Abstract
We describe the isolation and molecular characterization of a novel glucose-lysine dimer crosslink 1,3-bis-(5-amino-5-carboxypentyl)-4-(1′,2′,3′,4′-tetrahydroxybutyl)-3H-imidazolium salt, named GLUCOLD. GLUCOLD was easily formed from the Amadori product (fructose–lysine). However, when BSA was incubated with 100 mM glucose for 25 days, the levels of the lysine-lysine glucose crosslinks GLUCOLD and CROSSLINE were only 21 and <1 pmol/mg, respectively, compared to 611 pmol/mg protein for the lysine-arginine GLUCOSEPANE crosslink, in spite of more than 20 potential lysine-lysine crosslinking sites in the protein. Mechanistic investigation revealed that metal-free phosphate ions catalyzed formation of fructose–lysine and all three crosslinks from amino acids, while cationic MOPS buffer had an opposite effect. This together with the rapid formation of N6-1,4-dideoxy-5,6-dioxoglucosone derivatives by dicarbonyl trapping agents, such as 1,2-diaminobenzene or γ-guanidinobutyric acid, strongly suggests that enolization of the Amadori product and trapping of the 5,6-dioxo derivative by arginine residues constitutes the major pathway for glucose-mediated crosslinking in proteins.
Keywords: Advanced glycation end-product, Maillard reaction, Glycation, Crosslink, Diabetes
Introduction
Nonenzymatic glycation (the Maillard reaction) in vivo is initiated by the reaction of reducing sugars such as glucose and the ε-amino groups of the lysine side chain followed by Schiff base formation. This adduct rearranges into a derivate also known as the Amadori product, which further degrades into more stable advanced glycation end products (AGEs). Increasing evidence suggests a role for AGE-induced oxidative stress in the pathogenesis of normal aging and many age-related chronic diseases, including diabetes, end-stage renal disease, and cardiovascular disease (Ahmed and Thornalley 2007; Goldin et al. 2006; Monnier et al. 1992; Stern et al. 2002; Tan et al. 2007).
One of the major consequences of advanced nonenzymatic glycation is the formation of protein crosslinks. Free α-oxoaldehydes, intermediates of the Maillard reaction, such as 3-deoxyglucosone (3-DG), glyoxal (GO), and methylglyoxal (MGO), are known intermediates in cross-link formation. Moreover, lysine-lysine imidazolium crosslinks from GO (GOLD) (Wells-Knecht et al. 1995), MGO (MOLD) (Brinkmann et al. 1995) and 3-DG (DOLD) (Skovsted et al. 1998) and the lysine-arginine derived crosslinks GODIC (Lederer and Klaiber 1999), MODIC (Lederer and Klaiber 1999) or DOGDIC (Biemel et al. 2001b) isolated from different model reactions are present in numerous biological systems (Biemel et al. 2001b; Chellan and Nagaraj 1999; Frye et al. 1998; Nagaraj et al. 1996; Odani et al. 1998; Thornalley et al. 2003). Glyoxal-derived lysine-lysine crosslinks GOLA and GALA have also been described (Glomb and Pfahler 2001; Sell and Monnier 1989). In addition, the fluorescent crosslinks pentosidine (Sell and Monnier 1989), vesperlysine A (Nagaraj and Monnier 1992; Tessier et al. 1999), and crossline (Yamaguchi et al. 1998) have also been identified in vivo, evidence for the latter, however, was based on immunological rather than mass spectrometry method.
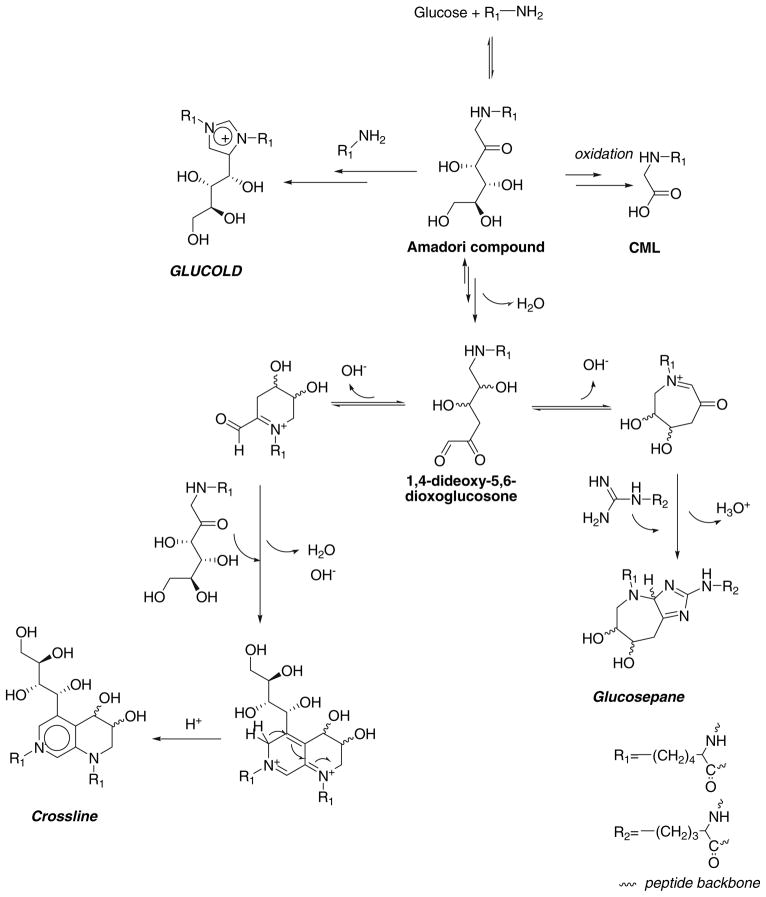
Evidence for a major role of glucose in crosslink generation came with the discovery of glucosepane by Lederer et al. (Biemel et al. 2002a). Its formation implies existence of a new α-oxoaldehyde precursor, i.e., N6-1,4-dideoxy-5,6-dioxoglucosone-lysine, which is formed from the Amadori product through a non-oxidative pathway by intramolecular enolisation and loss of a water molecule (Reihl et al. 2004). Moreover, it was shown that glucose-pane represents a single major crosslink in skin and glomerular basement membrane collagen from diabetic individuals (Sell et al. 2005) suggesting that the 5,6-dioxoglucosone derived from the Amadori product could also be the intermediate compound in numerous, yet undiscovered crosslinks. As an example, crossline formation was suggested to proceed through the Amadori product and N6-1,4-dideoxy-5,6-dioxoglucosone-lysine (Biemel et al. 2002b) (Scheme 1).
Scheme 1.
These above findings together with the well-documented formation of carboxymethyl-lysine (CML) from the Amadori product (Fu et al. 1996) and its involvement in the progression of diabetic complications through interaction with RAGE (Kislinger et al. 1999) brought the Amadori product back at the center of glucose-mediated protein damage. Therefore, the work below was initiated with the aim of addressing the question of whether other major Amadori-derived glucose crosslinks forming under non-oxidative conditions remained to be discovered. We now report the existence of a novel lys-lys crosslink GLUCOLD, a mechanism of its formation, and role for protein crosslinking in relationship to the previously described glucose-mediated crosslinks crossline and glucosepane.
Materials and methods
Materials
Reagents were obtained from Sigma (St. Louis, MO, USA), unless indicated otherwise. Deionized water (18.2 MΩ/cm2) was used for all experiments. For LC–ESI–MS/MS experiments ultrapure solvents were obtained from Burdick&Jackson (Muskegon, MI, USA). Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid, Nα-Boc-Nε-(1-deoxy-D-fructos-1-yl)-L-lysine (Amadori products of ε-amino caproic acid and Boc-lysine), and quinoxaline product of N6-1,4-dideoxy-5,6-dioxoglucosone-ε-amino caproic acid were synthesized as previously described (Biemel et al. 2002a; Gerhardinger et al. 1994; Thornalley et al. 1999).
Preparative high-performance liquid chromatography (HPLC)
The preparative HPLC system consisted of a Waters HPLC instrument (Waters Chromatography Div., Milford, MA, USA) with two pumps (Model 510), automatic injector (Model 712 WISP), and semi-preparative column Gemini (250 × 10 mm; 5 μm; Phenomex, USA) (column 1); injection volume 500 μl; flow rate 2 ml/min.
NMR
NMR spectra were recorded on a Varian Inova 600 NMR spectrometer, operating at 150.91 MHz for 13C and 600.13 MHz for 1H nuclei. The spectra were measured in D2O solution at 25°C. Chemical shifts in parts per million were referenced to SiMe4 (TMS). Spectra were assigned based on 1D (1H, 13C, DEPT) and 2D homonuclear (COSY), and heteronuclear (HMQC, HMBC) experiments.
Model incubation systems
ε-Amino caproic acid (100 mM) and glucose (100 mM or 300 mM) or ε-amino caproic acid (100 mM) and Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (100 or 300 mM) were incubated under different molar ratios (1:1; 1:3) and conditions (100, 200, and 500 mM sodium phosphate buffer, pH 7.0–8.5, 50°C; with 0.1–0.5 mM diethylene-triaminepentaacetic acid (DTPA) or methanol:water 9:1, pH 8.0 (adjusted with solid sodium bicarbonate); 50°C under nitrogen up to 3 weeks. Incubation mixtures at different time points in phosphate buffer were diluted in 1% trifluoroacetic acid (TFA), while incubations in methanol were first evaporated under reduced pressure and then reconstituted in 0.1% TFA and then applied on preparative HPLC. Every 0.5-min fractions were collected and 10 μL was analyzed by loop injection into 2695 Separation Module with a Micromass Ultima triple quadrupole mass spectrometric detector (Waters-Micromass, Manchester, UK), operating in MS mode at 60 V, screening mass m/z from 200 to 700, the mobile phase (0.1% TFA in water) was ran under isocratic conditions at a flow rate of 200 μL/min.
GLUCOLD analog isolation
Nε-(1-Deoxy-D-fructos-1-yl)-amino caproic acid (500 mg) and ε-amino caproic acid (223 mg) were incubated in methanol:water 9:1 (10 mL), under nitrogen at pH 8.0 (adjusted with solid sodium bicarbonate) at 50°C for 15 days. Methanol from the incubation mixture was first removed by evaporation under reduced pressure. The residue was dissolved in 0.1% TFA and applied to an SPE column (Waters; Sep-Pack; 35 cc tC-18; 100 g) previously washed with methanol (50 ml) and equilibrated with 0.1% TFA in water (100 ml). The column was first washed with 0.1% TFA in water (100 ml), then with 50% methanol in 0.1% TFA in water (100 ml) and 100% methanol (100 ml). Methanol from the second fraction was removed by evaporation under reduced pressure. Four incubation mixtures were combined and purified by preparative RP HPLC. Mobile phase was 0.1% TFA in water (solvent A) and 0.1% TFA in 50% methanol 50% water (solvent B). Gradient was applied as follows: gradient 1 0 min 90% A (10% B); 0–15 min 90% A (10% B); 15–25 min 90% A (10% B)–10% A (90% B); 25–40 min 10% A (90% B); gradient 2 0 min 95% A (5% B); 0–15 min 95% A (5% B), 15–25 min 95% A (5% B)–50% A (50% B); 25–40 min 50% A (50% B). MS/MS was performed on the m/z 417. Major fragments are: m/z 303; 267; 225; 209; 153, and 111. For NMR assignments see Table 1.
Table 1.
1H and 13C NMR chemical shifts (δ/ppm) of GLUCOLD analogue (1) in D2O
| Positiona | 1H (δ/ppm) | 13C (δ/ppm) |
|---|---|---|
| 2 | 8.66 | 135.8 |
| 4 | – | 135.0 |
| 5 | 7.42 | 121.1 |
| 1′ | 5.01 | 62.5 |
| 2′ | 3.60 | 71.9 |
| 3′ | 3.65 | 70.7 |
| 4a′ | 3.70 | 62.9 |
| 4b′ | 3.52 | 62.9 |
| 1″ | 4.04 | 47.4 |
| 2″ | 1.74 | 28.8 |
| 3″ | 1.14 | 24.8 |
| 4″ | 1.47 | 23.5 |
| 5″ | 2.24 | 33.5 |
| 6″ | – | 178.8 |
| 1‴ | 4.10 | 49.6 |
| 2‴ | 1.74 | 29.0 |
| 3‴ | 1.20 | 25.0 |
| 4‴ | 1.47 | 23.7 |
| 5‴ | 2.24 | 33.5 |
| 6‴ | – | 178.8 |
Numeration see in Fig. 3
Chemical shifts are relative to SiMe4
Crossline analog isolation
ε-Amino caproic acid (500 mg) and glucose (687 mg) were incubated in phosphate buffer (100 mM, pH 8, 5 mL) in the presence of 0.1 mM DTPA, under nitrogen at 50°C for 15 days. The reaction mixture was diluted five times in 1% TFA and applied to the preparative HPLC. Mobile phase was 0.1% TFA in water (solvent A) and 0.1% TFA in 70% methanol 30% water (solvent C). Gradient was applied as follows: gradient 3 0 min 90% A (10% C); 0–30 min 90% A (10% C)–10% A (90% C); 30–45 min 10% A (90% C); gradient 4 0 min 90% A (10% C); 0–20 min 90% A (10% C)–15% A (85% C); 20–21 min 15% A (85% C)–5% A (95% C); 21–50 min 5% A (95% C). 1H NMR (600 MHz, D2O) δ(ppm): 7.85 (aromatic CH), 7.80 (aromatic CH), 5.40 (R or S CHOH), 4.35 (2× CαH2), 4.0–3.0 (CHOH, CH2OH, CεH2), 1.4–2.2 (CβγδCH2). 13C NMR (150 MHz, D2O) δ(ppm): 178.87 (COOH), 178.72 (COOH), 142.98 (aromatic Cq), 142.71 (aromatic Cq), 129.64 (aromatic Cq), 129.29 (aromatic CH), 124.85 (aromatic CH), 73.32 (CHOH), 71.47 (CHOH), 65.91 (R or S CHOH), 63.11 (ring CHOH), 62.56 (CH2OH), 62.11 (CεH2), 61.27 (ring CHOH), 52.11 (CεH2), 50.61 (2 × CαH2), 47.27 (ring CH2), 33.67 (CβH2), 33.26 (CβH2), 25.53 (CδH2), 24.98 (CδH2), 24.22 (CγH2), 23.81 (CγH2). Mass spectrum: m/z 514.99, fragments m/z: 468.36; 404.99; 388.95; 377.12; 360.89; 276.96; 274.76; 236.13; 160.64.
In vitro incubation of ε-amino caproic acid and glucose in the presence of glyoxal (GO) and glycolaldehyde (GA)
Incubation of ε-amino caproic acid (100 mM) and glucose (500 mM) was performed in the presence of 0.0; 0.1; 0.5, and 1.0 mM glyoxal (GO) or glycolaldehyde (GA) in phosphate buffer (100 mM; 0.02% sodium azide) in the presence of 0.1 mM DTPA at 37°C for 1, 3, 5, 9, 15, 20, and 25 days. All incubations were performed in triplicate. All samples at different time points were frozen at −80°C and thawed immediately before analysis. The samples were diluted two times and 30 μl was injected for LC–MS/MS analysis.
Determination of GLUCOLD and crossline in proteins incubated with glucose in vitro or isolated from biological and food samples. LC–(ESI)–MS/MS analysis 1
GLUCOLD and crossline standards were prepared from ε-fructosyl-α-Boc-lysine or and Boc-lysine and glucose, respectively, under the same conditions as their analogs following the same purification procedures. The protective Boc-groups were cleaved with TFA:water (9:1, 1 mL) at room temperature. LC–MS measurements showed molecular ion at m/z 447 for GLUCOLD and m/z 545 for crossline. The LC–MS/MS analysis 1 conditions were as previously published (Thornalley et al. 2003) whereby conditions for GLUCOLD and crossline were as follows: GLUCOLD: parent ion m/z 447.32; daughter ions m/z 84.08, 10.97 and 402.11; collision energy 40, 40 and 27 eV, respectively; retention time 23.8 min; crossline: parent ion m/z 545.45; daughter ion m/z 500.36; collision energy 35 eV; retention time 24.7 min. BSA (25 mg/ml) incubated with glucose (0–100 mM) in vitro (200 mM phosphate buffer in the presence/absence of 0.2 mM DTPA, pH 7.4, 37°C, 5 and 25 days) and proteins isolated from different tissues or biological fluid (skin, lens, kidney and serum) were enzymatically digested as previously described (Ahmed et al. 2002; Fan et al. 2009; Sell et al. 2005). Food samples [different types of milk (12), protein bars (4), cookies (2), bread crumbs, cereals, baby foods (4), and chicken broth] were purchased from a local supermarket. Proteins from the samples were precipitated with TCA, washed, delipidated, freeze-dried, and then enzymatically digested followed the procedure for non-soluble lens crystallins as previously described (Fan et al. 2009).
In vitro incubation of ε-amino caproic acid and glucose or Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid in the presence/absence of γ-guanidino butyric acid
Incubation of ε-amino caproic acid (100 mM) and glucose (100 mM) was performed in the presence of 0.0 and 100 mM of γ-guanidino butyric acid 100, 200 and 500 mM sodium phosphate buffer (pH 7.4 with 0.02% sodium azide and 0.1, 0.2 and 0.5 mM DTPA, respectively) and in 200 mM MOPS buffer (pH 7.4 with 0.02% sodium azide and 0.2 mM DTPA) at 37°C for 0, 3, 10, 15, 20, and 25 days. Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (100 mM) was incubated in the presence/absence of ε-amino caproic (100 mM) or γ-guanidino butyric acid (100 mM) in phosphate buffer (200 mM pH 7.4 with 0.02% sodium azide 0.2 mM DTPA) and in MOPS buffer (200 mM pH 7.4 with 0.02% sodium azide 0.2 mM DTPA) at 37°C for the same period of times. All incubations were performed in triplicates. All samples at different time points were frozen at −80°C and thawed immediately before LC–(ESI)–MS/MS analysis 2. The samples were diluted ten times and 30 μl was injected for LC–MS/MS analysis 2.
In vitro incubation of ε-amino caproic acid and glucose or Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (Amadori product) in the presence/absence 1,2-diaminobenzene (OPD)
ε-Amino caproic acid (100 mM) and glucose (100 mM) or Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (100 mM) were incubated under the same conditions as described above in the presence of OPD (100 mM). Additional incubation was performed initially without OPD which was added into the incubation mixtures at different time points and incubated for 8 h at room temperature. All incubations were performed in triplicates. All samples at different time points were frozen at −80°C and thawed immediately before LC–(ESI)–MS/MS analysis 3. The incubations mixtures of ε-amino caproic acid and glucose were diluted ten times, while the Amadori product incubation mixtures were diluted 100 times before analysis; in both cases 10 μl was injected for LC–MS/MS analysis 3.
LC–(ESI)–MS/MS analysis of GLUCOLD, crossline, glucosepane analogs, and Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (Amadori product): LC–(ESI)–MS/MS analysis 2
The LC–ESI–MS/MS analysis 2 was run using a Model 2695 Separation Module with a Micromass Ultima triple quadrupole mass spectrometric detector (Waters-Micromass, Manchester, UK) and 5 μm Hypercarb™ column (Thermo Hypersil, Runcorn, Cheshire, UK) 2.1 × 50 mm (column 2) was used. Solvents for analysis of the incubation mixtures were 1% formic acid in water buffered with ammonia to pH 3.6 (solvent D) and 90% acetonitrile in water (solvent E). Gradient was applied as follows: gradient 5 0 min 100% D (0% E); 0–15 min 100% D (0% E)–50% D (50%E); 15–20 min 50% D (50% E); after each run, the column was washed with 0.1% formic acid in THF: water 50:50 for 10 min, and then equilibrated with buffer D for 20 min. All flow rates were 0.2 ml/min. Electronspray positive ionization-mass spectrometric multiple-reaction monitoring (ESI+/MRM) experiments were used for GLUCOLD, crossline, glucosepane analog, and the Amadori product in the incubation mixtures The ionization source temperature was 120°C and the desolvation gas temperature 300°C. The cone gas and desolvation gas-flow rates were 660 and 85 l/h, respectively. The capillary voltage was 3.50 kV. Argon gas was in the collision cell. Programmed molecular ion and fragment ion masses were optimized to ±0.1 Da for multiple-reaction-monitoring detection of analyte. Cone voltage was 60 V. For GLUCOLD analog (parent ion m/z 417.20) collision energies for fragments m/z 224.90 and 110.90 were 35.00 and 43.00 eV, respectively, for crossline (parent ion m/z 515.20) collision energy for both fragments (m/z 377.13 and 388.95) was 50 eV, for Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (parent ion m/z 293.60) collision energies for fragments m/z 144.08 and 210.00 were 21.00 and 20.00 eV, respectively, while for glucosepane analog (parent ion m/z 385.30), collision energies for both fragments (m/z 178.90 and 253.10) were 42.00 eV. The retention times for GLUCOLD, crossline, glucosepane analog, and the Amadori product were 12.2, 13.5, 15.0, and 8.6 min, respectively.
LC–(ESI)–MS/MS analysis of quinoxaline product of N6-1,4-dideoxy-5,6-dioxoglucosone-lysine: LC–(ESI)–MS/MS analysis 3
Atlantis dC-18 column (2.1 × 50 mm; 3 μm; Waters, Milford, MA, USA) (column 3) was used and the same LC–MS/MS system as previously described. Solvents for analysis of the incubation mixtures were 0.1% TFA in water (solvent F) and 90% acetonitrile (solvent E). Gradient was applied as follows: gradient 6 0–5 min 100% F (0% E); 5–15 min 100% F (0% E)–60% F (40% E); 15–16 min 60% F (40% E)–20% F (80% E); 16–20 min 20% F (80% E). ESI+/MRM experiments were used where parameters were set as described above. Collision energies for the quinoxaline (parent ion m/z 348.03) fragments m/z 144.00 and 186.83 were 23.00 and 18.00 eV, respectively. Retention time for the quinoxaline was 14.9 min.
In vitro incubation of glycated BSA with nucleophilic amines and GC/MS analysis of furosine (fructose–lysine)
BSA (50 mg/mL) and glucose (500 mM) were incubated in phosphate buffer (100 mM, pH 7.4) containing DTPA (0.2 mM) at 37°C for 10 days (pre-glycation). After incubation BSA was dialyzed against cold water (4°C) for 48 h with change of water after 24 h. The dialyzate was lyophilized. The pellet (50 mg/mL) was re-incubated in phosphate buffer (200 mM, pH 7.4, 0.2 mM DTPA) or MOPS (200 mM, pH 7.4, 0.2 mM DTPA) in the presence of ε-amino caproic acid, guanidino butyric acid or OPD (50 mM for 20 days). All incubations were performed in triplicate. After incubation, BSA was dialyzed against cold water (4°C) for 48 h with change of water after 24 h. After dialysis, 3 mg of the sample was placed into a 13 × 100 mm borosilicate glass tube fitted with a PTFE (polytetrafluoroethylene)-faced, rubber-lined screw cap. Each sample was acid hydrolyzed for 18 h with 3 ml of 6 M HCl as detailed elsewhere (Sell et al. 2007). In addition, the acid was degassed under vacuum followed by purging with argon by bubbling for at least 20 min. The acid was immediately pipetted into each tube followed by thoroughly purging the tube with argon before sealing with the screw cap. Furosine was determined in acid hydrolysates of processed BSA samples, derivatized as their tri-fluoroacetyl methyl esters, by selected ion monitoring gas chromatography (GC)/MS as previously described (Sell et al. 2007).
Statistical analysis
All data are expressed as mean ± SD. Statistical differences between groups were analyzed by the Student’s t test. P values less than 0.05 were considered statistically significant.
Results
The initial purpose of this study was the isolation and structural characterization of novel glucose-derived lysine-lysine crosslinks and the elucidation of their formation pathway starting from the Amadori compound. In doing so, we describe the discovery of the novel crosslink GLUCOLD, whose formation was strongly dependent on the incubation conditions which led us to undertake a comprehensive analysis of the glycation pathways under metal-free conditions.
Isolation and characterization of glucose crosslinks from model reaction systems
Incubation of the Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid (Amadori product) and ε-amino caproic acid (structural analogue of lysine) (molar ratios 1:1 and 3:1) was performed in 100, 200 or 500 mM sodium phosphate buffer pH 7.0–8.5 in the presence of 0.1–0.5 mM DTPA at 50 °C under nitrogen in order to suppress glycoxidation. ESI–MS analysis of the crude reaction mixtures showed that a compound with m/z 417 represented a prominent signal. On the other hand, when ε-amino caproic acid was incubated with glucose in the same buffers, a compound with m/z 515 was observed as the major signal. After isolation by preparative HPLC, mass and NMR analysis it was confirmed that the compound with m/z 515 was a previously described lysine-lysine crosslink crossline (Nakamura et al. 1992).
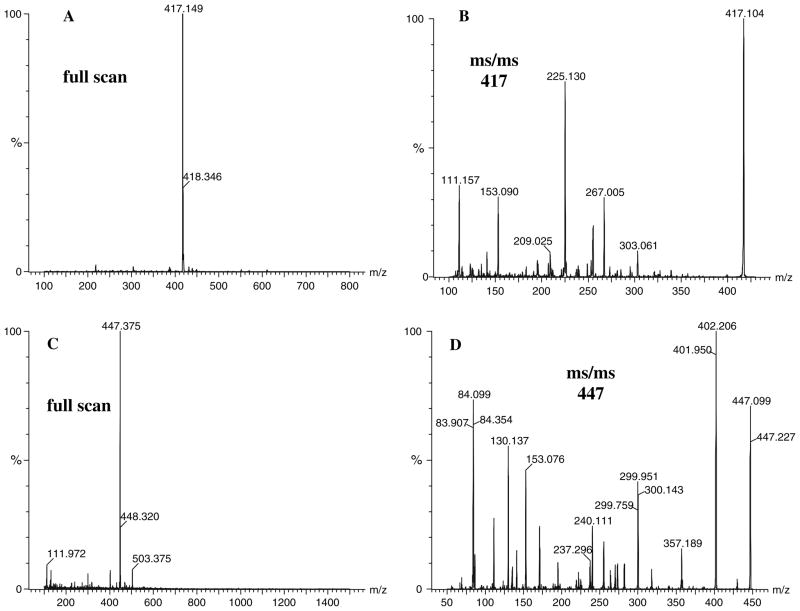
Aiming to obtain a better yield on the compound with m/z 417, different solvent and pH conditions were examined. Incubation of Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid and ε-amino caproic acid (1:1) in methanol:water 9:1, pH 8.0 at 50°C under nitrogen gave the best results. After 15 days of incubation, the compound of interest (m/z 417) was purified by SPE and RP HPLC in gradient 1 followed by gradient 2. Fractions with acceptable purity, as determined by ESI–MS total ion scan (Fig. 1a), were pooled, freeze-dried and analyzed by ESI–MS/MS spectrometry (Fig. 1b), and NMR spectroscopy (Fig. 2).
Fig. 1.
Electron spray ionization-MS (a, c) and MS/MS (b, d) spectra of GLUCOLD analogue (a, b) and GLUCOLD (c, d)
Fig. 2.
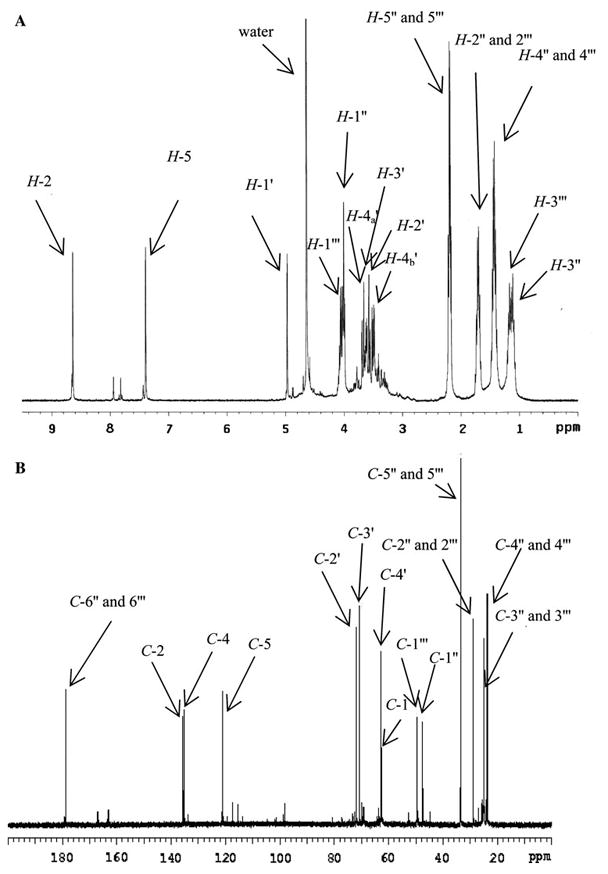
1H (a) and 13C (b) NMR spectra of GLUCOLD analogue (10 mg in 500 μl of D2O, 600 MHz, 25°C). For numeration see Fig. 3
1H NMR spectrum (Fig. 2a) showed two aromatic proton signals at 8.64 (H-2) and 7.39 (H-5) ppm bound to two C-atoms at 135.8 (C-2) and 121.1 (C-5) ppm, respectively (Fig. 2b). Additional signal in the 13C NMR spectrum at 135.0 ppm and a coupling constant between H-2 and H-5 of 1.6 Hz, which is previously demonstrated in GOLD and MOLD crosslinks (Biemel et al. 2002b), confirm an imidazolium ring in the structure. The newly isolated compound also showed three CH-atoms and one CH2 between 62 and 72 ppm (Fig. 2b) together with four proton signals between 3.4 and 3.8 ppm (Fig. 2a), chemical shifts characteristic for sugars. One additional signal at 5.01 ppm in 1H NMR spectrum (H-1′) showed in COSY spectrum coupling with proton at 3.60 ppm, while in HSQC spectrum correlation with C-atom at 62.5 ppm (C-1′). By analogy with the CHOH signals in crossline, in which the sugar portion is directly bound on the aromatic ring and has similar chemical shifts [5.64 ppm (1H) and 67.3 ppm (13C)] (Nakamura et al. 1992), as well as in vesperlysine C [4.97 ppm (1H) and 61.5 ppm (13C)] (Nakamura et al. 1997), in GA-pyridine [4.81 ppm (1H) and 58.0 ppm (13C)] (Nagai et al. 2002) and histidino-threosidine [4.70 ppm (1H) and 66.0 ppm (13C)] (Dai et al. 2007), we propose that the sugar part of the structure is directly bound to the imidazolium ring. Other proton signals between 0.5 and 1.7 ppm together with carbon signals between 20 and 30 ppm were assigned as aliphatic chains of ε-amino caproic acid. Hydrogen/carbon assignments were confirmed by 1H, 13C, COSY, 13C-DEPT, HSQC, and HMBC experiments. The NMR data are summarized in Table 1.
ESI–MS/MS analysis of molecular ion at m/z 417 (Fig. 1b) showed m/z 303 [loss of (CH2)5COOH)]; 267 [loss of (CH2)5COOH and two water molecules]; 225 [loss of (CH2)5COOH, a carboxyl and two hydroxyl groups]; 209 [loss of (CH2)5COOH, a carboxyl and two hydroxyl groups]; 153 [loss of two (CH2)5COOH and two waters]; 111 [loss of two (CH2)5COOH, CHOHCH2OH and a hydroxyl group] as major fragments.
Together, NMR and MS/MS data unequivocally prove 1,3-bis-(5-amino-5-carboxypentyl)-4-(1′,2′,3′,4′-tetrahydroxybutyl)-3H-imidazolium salt as a structure for newly isolated lysine-lysine crosslink (Fig. 3). Based on the structural analogy with GOLD, MOLD, and DOLD, we propose the trivial name GLUCOLD (GLUCOse-derived Lysine-lysine Dimer).
Fig. 3.
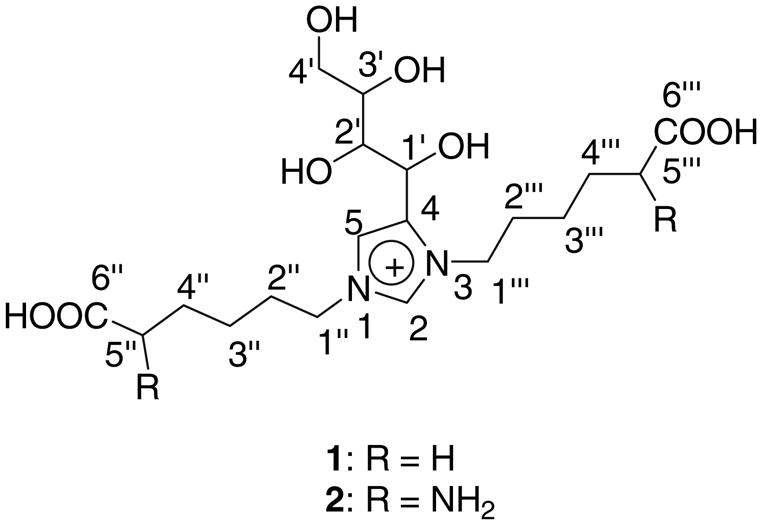
Proposed chemical structure for GLUCOLD analogue (1) and GLUCOLD (2)
GLUCOLD and crossline in proteins modified with glucose in vitro and biological samples
An LC–MS/MS method for GLUCOLD and crossline quantification in biological samples was developed (LC–MS/MS analysis 1). Limit of detections, determined form signal to noise ratio by adding a known amount of standards into blank protein digests (50 μg), were for GLUCOLD: 0.089, 0.068, and 0.072 pmol for 447.32/84.08; 447.32/111.00 and 447.32/402.2 transitions, respectively, while for crossline 0.086 pmol for 545.45/500.36 transitions.
In proteins incubated in vitro (BSA) with glucose the compound eluting at 23.7 min was identified as GLU-COLD, based on its co-elution with the standard and by comparison of signal ratios from three different fragments with the standard.
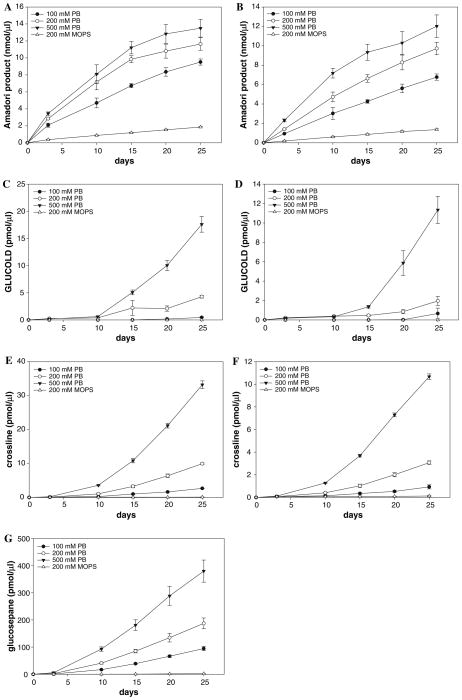
GLUCOLD was detected only when high concentrations of glucose were used (>50 mM) under prolonged incubation period (Fig. 4). Levels of GLUCOLD after 25 days of incubation in the presence of 100 mM glucose reached only 21 pmol/mg of protein (GLUCOLD was quantified by spiking the samples with known amount of the standard) while the level of glucosepane was 611 pmol/mg of protein. Crossline, surprisingly, was not detected under those conditions. Moreover, in further investigations neither GLUCOLD nor crossline was detected in proteins isolated from biological tissues or commercial food samples.
Fig. 4.
Detection of GLUCOLD (m/z 447.32) by LC–ESI/MS/MS analysis 1 in enzymatically digested BSA (25 mg/ml) incubated with glucose (100 mM) in vitro in phosphate buffer (200 mM, pH 7.4, 37°C, 25 days) (a) versus standard (b). Multiple-reaction monitoring (MRM) with m/z 84.08; 111.00 and 402.2 as characteristic fragment ions
In vitro incubation of ε-amino caproic acid and glucose or Nε-(1-deoxy-D-fructos-1-yl)-amino caproic acid in the presence/absence of γ-guanidinobutyric acid or OPD
In order to clarify the unexpected results, GLUCOLD, crossline, and glucosepane together with the Amadori product were measured in different reaction systems (LC–MS/MS analysis 2). As previously shown by Watkins et al. phosphate ions accelerated the Amadori product formation by catalyzing glucose–lysine Schiff base rearrangement (Watkins et al. 1987) (Fig. 5a, b). The catalytic effect of phosphate ions was observed for all three crosslinks. In cationic MOPS buffer lower yields were obtained in comparison with the phosphate buffer (Fig. 5c–g). Although the Amadori product is a precursor of all examined crosslinks, and increased their generation in a concentration-dependent manner, PO43− also catalyzed Amadori product rearrangements needed for crosslink formation.
Fig. 5.
Influence of buffer ions [MOPS (200 mM; pH 7.4 with 0.02% sodium azide and 0.2 mM DTPA) versus phosphate buffer (100, 200 and 500 mM; pH 7.4 with 0.02% sodium azide and 0.1, 0.2 and 0.5 mM DTPA, respectively)] and γ-gunidino butyric acid on Amadori product (Panels a, b), GLUCOLD (Panels c, d), crossline (Panels e, f) and glucosepane (Panel g) formation. Panels a, c and e are from glucose (100 mM) and ε-amino caproic acid (100 mM) incubation systems, while panels b, d, f and g from glucose (100 mM), ε-amino caproic acid (100 mM) and gunidino butyric (100 mM) incubation systems. Data are expressed as mean value ± SD (n = 3)
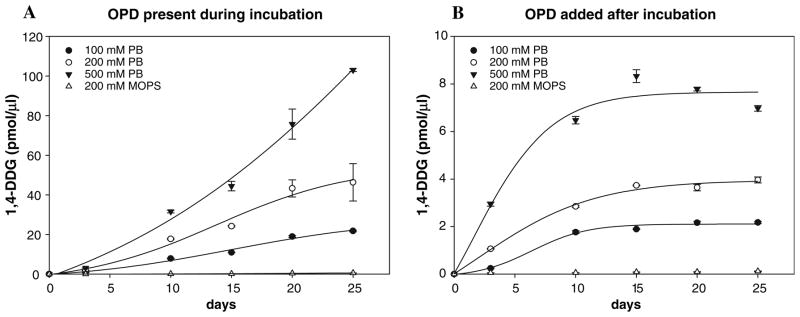
According to the mechanism proposed by Biemel et al. glucosepane and crossline share the same precursor—N6-1,4-dideoxy-5,6-dioxoglucosone in a seven- and six-member ring form, respectively (Scheme 1). N6-1,4-Dideoxy-5,6-dioxoglucosone level (measured as its quinoxaline; LC–MS/MS analysis 3) is increased in systems with higher phosphate content, regardless of whether the trapping agent (OPD) was added to the ε-amino caproic acid–glucose incubation mixtures prior to the incubation (Fig. 6a), after the incubation (Fig. 6b), or to the solutions of the Amadori product (data not shown). Interestingly, the N6-1,4-dideoxy-5,6-dioxoglucosone level was approximately ten times higher in the amine-glucose model system performed in the presence of OPD in comparison with systems where the trapping reagent OPD was added after incubation (Fig. 6a vs. b). The difference is even more pronounced in the incubation system where incubation was started with the Amadori product.
Fig. 6.
N6-1,4-Dideoxy-5,6-dioxoglucosone (1,4-DDG) generated during incubation of ε-amino caproic acid (100 mM) and glucose (100 mM) in phosphate buffer (100, 200 and 500 mM; pH 7.4 with 0.02% sodium azide and 0.1, 0.2 and 0.5 mM DTPA, respectively) and in MOPS buffer (200 mM; pH 7.4 with 0.02% sodium azide and 0.2 mM DTPA) in the presence of 1,2-diaminobezene (OPD) (100 mM) through whole incubation period (panel a) and incubation performed without OPD during given time, but it was added into the incubation mixtures after given time point. Data are expressed as mean value ± SD (n = 3)
Contrary to the Amadori product where the presence of a guanidino compound did not significantly influence its production, addition of γ-guanidino butyric acid to the amine-glucose reaction system reduced GLUCOLD and crossline generation by approximately 40 and 70%, respectively. The results for crossline are in agreement with the proposed mechanism for its formation by Biemel et al., where guanidinobutyric acid, as dicarbonyl compound trapping agent, reacts with N6-1,4-dideoxy-5,6-dioxoglucosone and reduces its availability for crossline ring closures (Biemel et al. 2002b). Reduction of GLUCOLD production by γ-guanidino butyric, on the other hand, suggests that dicarbonyl precursors are also needed for GLUCOLD formation.
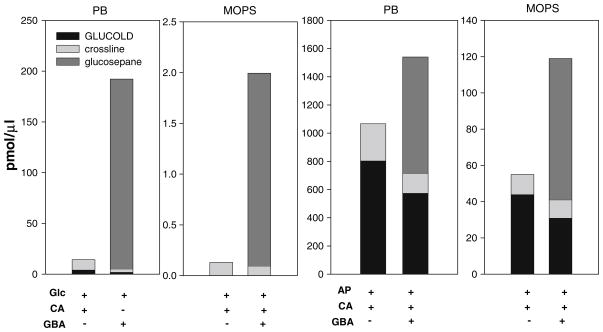
After 25 days of incubation of ε-amino caproic acid with glucose, ratios of GLUCOLD and crossline were 1:6; 1:2.5 and 1:2 in 100, 200 and 500 mM phosphate buffer, respectively. In contrast, the ratios among GLUCOLD, crossline and glucosepane in the same systems with added γ-guanidinobutyric acid were 1:1.5:150; versus 1: 0.02: 95; and 1: 0.9: 35 (Figs. 5, 7). In 200 mM MOPS GLUCOLD was not detected and ratio between crossline and glucosepane at the end of the incubation was 1:20 (Fig. 7). In the Amadori product incubations the ratio among crosslinks did not follow the same trend. Moreover, GLUCOLD which was the minor product in the amine-glucose systems was formed in amounts three and four times higher than crossline in 200 mM phosphate buffer and MOPS, respectively, while the ratios between GLUCOLD and glucosepane, under the same conditions were 1:4 and 1:2.5 (Fig. 7).
Fig. 7.
Comparison of crosslinks (GLUCOLD, crossline, glucold) amine-glucose versus Amadori product model incubation systems [phosphate buffer (PB) or MOPS; (200 mM, pH 7.4 with 0.02% sodium azide and 0.2 mM DTPA)], after 25 days of incubation at 37°C. Glc, glucose; CA, ε-amino caproic acid; GBA, γ-guanidino butyric acid; AP, Amadori product
Mechanism of GLUCOLD formation
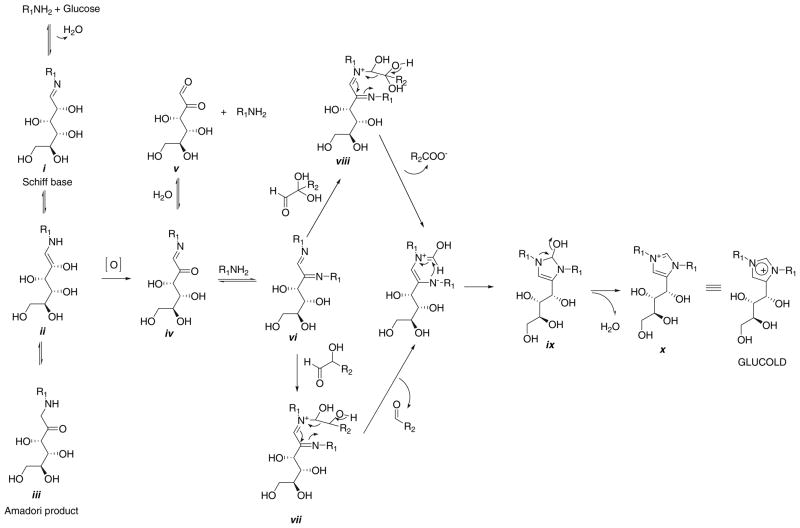
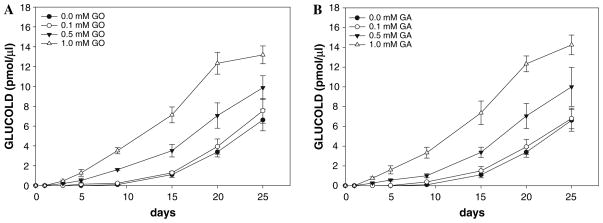
The structure of GLUCOLD suggests that for its formation, in addition to glucose and two lysine residues, an extra aldehyde that serves as the donor of the C2 carbon is needed. A possible mechanism is shown on Fig. 8. The enol-enamine species formed during reaction of lysine with glucose ii can undergo autoxidation in the reaction mixture and give 2-oxoaldehyde Schiff base iv. Intermediate iv can then react with the second lysine residue and give α-diimine intermediate vi. Further reaction of vi with a 2-hydroxylaldehyde or 2-oxoaldehyde will give adducts vii and viii, respectively. Adducts vii and viii after loss of an aldehyde or carboxylic acid, respectively, generate species ix that readily dehydrates into GLUCOLD (x). Increased formation of GLUCOLD in the presence of glycolaldehyde (hydroxylaldehyde) and glyoxal (dicarbonyl) as shown in Fig. 9 proves that these types of compounds can participate in GLUCOLD production.
Fig. 8.
Proposed reaction pathway for the GLUCOLD formation. R1 represents lysine residue, while R2 hydroxyaldehyde or dicarbonyl residue
Fig. 9.
Effect of glyoxal (GO) (panel a) and glycolaldehyde (GA) (panel b) on GLUCOLD analogue formation. Increased concentrations of GO (a) and GA (b) (0; 0.1; 0.5 and 1.0 mM) were initially added to the incubation mixture of ε-amino caproic acid (100 mM) and glucose (500 mM) in phosphate buffer (100 mM with 0.02% sodium azide and 0.1 mM DTPA). Incubations were performed at 37°C. Data are expressed as mean ± SD (n = 3)
2-Oxoaldehyde Schiff base iv can be easily hydrolyzed into glucosone (v), the well-described dicarbonyl intermediate of the Maillard reaction whose production is higher under oxidative versus non-oxidative conditions (Gobert and Glomb, 2009). In addition, dicarbonyls such as glyoxal that increase the level of GLUCOLD are also products of oxidative pathways (Thornalley et al. 1999). Incubation of glucose with ε-amino caproic acid for 10 days at 37°C generated ten times more GLUCOLD in the incubation mixtures in the absence of metal chelating agent (DTPA) versus incubation mixtures with DTPA (data not shown). All of this together, with the observation that guanidino butyric acid reduced GLUCOLD formation (see Fig. 5c vs. 5d), supports involvement of dicarbonyl forms in GLUCOLD formation.
Protein-bound Amadori product stability in the presence of amine, guanidine or diaminobenzene
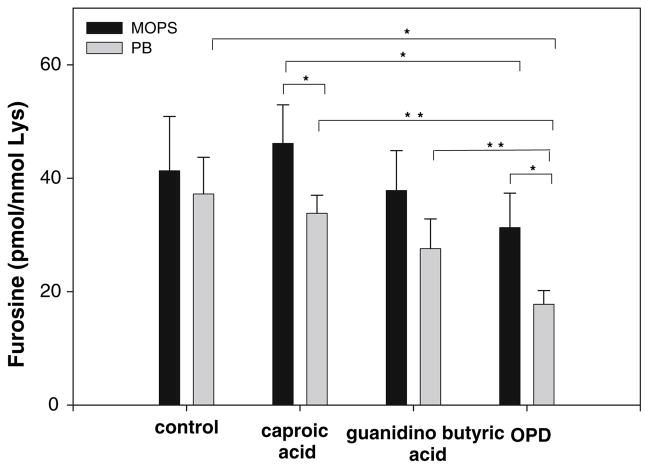
Aiming to investigate the protein-bound Amadori product stability under different conditions, glycated BSA was reincubated with ε-amino caproic acid, γ-guanidino butyric acid, and OPD. Figure 10 shows the Amadori product levels (measured as furosine) in BSA acid hydrolyzates. The Amadori product showed very high stability after 20 days of incubation with amine, but lower with guanidine and the lowest stability in the presence of OPD.
Fig. 10.
Amadori product stability in glycated BSA (50 mg/mL) in the presence of ε-amino caproic acid (CA) (50 mM), γ-guanidno butyric acid (GBA) (50 mM) and 1,2-diaminobenzene (OPD) (50 mM) in phosphate buffer (PB) and MOPS (200 mM with 0.02% sodium azide and 0.2 mM DTPA) at 37°C for 20 days. Amadori product was measured by GC–MS in acid hydrolyzed samples as furosine. Data are expressed as mean value ± SD (n = 3). *P < 0.05, **P < 0.005
Discussion
The first and important finding in the present study is that glucose can form GLUCOLD, i.e., a novel lys-lys imidazolium crosslink belonging to the same molecular crosslink family as the previously reported GOLD, MOLD, and DOLD. Interestingly, the new crosslink, unlike glucosepane and crossline, does not require the Amadori product rearrangement through intramolecular enolisation reactions along the entire carbohydrate backbone and N6-1,4-dideoxy-5,6-dioxoglucosone-lysine (dicarbonyl) formation. It is formed from the oxidized enol form of the Amadori product with participation of an additional 2-oxoaldehyde or 2-hydroxyaldehyde (Scheme 1, Fig. 5), whereby glucosone is likely an important precursor.
While GLUCOLD could easily be obtained from low-molecular-weight precursors, the major surprise was that neither GLUCOLD nor crossline was detected in proteins isolated from biological systems or commercial food products by liquid chromatography with tandem mass spectrometry. Surprisingly, crossline was previously detected in human and bovine serum albumin incubated with glucose in vitro and in rat lens samples (Obayashi et al. 1996) as well as in serum and erythrocyte membrane proteins (Aoki et al. 2000) using crossline polyclonal antiserum. The complete absence of crossline by LC–MS/MS MRM (analysis 1) from our samples suggests that the antibody cross-reacted with other AGEs or it reached better sensitivity than our LC–MS/MS. This discrepancy raised the question of which factors affect the reactivity of the Amadori product.
There may be several reasons why neither GLUCOLD nor crossline was detected in glycated proteins. Our systematic investigation of the incubation mixtures showed that in all amine–guanidine–glucose model systems glucosepane represented the major crosslink. However, while the levels of crossline and GLUCOLD were ca 100 times lower (Fig. 5), as such they should still be measurable in proteins. Moreover, in the Amadori product–amine–guanidine model systems GLUCOLD level was only two to four times lower than glucosepane showing that very high concentration of the Amadori product favors GLUCOLD formation (Fig. 7). One possibility is that the latter conditions are very difficult to reach in proteins. However, in vitro glycated albumin can accumulate as much as 8 mol fructose–lysine per mol protein (Watkins et al. 1987). A second possibility is that the configuration of lysyl residues in the protein is not favorable. However, we found in human serum albumin no less than 20 pairs of lysine residues less than 7 Å apart, i.e., sufficiently close for crosslinking by glycation to occur (Dai et al. 2008).
Additional reasons for the lack of GLUCOLD as well as crossline in biological proteins could be the tetramolecular nature of the reaction, i.e., the requirement of two lysine residues and two sugar molecules. On the other hand, tetramolecular-crosslinks are not unknown in the Maillard reaction, as exemplified by GOLD and MOLD (Biemel et al. 2001a; Chellan and Nagaraj 1999; Thornalley et al. 2003). A third potential reason for the presence of GLUCOLD and crossline in proteins below 1.5 pmol/mg protein is perhaps that the in vitro incubation conditions we have used favor the accumulation of highly levels of reactive intermediates of the Maillard reaction that are not found in the living organism. They could be constantly detoxified through enzymatic pathways or because they are present in very low levels.
The failure of glycation to generate significant amounts of GLUCOLD and crossline in proteins, however, is best explained by the reactivity of the Amadori product and the low yield of glucosone under physiological conditions. Both the data from Lederer’s group and our data strongly implicate (1) the ability of the Amadori product to form the reactive intermediate N6-1,4-dideoxy-5,6-dioxoglucosone, (2) the role phosphate in the enolization reaction of fructose–lysine, and (3) the role of guanidino groups in the trapping of the 5,6-dioxo precursor of glucosepane.
The latter constantly removes the 5,6-dioxo compound from the equilibrium with the Amadori product and catalyzes N6-1,4-dideoxy-5,6-dioxoglucosone generation. This is supported by our previous structural studies in which we observed that the Amadori product has a higher rate of transformation into N6-1,4-dideoxy-5,6-dioxoglucosone when it is in the vicinity of an arginine residue (Dai et al. 2008). Gobert and Glomb observed the same phenomenon not only during N6-1,4-dideoxy-5,6-dioxoglucosone generation, but also during release of the whole set of dicarbonyls from amine–glucose incubation systems (Gobert and Glomb 2009). These phenomena can be the major reason for accumulation of glucosepane in the proteins.
The above reasoning leads to a number of hypothesis and conclusions. First, we hypothesize that the Amadori product in proteins should be more stable in proximity of an amine than in the presence of guanidino compound. This is supported by our data on the incubation of glycated BSA in different surroundings under non-oxidative conditions (Fig. 10). Second, we come to the conclusion that efficient protein crosslink formation from Amadori product under non-oxidative conditions requires its enolization into N6-1,4-dideoxy-5,6-dioxoglucosone. Alternatively, either oxidative fragmentation or participation of more reactive C2 and C3 fragments is required to form the “OLD” or “ODIC” series of crosslinks (Biemel et al. 2001a; Biemel et al. 2001b; Lederer et al. 1998; Lederer and Klaiber 1999), though even these crosslinks are very minor compared to glucosepane (Sell et al. 2005).
Third, from the above studies it is readily apparent that chemistry of the Amadori product in free form is different from the chemistry of the Amadori product in proteins. In particular, it appears that nucleophilic addition to the Amadori product is disfavored unless it can be rearranged into more reactive species.
Acknowledgments
This work was supported by NEI grant EY 07099. I.N. is a recipient of a fellowship from the Juvenile Diabetes Research Foundation International.
References
- Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Hasegawa G, Shigeta H, Obayashi H, Fujii M, Kimura F, Moriwaki A, Nakamura N, Ienaga K, Nakamura K, Kondo M. Crossline levels in serum and erythrocyte membrane proteins from patients with diabetic nephropathy. Diabetes Res Clin Pract. 2000;48:119–125. doi: 10.1016/s0168-8227(99)00148-5. [DOI] [PubMed] [Google Scholar]
- Biemel KM, Buhler HP, Reihl O, Lederer MO. Identification and quantitative evaluation of the lysine-arginine crosslinks GODIC, MODIC, DODIC, and glucosepane in foods. Nahrung. 2001a;45:210–214. doi: 10.1002/1521-3803(20010601)45:3<210::AID-FOOD210>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Biemel KM, Reihl O, Conrad J, Lederer MO. Formation pathways for lysine-arginine cross-links derived from hexoses and pentoses by Maillard processes: unraveling the structure of a pentosidine precursor. J Biol Chem. 2001b;276:23405–23412. doi: 10.1074/jbc.M102035200. [DOI] [PubMed] [Google Scholar]
- Biemel KM, Conrad J, Lederer MO. Unexpected carbonyl mobility in aminoketoses: the key to major Maillard crosslinks. Angew Chem Int Ed Engl. 2002a;41(5):801–804. doi: 10.1002/1521-3773(20020301)41:5<801::aid-anie801>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major Maillard cross-links in human serum albumin and lens protein evidence for glucosepane as the dominant compound. J Biol Chem. 2002b;277:24907–24915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- Brinkmann E, Wells-Knecht KJ, Thorpe SR, Baynes JW. Characterization of an imidazolium compound formed by reaction of methylglyoxal and N.alpha.-hyppuryllysine. J Chem Soc Perkin Trans. 1995;1:2817–2818. [Google Scholar]
- Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999;368(1):98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- Dai Z, Nemet I, Shen W, Monnier VM. Isolation, purification and characterization of histidino-threosidine, a novel Maillard reaction protein crosslink from threose, lysine and histidine. Arch Biochem Biophys. 2007;463:78–88. doi: 10.1016/j.abb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Wang B, Sun G, Fan X, Anderson VE, Monnier VM. Identification of glucose-derived cross-linking sites in ribonuclease A. J Proteome Res. 2008;7:2756–2768. doi: 10.1021/pr700874a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Zhang J, Theves M, Strauch C, Nemet I, Liu X, Qian J, Giblin FJ, Monnier VM. Mechanism of lysine oxidation in human lens crystallins during aging and in diabetes. J Biol Chem. 2009;284:34618–34627. doi: 10.1074/jbc.M109.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem. 1998;273:18714–18719. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C, Taneda S, Marion MS, Monnier VM. Isolation, purification, and characterization of an Amadori product binding protein from a Pseudomonas sp soil strain. J Biol Chem. 1994;269:27297–27302. [PubMed] [Google Scholar]
- Glomb MA, Pfahler C. Amides are novel protein modifications formed by physiological sugars. J Biol Chem. 2001;276:41638–41647. doi: 10.1074/jbc.M103557200. [DOI] [PubMed] [Google Scholar]
- Gobert J, Glomb MA. Degradation of glucose: reinvestigation of reactive alpha-dicarbonyl compounds. J Agric Food Chem. 2009;57:8591–8597. doi: 10.1021/jf9019085. [DOI] [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Lederer MO, Klaiber RG. Cross-linking of proteins by Maillard processes: characterization and detection of lysine-arginine cross-links derived from glyoxal and methylglyoxal. Bioorg Med Chem. 1999;7:2499–2507. doi: 10.1016/s0968-0896(99)00212-6. [DOI] [PubMed] [Google Scholar]
- Lederer MO, Gerum F, Severin T. Cross-linking of proteins by Maillard processes—model reactions of D-glucose or methyl-glyoxal with butylamine and guanidine derivatives. Bioorg Med Chem. 1998;6:993–1002. doi: 10.1016/s0968-0896(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Sell DR, Nagaraj RH, Miyata S, Grandhee S, Odetti P, Ibrahim SA. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41(Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- Nagai R, Hayashi CM, Xia L, Takeya M, Horiuchi S. Identification in human atherosclerotic lesions of GA-pyridine, a novel structure derived from glycolaldehyde-modified proteins. J Biol Chem. 2002;277:48905–48912. doi: 10.1074/jbc.M205688200. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Monnier VM. Isolation and characterization of a blue fluorophore from human eye lens crystallins: in vitro formation from Maillard reaction with ascorbate and ribose. Biochim Biophys Acta. 1992;1116:34–42. doi: 10.1016/0304-4165(92)90125-e. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996;271:19338–19345. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hasegawa T, Fukunaga Y, Ienaga K. Crosslines A and B as candidates for the fluorophores in age- and diabetes-related cross-linked proteins, and their diacetates produced by Maillard reaction of N.alpha.-acetyl-lysine with glucose. J Chem Soc Chem Commun. 1992:992–994. [Google Scholar]
- Nakamura K, Nakazawa Y, Ienaga K. Acid-stable fluorescent advanced glycation end products: vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem Biophys Res Commun. 1997;232:227–230. doi: 10.1006/bbrc.1997.6262. [DOI] [PubMed] [Google Scholar]
- Obayashi H, Nakano K, Shigeta H, Yamaguchi M, Yoshimori K, Fukui M, Fujii M, Kitagawa Y, Nakamura N, Nakamura K, Nakazawa Y, Ienaga K, Ohta M, Nishimura M, Fukui I, Kondo M. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun. 1996;226:37–41. doi: 10.1006/bbrc.1996.1308. [DOI] [PubMed] [Google Scholar]
- Odani H, Shinzato T, Usami J, Matsumoto Y, Brinkmann Frye E, Baynes JW, Maeda K. Imidazolium crosslinks derived from reaction of lysine with glyoxal and methylglyoxal are increased in serum proteins of uremic patients: evidence for increased oxidative stress in uremia. FEBS Lett. 1998;427:381–385. doi: 10.1016/s0014-5793(98)00416-5. [DOI] [PubMed] [Google Scholar]
- Reihl O, Rothenbacher TM, Lederer MO, Schwack W. Carbohydrate carbonyl mobility—the key process in the formation of alpha-dicarbonyl intermediates. Carbohydr Res. 2004;339:1609–1618. doi: 10.1016/j.carres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19:77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix relationship with diabetes. J Biol Chem. 2005;280:12310–12315. doi: 10.1074/jbc.M500733200. [DOI] [PubMed] [Google Scholar]
- Sell DR, Strauch CM, Shen W, Monnier VM. 2-Aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochem J. 2007;404:269–277. doi: 10.1042/BJ20061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovsted IC, Christensen M, Breinholt J, Mortensen SB. Characterization of a novel AGE-compound derived from lysine and 3-deoxyglucosone. Cell Mol Biol (Noisy-le-grand) 1998;44:1159–1163. [PubMed] [Google Scholar]
- Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54:1615–1625. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Tan AL, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27:130–143. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1 relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J Biol Chem. 1999;274:20796–20804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, Dawnay A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J. 2003;375:581–592. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins NG, Neglia-Fisher CI, Dyer DG, Thorpe SR, Baynes JW. Effect of phosphate on the kinetics and specificity of glycation of protein. J Biol Chem. 1987;262:7207–7212. [PubMed] [Google Scholar]
- Wells-Knecht KJ, Brinkmann E, Baynes JW. Characterization of an imidazolium salt formed from glyoxal and N.alpha.-Hypuryllysine: a model for Maillard reaction crosslinks in proteins. J Org Chem. 1995:6246–6247. [Google Scholar]
- Yamaguchi M, Nakamura N, Nakano K, Kitagawa Y, Shigeta H, Hasegawa G, Ienaga K, Nakamura K, Nakazawa Y, Fukui I, Obayashi H, Kondo M. Immunochemical quantification of crossline as a fluorescent advanced glycation endproduct in erythrocyte membrane proteins from diabetic patients with or without retinopathy. Diabet Med. 1998;15:458–462. doi: 10.1002/(SICI)1096-9136(199806)15:6<458::AID-DIA601>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]