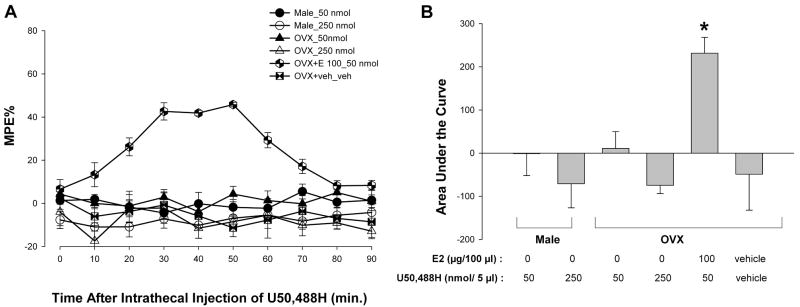
To determine whether the activation of KOR produces antinociception in animals treated with estradiol, the effects of U50,488H on the tail-flick reflex were tested in males, OVX, and estradiol-treated OVX females. ANOVA yielded significant main effects of time [F(9,189) = 3.02; p <0.05], group [F(5,21) = 20.02; p < 0.01], and an interaction between the two [F(45,189) =4.21; p < 0.01]. Post hoc comparisons revealed that U50,488H (50 nmol) significantly increased the MPEs only in OVX + E (10 μg) animals, but not in male or OVX groups (Fig. 2A). The effects of U50,488H peaked at 30–50 min and lasted for 70 min. ANOVA of the area under the curve yielded a significant main effect of group (F(5,26) = 23.16; p < 0.01). As shown in Figure 2B, post hoc comparisons revealed that the AUC was significantly higher in the OVX + E group treated with 50 nmol U50,488H as compared to male and OVX groups.
Figure 2.
U50,488H produced antinociception in OVX + E rats but not in male or OVX rats. A, U50,488H (50 nmol; given at time 0) produced a significant increase in MPEs in OVX + E rats compared with all other groups (all p < 0.05). No effects of U50,488H (50 nmol) were seen in the OVX and male groups. Values are given as percentage of maximum possible effect. B, U50,488H significantly increased the AUC of the estradiol-treated OVX group as compared to all other groups. * p < 0.01
Estradiol dose dependently enhances antinociception produced by U50,488H
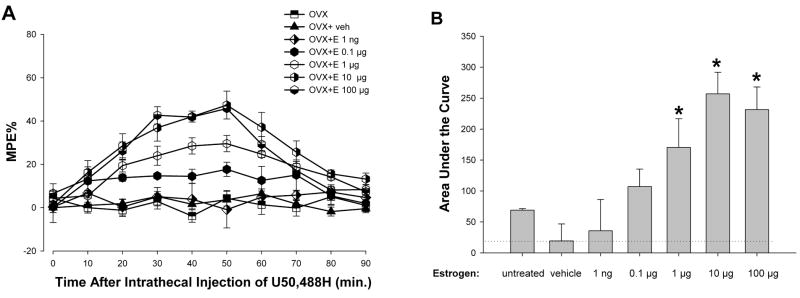
To assess whether estradiol dose dependently enhances the antinociceptive effects of KOR activation, an estradiol dose-response study was conducted in OVX animals. ANOVA yielded significant main effects of time [F(9,225) = 23.15; p <0.01], group [F(6,25) = 31.40; p < 0.01], and an interaction between the two [F(54,225) = 4.66; p < 0.01]. As shown in Figure 3A, post hoc comparisons indicate that estradiol (1–100 μg) significantly enhanced U50, 488H antinociception. However, the effects of U50, 488H (50 nmol) were not evident at 0.1 μg and 1 ng doses of estradiol. Evaluation of the overall effect of each dose of estradiol on the antinociceptive effects of U50,488H, assessed by measuring the area under the curve, demonstrates that U50,488H is significantly more effective in OVX groups treated with high doses (1,10, and 100 μg) of estradiol (Fig. 3B). ANOVA of the area under the curve yielded a significant main effect of group (F(6,31) = 32.30; p < 0.01).
Figure 3.
Estradiol dose-dependently enhances the antinociceptive effect of U50,488H (50 nmol) in OVX females. A, Dose response curve for the effects of varying doses of estradiol on intrathecal U50,488H in OVX rats. Values are given as percentage of maximum possible effect. B, AUC significantly increased in groups with estradiol doses of 1, 10, and 100 μg/100 μl as compared to those of all other groups. Further, the 10 and 100 μg doses had significantly higher AUC compared to 1 μg. Dotted line represents AUC of the control OVX group. *p < 0.01.
Activation of KOR produces agonist dose-dependent antinociceptive effects
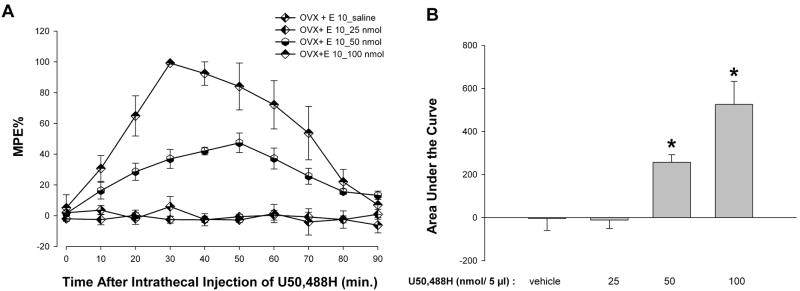
To assess whether the antinociceptive effect of KOR activation is agonist dose dependent, the impact of increasing doses of U50,488H (25, 50, and 100 nmol) was evaluated. ANOVA yielded significant main effects of group [F(3,16) = 72.74; p < 0.01], time [F(9,144) = 15.22; p < 0.01] and an interaction between the two [F(27,144) = 7.04; p < 0.01]. Post hoc comparison revealed that U50,488H significantly increased MPEs at the higher doses evaluated (50 and 100 nmol) but not at the lowest dose (25 nmol) in OVX + E (10 μg) animals (Fig. 4A). As shown in Figure 4B, U50, 488H significantly increased the AUC in OVX + E (10 μg) animals at higher doses in a dose-dependent manner. ANOVA of the area under the curve yielded a significant main effect of group [F(3,19)= 77.34; p < 0.01].
Figure 4.
A, U50,488H produces dose-dependent antinociceptive effects in estradiol (10 μg) – treated OVX rats. U50,488H enhanced the MPE at doses of 50 and 100 nmol, but not at 25 nmol compared to the vehicle groups. Values are given as percentage of maximum possible effect. B, U50,488H, at doses of 50 nmol and 100 nmol, significantly increased the AUC in OVX+E (10 μg) rats but had no effect at the 25 nmol dose compared to the vehicle group. Further, the AUC at the 100 nmol dose was significantly higher than AUC at 50 nmol. *p < 0.01 compared to all other groups.
Lack of antinociceptive effect of U-50,488H is neither dependent on high levels of testosterone nor on low levels of estrogen in the male
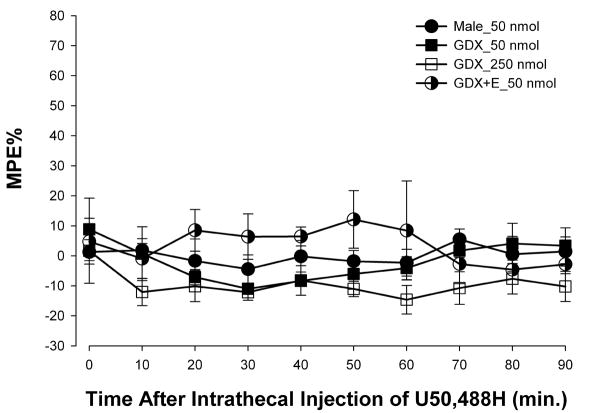
To determine whether KOR-mediated antinociception is negatively affected by testosterone, the effects of U50,488H on the heat stimulus evoked tail-flick reflex were tested in males, GDX, and estradiol-treated (10 μg) GDX males (Fig. 5). ANOVA yielded no significant main effect of time [F(9,99) = 0.79; p = 0.63] or group [F(3,11) < 1; p = 0.10]. Neither removal of testosterone nor administration of estradiol to GDX males altered the inability of U50,488H to produce antinociception in males.
Figure 5.
U50,488H (50 nmol) did not alter TFLs of males, gonadectomized (GDX), and estradiol (10 μg)-treated gonadectomized (GDX+E) males. Values are given as percentage of maximum possible effect.
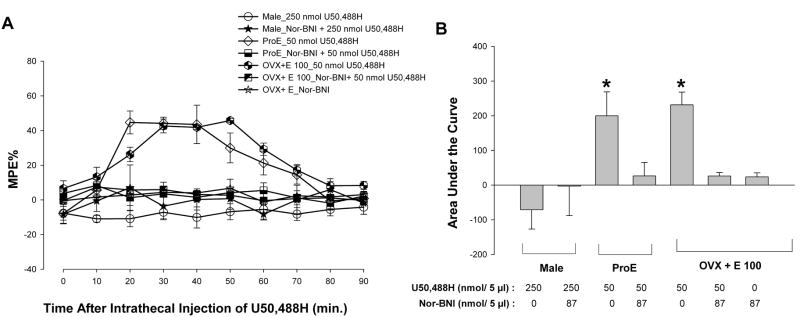
A selective antagonist of KOR, nor-BNI, blocks the antinociceptive effects of U50,488H
To confirm whether the observed effects of U50,488H are mediated via the selective activation of KOR, the effects of pretreatment with norbinaltorphimine (nor-BNI), a selective KOR antagonist, were examined on acute thermal nociception in OVX + E and proestrous animals treated with 50 nmol U50,488H. ANOVA yielded a significant main effect of time [F(9,144) = 3.88; p < 0.01], group [F(5,16) = 10.21; p < 0.01] and an interaction between the two [F(45,144) = 3.79; p < 0.01]. Post hoc comparisons revealed that Nor-BNI (87 nmol/5 μl, i.t.) administered 5 min prior to U50,488H completely abolished the antinociceptive effect of U50,488H (Fig. 6A). Further, nor-BNI alone failed to produce any significant effects in OVX +E rats. These data are consistent with the interpretation that the selective activation of KOR is responsible for the antinociceptive effects observed in this study. In Figure 6B, nor-BNI significantly reduced the AUC in ProE and OVX + E animals. ANOVA of the area under the curve yielded a significant main effect of group [F(5,21)= 11.16; p < 0.01].
Figure 6.
The antinociceptive effects of U50,488H are completely reversible by selective KOR blockade. A. Nor-BNI (87 nmol/5 μl), a selective KOR antagonist, given 5 min before U50,488H completely blocked the antinociceptive effects of U50,488H in proestrous and OVX +E groups. Given alone, Nor-BNI did not change MPEs in the OVX+E (100 μg) group. Values are given as percentage of maximum possible effect. B, Nor-BNI reversed U50,488H-induced increase in the AUC in proestrous and OVX+E rats (p < 0.01). *p < 0.01 compared to U50,488H-treated respective groups.
Sex-specific, estradiol-dependent modulation of inflammation-induced thermal hyperalgesia by U-50,488H
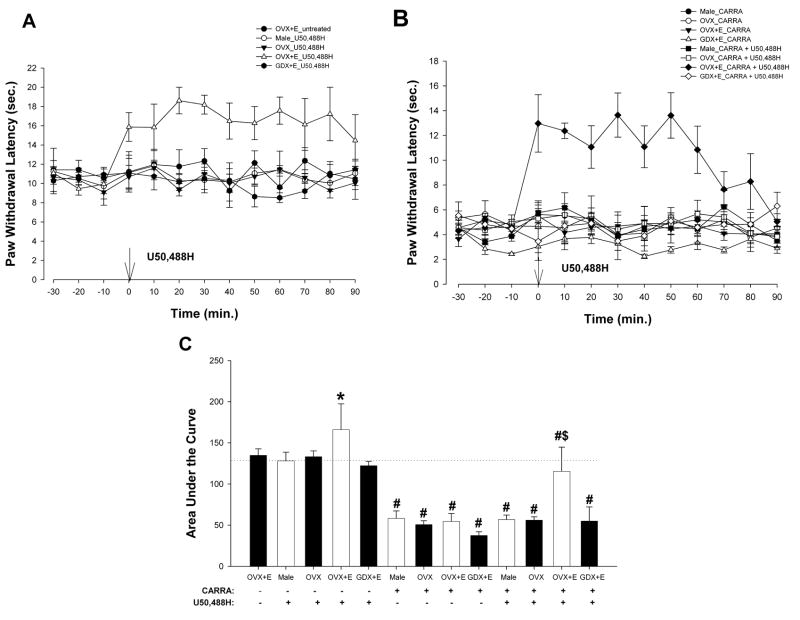
To assess whether activation of KOR results in sex-specific, estradiol-dependent modulation of thermal nociception in the presence of inflammation, the effects of U50,488H on the heat-evoked paw withdrawal reflex were tested in carrageenan-treated males, GDX+E (10 μg), OVX, and OVX+E (10 μg) rats (Fig. 7A,B). First, average baseline PWLs without carrageenan injection (Fig. 7A) were between 10 and 12 sec in control animals and did not differ between groups. ANOVA yielded significant main effects of group [F(4,13) = 14.07; p < 0.01] and an interaction between time and group [F(48,156) = 1.50; p < 0.05]. Post hoc comparisons revealed that U50,488H (50 nmol; given at time 0) produced significant increases in PWLs only in OVX+E (10 μg) group, starting at 0 min, and persisted for ~ 90 min as compared with male, OVX, and GDX + E (10 μg) rats (p < 0.05).
Figure 7.
U50,488H produced estradiol-dependent, sex-specific antinociception on inflammation-induced secondary thermal hyperalgesia. A, U50,488H (50 nmol; given at time 0) produced significant increases in PWLs in OVX+E (10 μg) rats (p < 0.05). B. U50,488H reversed the carrageenan-induced hyperalgesia in OVX + E rats. C. Carrageenan significantly reduced the AUC in male, OVX, GDX + E, and OVX +E rats. U50,488H significantly increased the AUC of the estradiol-treaded OVX groups, with or without carrageenan-induced inflammation, as compared to all other groups. Dotted line represents AUC of male + U50,488H group. * p < 0.05 compared to U50,488H-treated male, OVX, GDX+ E and OVX+E groups. # p < 0.01 compared to groups without carrageenan treatment. #$ p < 0.01 compared to all other carrageenan-treated groups.
Carrageenan injection significantly reduced PWLs to 4–6 sec., indicating hyperalgesia, in all groups (Fig 7B). ANOVA yielded significant main effects of time [F(12,300) = 2.86; p < 0.01], group [F(7,25) = 10.45; p < 0.01] and an interaction between time and group [F(84,300) = 3.07; p < 0.01]. Post hoc comparisons indicated that U50, 488H reversed the carrageenan-induced hyperalgesia in OVX + E rats, significantly increasing PWLs at time 0 and persisting for ~ 60 min. In contrast, U50, 488H did not alter PWLs in the carrageenan-treated male, GDX+E, or OVX groups. Carrageenan significantly reduced the AUC in all groups (Fig. 7C). U50,488H significantly increased the AUC only in OVX+E groups (with or without carrageenan treatment) compared to that of respective controls, male, GDX+E and OVX groups. ANOVA of the area under the curve yielded a significant main effect of group [F(7,25) = 10.83; p < 0.01].
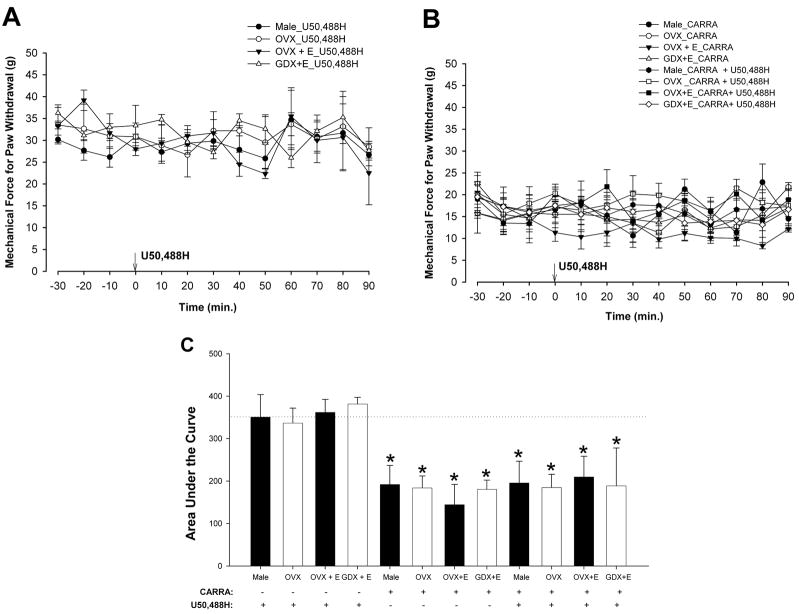
Mechanical thresholds or tactile hypersensitivity does not respond to U50,488H treatment with or without inflammation
To determine whether KOR activation results in sex-specific, estradiol-dependent modulation of mechanical thresholds under inflammatory conditions, the effects of U50,488H were tested in carrageenan-treated male, OVX, GDX+E, and OVX+E animals. Baseline mechanical forces required for paw withdrawal were between 25–40 g among all groups. U50,488H failed to produce an effect in any group (Fig. 8A). ANOVA did not yield a significant main effect of time [F(12,108) = 1.59; p = 0.11] or group [F(3,9) <1; p = 0.73]. As shown in Figure 8B, carrageenan significantly reduced the baseline mechanical thresholds of all groups, but U50,488H failed to produce an effect in any group. ANOVA did not yield a significant main effect of group [F(7,21) <1; p = 0.77]. AUC analysis (Fig. 8C) further confirmed these observations which emphasize an absence of sex-related differences in the modulation of mechanical threshold, with or without carrageenan-induced inflammation, by U50,488H. ANOVA of the area under the curve yielded a significant main effect of group [F(11,41) = 12.01; p < 0.01].
Figure 8.
U50,488H did not affect mechanical response thresholds in the presence or absence of carrageenan-induced inflammation. A, Intrathecal U50,488H did not affect mechanical thresholds in male, OVX, GDX + E, or OVX + E rats. B, Carrageenan injection reduced the mechanical response thresholds to hind paw stimulation of male, OVX, GDX + E, and OVX + E rats. U50,488H failed to produce any changes in mechanical response thresholds in any group. C. Carrageenan significantly reduced the AUC in male, OVX, GDX +E, and OVX +E rats. Dotted line represents AUC of male + U50,488H group. * p < 0.01
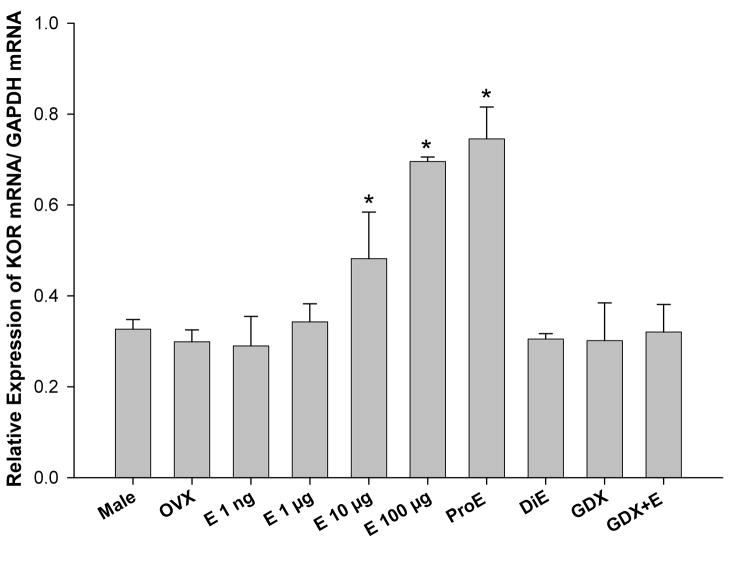
Expression of the KOR mRNA in the spinal cord directly correlates with KOR-mediated sex-specific modulation of pain
To determine if changes in expression of the KOR gene could explain the effect of estrogen to enhance the antinociceptive effects of U50,488H, the expression of the KOR gene relative to that of GAPDH was determined in the lumbosacral region of the spinal cord in the male, OVX, OVX+E (1 ng–100 μg), proestrous, diestrous, GDX, and GDX+E rats using real-time quantitative PCR. The male group was used as the control against which all other groups where compared. ANOVA revealed a significant main effect of group [F(9,29) = 9.040; p < 0.01]. Post hoc comparisons showed that KOR mRNA levels were significantly higher in OVX+E (10- and 100 μg) and ProE groups in comparison to all other groups (Fig. 9).
Figure 9.
Expression of the KOR gene in the lumbosacral spinal cord correlates with behavioral observations. Levels of KOR mRNA were quantified in the lumbar spinal cord using real-time quantitative PCR and normalized by levels of GAPDH mRNA. There was an increased expression of KOR in OVX+E (10- and 100 μg) and ProE rats in comparison to all other groups. * p < 0.01.
DISCUSSION
This is the first systematic study that investigated whether antinociception in the female or lack of antinociception in the male produced by selective activation of KOR in the spinal cord is, a) estradiol dose-dependent in the female, b) affected by fluctuations in endogenous levels of estrogen in the female and c) observed regardless of hormonal (estrogen or testosterone) status in the male. Further, effects of selective activation of KOR in the spinal cord were tested against acute thermal nociception as well as inflammation-induced thermal hyperalgesia and tactile hypersensitivity. This study demonstrated estrogen-dependent sex-related differences in KOR-mediated modulation of acute spinal nociception as well as inflammation-induced thermal hyperalgesia. This was demonstrated not only in estradiol treated ovariectomized females but also in normally cycling females during phases of high (proestrous) or low estrogen (diestrous). There were no differences in the basal nociception between sexes, suggesting that the perception of pain per se between sexes in the present study was similar. However, clear sex differences emerged in the modulation of nociception after the activation of KOR by U50,488H. These findings are consistent with previous data obtained in animal and human studies [2,6,19,20,21,22,34]. However, the information provided here advances our previous knowledge of KOR-mediated antinociception in some important ways. First, fluctuations in endogenous estrogen that occur normally during the estrous cycle were shown to modulate the KOR-mediated antinociception as well as the KOR gene expression. Second, based on our behavioral and molecular observations, neither the presence of high levels of testosterone nor the low levels of estrogen are responsible for the lack of KOR-mediated antinociception in the male. Third, the KOR-mediated antinociception in the female is estradiol dose dependent and estradiol dose-dependently enhanced the expression of the KOR gene expression. Fourth, estrogen-dependent sex-related differences are, indeed, produced by selective activation of KOR in the spinal cord. Fifth, the KOR-mediated antinociception selectively reduces acute thermal nociception as well as inflammation-induced thermal hyperalgesia but not mechanical thresholds or inflammation-induced tactile hypersensitivity.
Our study also revealed that the KOR-mediated antinociception is estradiol dose-dependent in OVX animals, and is observed in normally cycling females only during the proestrous phase when the estrogen levels are the highest but not during the diestrous phase when the estrogen levels are low. This is contrary to our previous findings that showed that estrogen abolished the antinociceptive effects mediated by other G protein coupled receptors (GPCRs) such as the opioid receptor like 1 receptor (ORL1) and α2-adrenoceptors [11,18,36,43]. Behavioral observations can be explained by estrogen-induced upregulation of the KOR gene as shown here i.e. higher levels of KOR mRNA in proestrous females and OVX+E females as compared to other groups (males or diestrous or OVX females) could explain the KOR-mediated antinociceptive effect in high estrogen groups (proestrous and OVX+E females). Although KOR protein levels could not be assessed in the present study because of the lack of specificity of commercially available antibodies, recent studies using an antibody raised in Dr. Chavkin’s laboratory (University of Washington) have reported that KOR immunoreactivity is significantly denser in the spinal cord in proestrous females as compared to males [26] and that KOR labeling in the L6-S1 segments of the spinal cord is significantly higher in the proestrous stage than in the diestrous phase of female rats [10]. Whether estrogen receptors are present in KOR containing neurons remains unknown and requires further investigation. It has been shown previously that estrogen receptors are present in neurons containing dynorphin in the dorsal horn [24]. Further, we have shown that estrogen receptor mRNA is co-localized in neurons containing the ORL1 mRNA in the trigeminal dorsal horn neurons [11]. We also examined the effects of gonadectomy in males on the U50,488H-induced antinociception. No effects of the drug were shown in GDX males or GDX rats treated with estradiol (GDX+E), suggesting that neither testosterone nor low levels of estrogen is responsible for the lack of KOR-mediated antinociceptive effects in the male. Interestingly, neither gonadectomy in the male nor administration of estradiol to GDX males enhanced the expression of the KOR gene which supports our conclusions drawn from behavioral studies.
Gonadectomy does remove both estrogen and progesterone, however, replacement of estrogen alone restored the antinociceptive effects of U50,488H in OVX rats. Progesterone given 8 hours prior to testing did not affect antinociceptive effects of α2-adrenoceptors (our unpublished observation). In addition, any compensatory changes due to gonadectomy failed to change baseline tail flick latencies in OVX and GDX rats compared to normal females and males.
We established that the analgesic effects of intrathecal U50,488H were mediated by the selective activation of KOR in the spinal cord. The actions of intrathecal U50,488H were fully antagonized by selective antagonist norbinaltorphimine. Nor-BNI did not affect nociception when administered alone.
The present investigation also examined the effects of selective activation of KOR in a model of inflammatory pain. Carrageenan reduced the mechanical thresholds for paw withdrawal in all groups, indicative of tactile hypersensitivity or tactile allodynia. In contrast to thermal hyperalgesia, U50,488H failed to reduce or suppress tactile allodynia in males, OVX, or OVX+E rats. KOR activation also failed to affect mechanical thresholds in animals not subjected to inflammation. It is not entirely surprising that tactile allodynia was not affected by KOR activation in the spinal cord since it has been shown previously that nerve injury-induced allodynia is suppressed only by opiates (including U50,488H) administered systemically or supraspinally but not spinally [32]. Since sex differences were observed with the heat stimulus but not with mechanical stimulation, we postulate that the effects of selective activation of KOR in animals (with and without carrageenan-induced inflammation) are stimulus-dependent. It is known that C- and Aδ - fibers mediate the transmission of heat stimuli, whereas thinly myelinated Aδ-fibers transmit mechanical stimulation [30]. It is possible that KORs may be expressed more abundantly on thermal nociceptive afferent fibers or on interneurons mediating responses to thermal stimulation as opposed to mechanical nociceptive afferent fibers or interneurons mediating responses to mechanical stimulation. Although previous studies have shown that peripherally acting kappa opioid agonists given systemically are more potent analgesics against mechanical stimuli in adjuvant-induced arthritic female rats than males [6], similar effects have not been observed with the intrathecal administration of U-50,488H until recently. Intrathecal U50,488H attenuated formalin-induced hyperalgesia in female Wistar rats [1], which is consistent with our findings. Many previous studies have also implicated peripheral KORs in mediating the anti-inflammatory actions of U-50, 488H [7,8,46,47].
The present study demonstrated that the selective activation of KOR in the spinal cord results in sex-specific, estrogen- and stimulus-dependent modulation of spinal nociception in the rat in acute nociception as well as inflammation-induced thermal hyperalgesia but not tactile hypersensitivity. The lack of KOR-mediated antinociception in the male does not result from the high levels of testosterone or low levels of estrogen. Estrogen-dependent effects on KOR signaling may contribute to sex-related differences in pain sensitivity and modulation in the spinal cord. These findings could provide new insights into the development of therapeutic interventions for the treatment of pain syndromes in women depending on the hormonal status during the life span i.e. pre- puberty, reproductive years, and postmenopausal years. Estrogen induced regulation of the KOR gene expression and function has a broad relevance for many brain functions considering the known role of KOR in the stress response, learning and memory, addiction and motor functions.
Acknowledgments
This study was supported by NIH grants SC1NS063951, RR03032, U54 NS041071, Meharry/Vanderbilt Alliance training grant T32 MH65782 and RISE 2R25GM59994.
Footnotes
The authors have no conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambriz-Tututi M, Rocha-González HI, Castañeda-Corral G, Araiza-Saldaña CI, Caram-Salas NL, Cruz SL, Granados-Soto V. Role of opioid receptors in the reduction of formalin-induced secondary allodynia and hyperalgesia in rats. Eur J Pharmacol. 2009;619:25–32. doi: 10.1016/j.ejphar.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282(2):769–778. [PubMed] [Google Scholar]
- 3.Berglund LA, Derendorf H, Simpkins JW. Desensitization of brain opiate receptor mechanisms by gonadal steroid treatments that stimulate luteinizing hormone secretion. Endocrinology. 1988;122(6):2718–2726. doi: 10.1210/endo-122-6-2718. [DOI] [PubMed] [Google Scholar]
- 4.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–380. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 5.Bileviciute-Ljungar I, Saxne T, Spetea M. Anti-inflammatory effects of contralateral administration of the kappa-opioid agonist U-50,488H in rats with unilaterally induced adjuvant arthritis. Rheumatology (Oxford) 2006;45(3):295–302. doi: 10.1093/rheumatology/kei156. [DOI] [PubMed] [Google Scholar]
- 6.Binder W, Carmody J, Walker J. Effect of gender on anti-inflammatory and analgesic actions of two kappa-opioids. J Pharmacol Exp Ther. 2000;292(1):303–309. [PubMed] [Google Scholar]
- 7.Binder W, Scott C, Walker JS. Involvement of substance P in the anti-inflammatory effects of the peripherally selective kappa-opioid asimadoline and the NK1 antagonist GR205171. Eur J Neurosci. 1999;11(6):2065–2072. doi: 10.1046/j.1460-9568.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 8.Binder W, Walker JS. Effect of the peripherally selective kappa-opioid agonist, asimadoline, on adjuvant arthritis. Br J Pharmacol. 1998;124(4):647–654. doi: 10.1038/sj.bjp.0701874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 10.Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in rat spinal cord vary across the estrous cycle. Brain Res. 2000;861(1):168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- 11.Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J Neurosci. 2006;26(50):13048–13053. doi: 10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Amour FE, Smith DL. A method of determining pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- 13.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107(1):83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 14.Dawson-Basoa ME, Gintzler AR. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain. 1996;64(3):608–15. doi: 10.1016/0304-3959(96)87175-2. [DOI] [PubMed] [Google Scholar]
- 15.Eisenach JC, Carpenter R, Curry R. Analgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain. 2003;101(1–2):89–95. doi: 10.1016/s0304-3959(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 16.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8(5):413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 18.Flores CA, Wang XM, Zhang KM, Mokha SS. Orphanin FQ produces gender-specific modulation of trigeminal nociception: behavioral and electrophysiological observations. Neuroscience. 2001;105(2):489–498. doi: 10.1016/s0306-4522(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 19.Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996a;205(3):207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 20.Gear RW, Gordon NC, Miaskowski C, Paul SM, Heller PH, Levine JD. Sexual dimorphism in very low dose nalbuphine postoperative analgesia. Neurosci Lett. 2003;339(1):1–4. doi: 10.1016/s0304-3940(02)01438-6. [DOI] [PubMed] [Google Scholar]
- 21.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996b;2(11):1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 22.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83(2):339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 23.Gintzler AR. Ovarian sex steroids activate antinociceptive systems and reveal gender-specific mechanisms. Seattle: IASP Press; 2000. [Google Scholar]
- 24.Gintzler AR, Schnell SA, Gupta DS, Liu NJ, Wessendorf MW. Relationship of spinal dynorphin neurons to delta-opioid receptors and estrogen receptor alpha: anatomical basis for ovarian sex steroid opioid antinociception. Pharmacol Exp Ther. 2008;326(3):725–31. doi: 10.1124/jpet.108.139816. [DOI] [PubMed] [Google Scholar]
- 25.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 26.Harris JA, Chang PC, Drake CT. Kappa opioid receptors in rat spinal cord: sex-linked distribution differences. Neuroscience. 2004;124(4):879–90. doi: 10.1016/j.neuroscience.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 27.Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol. 1991;194(2–3):135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23(9):3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117(3):433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 31.Kavaliers M, Innes D. Stress-induced opioid analgesia and activity in deer mice: sex and population differences. Brain Res. 1987;425(1):49–56. doi: 10.1016/0006-8993(87)90482-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee YW, Chaplan SR, Yaksh TL. Systemic and supraspinal, but not spinal, opiates suppress allodynia in a rat neuropathic pain model. Neurosci Lett. 1995;199(2):111–4. doi: 10.1016/0304-3940(95)12034-2. [DOI] [PubMed] [Google Scholar]
- 33.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci U S A. 2003;100(1):271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogil JS, Ritchie J, Smith SB, Strasburg K, Kaplan L, Wallace MR, Romberg RR, Bijl H, Sarton EY, Fillingim RB, Dahan A. Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet. 2005;42(7):583–587. doi: 10.1136/jmg.2004.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100(8):4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nag S, Mokha SS. Activation of alpha2-adrenoceptors in the trigeminal region produces sex-specific modulation of nociception in the rat. Neuroscience. 2006;142(4):1255–1262. doi: 10.1016/j.neuroscience.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290(3):1132–1140. [PubMed] [Google Scholar]
- 38.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res Mol Brain Res. 1995;28(1):61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- 39.Riviere PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141(8):1331–1334. doi: 10.1038/sj.bjp.0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984;228(1):1–12. [PubMed] [Google Scholar]
- 41.Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6(4):261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su X, Joshi SK, Kardos S, Gebhart GF. Sodium channel blocking actions of the kappa-opioid receptor agonist U50,488 contribute to its visceral antinociceptive effects. J Neurophysiol. 2002;87(3):1271–1279. doi: 10.1152/jn.00624.2001. [DOI] [PubMed] [Google Scholar]
- 43.Thompson AD, Angelotti T, Nag S, Mokha SS. Sex-specific modulation of spinal nociception by alpha2-adrenoceptors: differential regulation by estrogen and testosterone. Neuroscience. 2008;153(4):1268–1277. doi: 10.1016/j.neuroscience.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahab SA, Askalani AH, Amar RA, Ramadan ME, Neweigy SB, Saleh AA. Effect of some recent analgesics on labor pain and maternal and fetal blood gases and pH. Int J Gynaecol Obstet. 1988;26(1):75–80. doi: 10.1016/0020-7292(88)90199-3. [DOI] [PubMed] [Google Scholar]
- 45.Walker JS, Chandler AK, Wilson JL, Binder W, Day RO. Effect of mu-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm Res. 1996;45(11):557–563. doi: 10.1007/BF02342227. [DOI] [PubMed] [Google Scholar]
- 46.Walker JS, Howlett CR, Nayanar V. Anti-inflammatory effects of kappa-opioids in adjuvant arthritis. Life Sci. 1995;57(4):371–378. doi: 10.1016/0024-3205(95)00296-i. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JL, Nayanar V, Walker JS. The site of anti-arthritic action of the kappa-opioid, U-50, 488H, in adjuvant arthritis: importance of local administration. Br J Pharmacol. 1996;118(7):1754–1760. doi: 10.1111/j.1476-5381.1996.tb15601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]