Abstract
In the well-fed state a relatively high activity of the pyruvate dehydrogenase complex (PDC) reduces blood glucose levels by directing the carbon of pyruvate into the citric acid cycle. In the fasted state a relatively low activity of the PDC helps maintain blood glucose levels by conserving pyruvate and other three carbon compounds for gluconeogenesis. The relative activities of the pyruvate dehydrogenase kinases (PDKs) and the opposing pyruvate dehydrogenase phosphatases determine the activity of PDC in the fed and fasted states. Up regulation of PDK4 is largely responsible for inactivation of PDC in the fasted state. PDK4 knockout mice have lower fasting blood glucose levels than wild type mice, proving that up regulation of PDK4 is important for normal glucose homeostasis. In type 2 diabetes, up regulation of PDK4 also inactivates PDC, which promotes gluconeogenesis and thereby contributes to the hyperglycemia characteristic of this disease. When fed a high fat diet, wild type mice develop fasting hyperglycemia but PDK4 knockout mice remain euglycemic, proving that up regulation of PDK4 contributes to hyperglycemia in diabetes. These finding suggest PDK4 inhibitors might prove useful in the treatment of type 2 diabetes.
Keywords: Diabetes, Fasting, Glucose, Ketone bodies, Pyruvate dehydrogenase complex, Pyruvate dehydrogenase kinase, Steatosis
REGULATION OF PYRUVATE DEHYDROGENASE COMPLEX
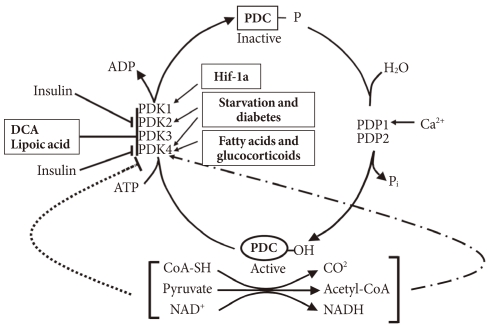
In the well-fed state the pyruvate dehydrogenase complex (PDC) is converted into its most active dephosphorylated state in order to direct pyruvate into the citric acid cycle. PDC activity is tightly regulated by phosphorylation by four pyruvate dehydrogenase kinases (PDKs) and dephosphorylation by two pyruvate dehydrogenase phosphatases (PDPs) (Fig. 1) [1,2]. The PDKs and PDPs exhibit unique tissue expression patterns, kinetic properties, and sensitivities to regulatory molecules [3,4]. All four PDKs are subject to regulation at the level of transcription. Expression of PDK1 and PDK3 is stimulated by HIF1α [5-7], making these PDKs of considerable interest in hypoxia and cancer. Expression of PDK2 and PDK4 is controlled by nutritional factors and hormones [8-16], making their roles of great interest in starvation and diabetes. PDK3 has received less attention, but is emerging as important since its expression is subject to regulation by HIF1α [7] and the carbohydrate response element binding protein (ChREBP) [17]. Although the PDKs are encoded by genes located in the nucleus, they are markedly different in sequence and three-dimensional structure from other serine protein kinases present in eukaryotic cells, which may facilitate the discovery of specific small molecule inhibitors. Phosphorylation of serine residues of the E1α component of PDC by PDKs inactivates the complex (Fig. 1). Dephosphorylation by PDPs activates the complex. The complex does not exist in any tissue in a completely active, dephosphorylated state nor in a completely inactive, phosphorylated state. The relative activities of the PDKs and the opposing PDPs determine the extent of phosphorylation and therefore the activity of the complex. Induced changes in activities of the kinases relative to the phosphatases during transitions to different nutritional and hormonal states result in substantial change in the phosphorylation state and therefore PDC activity. The products of the overall reaction, acetyl-CoA and NADH, indirectly inhibit the complex by activating the PDKs [18]. A high [NADH]/[NAD+] ratio caused by fatty acid oxidation reduces the lipoyl moieties of E2. A high [acetyl-CoA]/[CoA] ratio caused by fatty acid oxidation promotes acetylation of the reduced lipoyl moieties of E2. Binding of PDK to an E2 lipoyl domain in which the lipoyl group is reduced and acetylated results in maximum kinase activity (Fig. 1) [19]. The consequence is greater E1 phosphorylation and less PDC activity. Whereas the products of PDC promote inactivation of the complex, its substrates (pyruvate, NAD+, and CoA) induce activation by inhibition of the kinases (Fig. 1) [20]. Pyruvate is most important since its concentration fluctuates more dramatically in different nutritional states. Since PDK4 is less sensitive to pyruvate inhibition than PDK2, the shift to greater PDK4 expression during fasting and fat feeding decreases the effectiveness of pyruvate as an activator of PDC in these nutritional states [21].
Fig. 1.
Regulation of the pyruvate dehydrogenase complex (PDC) and its kinases (PDKs) and phosphatases (PDPs). DCA, dichloroacetate.
During fasting PDC is progressively shut down by phosphorylation in most tissues of the body. High serum levels of free fatty acids (FFAs) generated by lipolysis in the adipose tissue promote fatty acid oxidation in most tissues of the body. Reduced insulin levels and elevated levels of serum FFAs and glucocorticoids induce expression of PDK4 in muscle, kidney, liver, and heart, and PDK2 in liver and kidney. Inhibition of the conversion of pyruvate to acetyl-CoA by decreased PDC activity induced by the PDKs in peripheral tissues, especially skeletal muscle, heart, and liver, conserves three carbon compounds (pyruvate, lactate, and alanine) that are used by the liver to make glucose. Inactivation of the PDC in the liver blocks ketone body synthesis from three carbon compounds but allows ketone body synthesis from fatty acids and ketogenic amino acids. Inhibition of ketogenesis from three carbon compounds is critical because no pathway exists for the conversion of ketone bodies into glucose. Conservation of the compounds that can be used to synthesize glucose at the expense of compounds that can not be converted to glucose (fatty acids, acetyl-CoA, and ketone bodies) helps maintain the blood glucose levels required by the brain and red blood cells. Inactivation of the PDC in starvation indirectly conserves body protein because it minimizes the need for gluconeogenesis from gluconeogenic amino acids and prevents complete oxidation of the carbon skeletons of gluconeogenic amino acids. Indeed, survival during long-term starvation depends upon inactivation of PDC. If the complex remained active in the starved state, the three carbon compounds needed for gluconeogenesis would be converted to CO2 in peripheral tissues and to ketone bodies in the liver. Since survival requires maintenance of glucose levels, animals would have to consume their protein stores at a faster rate as a carbon source for the gluconeogenesis. Since pyruvate is an intermediate in the catabolism of several amino acids, much of the carbon coming from protein would be wasted. Thus, control of the PDC plays an important role in determining the fuel used by tissues in different nutritional and hormonal states.
IMPORTANCE OF REGULATION OF PDC BY PDK IN TYPE 2 DIABETES
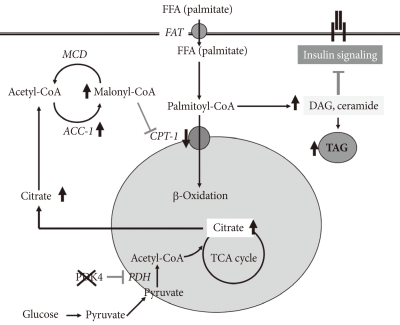
Remarkable upregulation of PDK4 occurs in humans with type 2 diabetes [13,22,23], in genetic animal models of type 2 diabetes [24], in animals fed a high fat diet [21], and in humans fed a high fat diet [25]. Type 2 diabetes is usually the result of a combination of insulin resistance and insulin insufficiency. In the insulin resistance state, the pancreas still makes insulin but the amount released is not enough to normalize blood glucose. Would a PDK inhibitor prove useful in the treatment of type 2 diabetes? Perhaps, but it is not certain. Boosting PDC activity in tissues with a PDK inhibitor will lower blood glucose levels which may reduce the toxic effects of hyperglycemia on the beta cells of the pancreas. However, if the prevailing hypothesis for the mechanism responsible for insulin resistance is correct (Fig. 2) [26], greater PDC activity might exacerbate insulin resistance. Elevated levels of FFAs are associated with inflammation and insulin resistance. A defect in mitochondrial fatty acid oxidation may be responsible. The combination of uptake of too much unesterified fatty acid in the face of reduced or defective fatty acid oxidation results in the accumulation of TAG as well as proinflammatory lipids (fatty acyl-CoA, diacylglycerol, and ceramide) that activate the serine/threonine stress kinases (JNK, IKKβ, and PKCθ) that are responsible for inactivation of components of the insulin signaling cascade [27]. PDK4 deficiency increases blood levels of FFAs and inhibits fatty acid oxidation [28], a combination that would be expected to induce insulin resistance by this mechanism. As to how PDK4 deficiency inhibits fatty acid oxidation, greater PDC activity may lead to an increase in malonyl-CoA, inhibitor of carnitine palmitoyltransferase I (CPT1), the rate-limiting enzyme in fatty acid oxidation. Since long chain acyl-CoA esters are converted to carnitine esters by CPT1, inhibition of CPT1 by malonyl-CoA should increase long chain acyl-CoA esters which in turn may increase diacylglycerol and ceramide, resulting in insulin resistance. It is known that inhibition acetyl-CoA carboxylase which produces malonyl-CoA protects against diet-induced insulin resistance [29]. In spite of what on paper seems should happen, PDK4 deficiency partially protects mice against diet-induced insulin resistance [29]. Although this finding doesn't fit with the model giving in Fig. 2, there are other findings that challenge the current view of the mechanism by which fatty acids induce insulin resistance. For example, data obtained with malonyl-CoA decarboxylase knockout mice also do not support this model [30]. Theoretically, elevated levels of malonyl-CoA in the malonyl-CoA decarboxylase knockout mouse should inhibit CPT1 and thereby increase long chain acyl-CoA esters, diacylglycerol, and ceramide, which in turn should induce insulin resistance by activation of the stress kinases. Instead, malonyl-CoA decarboxylase knockout mice are partially protected, like PDK4-/- mice, against the development of insulin resistance. Based on these findings, Koves et al. [30] proposed that high levels of FFAs may induce a mitochondrial overload or stress by exceeding the capacity of the mitochondria for fatty acid oxidation. They further proposed that mitochondrial overload may induce the accumulation of "incomplete products of fatty acid oxidation," e.g., acyl carnitine ester intermediates that function as proinflammatory compounds responsible for activation of the stress kinases.
Fig. 2.
Current model for mechanism responsible for insulin resistance. FFA, free fatty acid; DAG, diacylglycerol; MCD, malonyl-CoA decarboxylase; ACC, acetyl-CoA carboxylase; TAG, triacylglycerol.
ARE THE PDKs VIABLE TARGETS FOR THE TREATMENT OF TYPE 2 DIABETES?
Upregulation of PDK4 in diabetes begs the question of whether PDK4 and the other PDKs should be considered therapeutic targets for the treatment of diabetes. PDK4-/- mice, generated in an attempt to answer this question, have lower than normal fasting blood glucose levels and slightly but significantly better glucose tolerance [28]. This is observed in both chow-fed PDK4-/- mice that have normal insulin sensitivity [28] and diet-induced obese PDK4-/- mice that are insulin resistant [31]. Dichloroacetate, a well-established PDK inhibitor, decreases fasting blood glucose levels but has relatively low potency and long term treatment causes peripheral neuropathy [32-34]. 2-chloroproprionate also inhibits the PDKs, lowers fasting blood glucose levels, but also induces peripheral neuropathy [35]. α-Lipoic acid also inhibits the PDKs [36], stimulates pyruvate oxidation and inhibits fatty acid oxidation and gluconeogenesis by hepatocytes [37]. These effects likely explain why lipoic acid increases insulin-stimulated glucose disposal in patients with type 2 diabetes [38] and decreases blood levels of lactate and pyruvate [39]. Nevertheless, the mechanism of action of lipoic acid is complicated by its conversion to a CoA ester which inhibits mitochondrial processes by sequestering CoA in the mitochondrial matrix space [40]. The latter inhibits gluconeogenesis [41] and may increase the concentration of AMP and thereby stimulate AMP-activated protein kinase [41]. A synthetic inhibitor of the PDKs, SDZ048-619, increases PDC activity in tissues of the hyperglycemic Zucker diabetic rat and reduces blood lactate but, surprisingly, does not lower blood glucose [42]. In contrast, AZD7545, originally reported to be a specific inhibitor of PDK2 [43], but which we now know also inhibits PDK1 and PDK3 [44], markedly lowers blood glucose in hyperglycemic Zucker diabetic fatty rats [45]. Leelamine, another synthetic inhibitor of the PDKs [46] looks particularly promising as a lead compound. It lowers blood glucose in ob/ob mice [46], and inhibits glyceroneogenesis in isolated adipocytes by activating PDC [47].
ROLE OF GLYCERONEOGENESIS IN ACCUMULATION OF FAT IN TISSUES
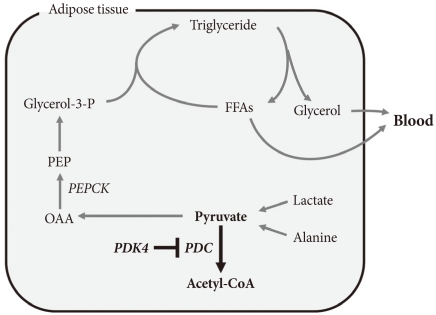
The term glyceroneogenesis refers to a pathway for the synthesis of the glycerol moiety of TAG from precursors other than glucose (Fig. 3) [48]. The pathway of glyceroneogenesis from lactate, pyruvate, and alanine shares many steps with gluconeogenesis from these substrates. Indeed, like gluconeogenesis, glyceroneogenesis is of greater importance in the fasted state than in the well-fed state. Dietary fat, absorbed as chylomicrons, is cleared from the circulation by adipose tissue lipoprotein lipase which releases FFAs. FFAs are esterified with glycerol 3-phosphate derived from glycolysis (using glucose taken up via insulin-stimulated GLUT4). During fasting, low insulin levels permit the activation of TAG lipases by protein kinase A, hydrolysis of stored TAG, and release of FFAs and glycerol. Much of this FFA is recycled within adipose tissue by re-esterification with glycerol 3-phosphate. Since insulin levels are low, glucose is not available for synthesis of glycerol 3-phosphate. Glycerol 3-phosphate therefore must be generated either by "glyceroneogenesis" from pyruvate, lactate, or alanine, or by phosphorylation of glycerol by glycerol kinase. Glyceroneogenesis is quantitatively much more important than the former [48]. Indeed, like gluconeogenesis, glyceroneogenesis depends on the activity of cytosolic phosphenolpyruvate carboxykinase (PEPCK), which converts oxaloacetate to phosphoenolpyruvate (PEP). Knocking out PEPCK expression in white adipose tissue abolishes glyceroneogenesis from pyruvate [49], resulting in lipodystrophic mice. Conversely, over expression of PEPCK in white adipose tissue increases the rate of glyceroneogenesis from pyruvate, resulting in obese mice [50]. Likewise, over-expression of PEPCK in skeletal muscle results in massive fat accumulation in muscles [51]. Thus, the pathway of glyceroneogenesis plays an important role in intracellular fat accumulation. Although not directly on the pathway of glyceroneogenesis, PDC is positioned to interfere with glyceroneogenesis by the same mechanism that it interferes with gluconeogenesis, i.e., by reducing the availability of lactate, pyruvate, and alanine (Fig. 3). Furthermore, glyceroneogenesis has also been shown to be the most important source of glycerol 3-phosphate for the synthesis of VLDL in the human liver [52]. It is estimated that glucose via glycolysis provides ~15% of glycerol 3-phosphate required for TAG synthesis whereas direct phosphorylation of free glycerol provides ~35% and glyceroneogenesis provides ~50%. Therefore, our working hypothesis is that abolishment of PDK activity can reduce adiposity (and therefore body weight) by limiting the availability of lactate, pyruvate, and alanine for glyceroneogenesis in adipose, liver, and other tissues. Indeed, activation of PDC by inhibition of PDK inhibits glyceroneogenesis in isolated adipocytes [47].
Fig. 3.
Pathway of glyceroneogenesis from three carbon compounds. FFAs, free fatty acids; OAA, oxaloacetate; PEP, phosphoenolpyruvate.
KNOCKING OUT PDK4 REDUCES GLUCOSE LEVELS IN FASTING AND STARVATION
Strong upregulation of PDK4 during fasting and starvation suggests a dominant role for this PDK in regulation of fuel homeostasis in these conditions. Studies with PDK4-/- mice support this conclusion [28,31,53]. Blood glucose levels are invariably lower in overnight-fasted PDK4-/- mice than overnight-fasted wild type mice. Serum concentrations of lactate, pyruvate, and alanine are also always lower in PDK4-/- mice, consistent with either a faster rate of oxidation by PDC or a reduced rate of production of these compounds. In contrast, higher serum concentrations of FFAs, acetoacetate, β-hydroxybutyrate, and TAG are induced by starvation in PDK4-/- mice relative to wild-type mice. Branched-chain amino acids (BCAAs) are also higher in the blood in starved PDK4-/- mice, consistent with lower blood alanine levels and the importance of BCAAs as a source of amino groups for alanine formation.
Liver glycogen levels are the same in wild-type and PDK4-/- mice in the fed state but are lost more rapidly from the liver of PDK4-/- mice during fasting. Concentrations of glucose and intermediates of the gluconeogenesis pathway are likewise lower (glucose 6-phosphate, fructose 1,6-bisphosphate, pyruvate, lactate, and citrate) or not different (dihydroxyacetone phosphate, glyceraldehyde 3-phosphate, phosphoenolpyruvate) compared to that of wild-type mice. Ketone bodies are elevated in concentration but with a decrease in the β-hydroxybutyrate to acetoacetate ratio.
Glucose oxidation rate, measured by 14CO2 production from [U-14C]glucose, was greater in diaphragms from PDK4-/- mice relative to diaphragms from wild-type mice. Rates of lactate release and net glycolysis, measured by 3H2O production from [5-3H]glucose, are significantly reduced. Rates of fatty acid oxidation, measured either by 14CO2 production or acid-soluble product formation from [14C]-Palmitate, are lower in diaphragms obtained from PDK4-/- mice than in diaphragms from wild-type mice. Starvation markedly decreases actual PDC activities in wild-type mice without significantly affecting total PDC activity, thereby decreasing the activity states of PDC to values typical of those induced in tissues of starved rats. A significantly higher PDC activity state is observed in tissues of PDK4-/- mice, consistent with a major role for PDK4 in control of the activity of PDC.
KNOCKING OUT PDK2 HAS NO EFFECT ON BLOOD GLUCOSE LEVELS
PDK2-/- mice have also been produced to examine the physiological role of PDK2 in glucose homeostasis (N. H. Jeoung and R. A. Harris, unpublished). They are viable with normal growth characteristics on mouse chow diet. Surprisingly, blood glucose levels are not different from wild-type mice in the fasted state. No difference in glucose levels are observed during the glucose tolerance test relative to age matched wild-type mice. Insulin sensitivity test likewise reveals no difference. PDK2-/- mice maintain normal PDC activities in major tissues and normal blood glucose levels during fasting, perhaps because upregulation of PDK4 compensates for lack of PDK2.
KNOCKING OUT PDK4 LOWERS FASTING BLOOD GLUCOSE LEVELS, IMPROVES GLUCOSE TOLERANCE, AND IMPROVES INSULIN SENSITIVITY IN MICE FED A HIGH UNSATURATED-FAT, HIGH-GLUCOSE DIET
Experiments to determine the effects of knocking out PDK4 on diabetes were carried out with mice fed a high-unsaturated fat, high sucrose diet that is known to induce hyperglycemia and insulin resistance [31]. No differences in blood metabolites were found wild-type and PDK4-/- mice maintained on this diet were compared in the fed state. Significant differences emerged, however, when blood obtained from overnight fasted mice was analyzed [31]. Fasting blood glucose levels were significantly lower in the PDK4-/- mice throughout an 18-week feeding period. PDK4-/- mice were significantly more glucose tolerant than wild-type mice after 16 weeks on the high-fat diet. Insulin levels increased slightly in both groups during the test without significant differences. Nevertheless, insulin tolerance tests revealed significantly greater insulin sensitivity in the PDK4-/- mice after 17 weeks on the diet. Glucose levels were 20% lower in the blood of PDK4-/- mice at the time of their sacrifice after 18 weeks on the high-fat diet. Gluconeogenic precursors (lactate, pyruvate, and alanine) were lower in the PDK4-/- mice but FFAs, TAGs, 3-hydroxybutyrate, acetoacetate, and BCAAs were higher.
Total PDC activities (completely dephosphorylated state) did not differ between PDK4-/- mice and wild-type mice regardless of whether fed or fasted. The activity states of PDC were significantly higher in the fed state in skeletal muscle and diaphragm of PDK4-/- mice. Relative to the fed state, the activity state of PDC was reduced by overnight fasting in all tissues of PDK4-/- mice with the exception of the kidney. Likewise, in the fasted state the activity state of PDC was significantly higher in the tissues of PDK4-/- mice.
Diaphragms from PDK4-/- mice oxidize glucose at a rate greater than diaphragms from wild-type mice. Less pyruvate accumulates in the incubation medium with diaphragms from PDK4-/- mice. Palmitate inhibited glucose oxidation in diaphragms from wild-type mice but exerts no inhibition with diaphragms from PDK4-/- mice, indicating inhibition of glucose oxidation by palmitate requires induction of PDK4. The rate of palmitate oxidation is lower in diaphragms isolated from PDK4-/- mice relative to diaphragms from wild-type mice.
KNOCKING OUT PDK4 REDUCES ADIPOSITY AND HEPATIC STEATOSIS IN MICE FED A HIGH-SATURATED FAT DIET
The experiment described above with high unsaturated-fat, high-sucrose fed mice was followed by studies in which wild-type and PDK4-/- mice were fed a high-saturated fat diet (no sucrose) [53] that induce high blood glucose levels, insulin resistance, and (in contrast to high unsaturated-fat, high-sucrose diet) hepatic steatosis [54]. Wild-type and PDK4-/- mice were maintained on this diet for 28 weeks. Fasting blood glucose levels were lower, glucose tolerance improved, and insulin sensitivity greater in the PDK4-/- mice [53]. The activity state (% active) of the PDC was significantly greater in liver and skeletal muscle of PDK4-/- mice after overnight fasting. Serum FFAs and ketone bodies were elevated more in PDK4-/- mice, consistent with slower rates of oxidation. Interestingly, PDK4-/- mice gained less weight than wild-type mice on the diet. Although the two types of mice gained similar amounts of weight for the first 8 weeks on the diet, the wild-type mice gained weight at a faster rate after this point, even though no difference in food consumption could be detected between the two groups. Furthermore, adiposity, as measured by weight of the adipose tissue relative to the body weight, was found to be 13% less in PDK4-/- mice after 28 weeks on the diet. In addition, macrovesicular accumulation of fat was apparent in the livers of wild-type mice by oil red O staining. Livers of PDK4-/- mice also exhibited hepatic steatosis but the fat was microvesicular and less than the amount present in the liver of wild-type mice [53].
KNOCKING OUT PDK4 INCREASES HEPATIC EXPRESSION OF PGC-1α
Hepatic steatosis occurs when the amount of fat delivered to the liver or synthesized de novo exceeds the capacity of the liver to oxidize or secrete. Inhibition of fatty acid oxidation by a mitochondrial poison induces hepatic steatosis. Decreased expression of nuclear-encoded mitochondrial genes is believed responsible for lipid accumulation in skeletal muscle in type 2 diabetes. Reduced mitochondrial capacity for fatty acid oxidation may also be responsible for hepatic steatosis in rodents fed a high saturated-fat diet [55]. PGC-1α, transcription coactivator for several nuclear transcription factors, is a key regulator of mitochondrial biogenesis [56]. Disruption of the PGC-1α gene reduces expression of mitochondrial enzymes, reduces the number of mitochondria, and induces hepatic steatosis [57]. Conversely, activation of PGC-1α by over expression of SIRT1 protects the liver from the accumulation of fat in mice fed a high fat diet. Furthermore, hepatic levels of PGC-1α are reduced in mice fed a high saturated fat diet relative to mice fed a polyunsaturated fat diet [58]. Because an inverse correlation between PGC-1α and tissue fat has been shown in other studies, we determined the amount of PGC-1α present in the livers of wild-type and PDK4-/- mice [53]. As reported previously for liver and muscle of mice fed high saturated-fat diets [58,59], PGC-1α protein was reduced in the liver of wild-type mice fed the high saturated-fat diet compared to wild-type mice fed chow diet. In contrast, a normal amount of PGC-1α was present in the liver of PDK4-/- mice fed the high saturated-fat diet [53]. Since PGC-1α controls expression of mitochondrial enzymes [56,57], these findings suggest that knocking out PDK4 creates conditions that up regulate expression of PGC-1α and therefore genes that encode mitochondrial enzymes in the liver. This is a novel finding that may help explain why knocking out PDK4 reduces fat accumulation in the liver of mice fed a high saturated-fat diet.
KNOCKING OUT PDK2 TOGETHER WITH PDK4 INDUCES GREATER EFFECTS ON BLOOD GLUCOSE LEVELS, GLUCOSE TOLERANCE, AND INSULIN SENSITIVITY THAN KNOCKING OUT ONLY PDK4
PDK2/PDK4 double knockout (DKO) mice are viable and appear normal (N. H. Jeoung and R. A. Harris, unpublished studies). Blood glucose levels of the PDK2/PDK4 DKO mice are lower than that of wild-type mice in both the fed and the fasted state. Overnight fasting induces lower blood levels of lactate, alanine, and pyruvate but higher blood levels of 3-hydroxybutyrate and acetoacetate. Glucose tolerance is remarkably better in the PDK2/PDK4 DKO mice. Insulin levels remain lower during the glucose tolerance test, suggesting the PDK2/PDK4 DKO mice are more sensitive to insulin.
SUMMARY AND PREDICTIONS
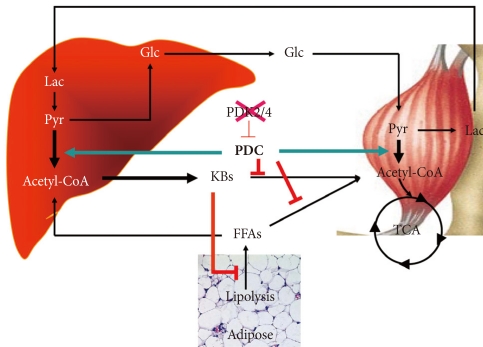
The schematic given in Fig. 4 summarizes how we visualize knocking out PDK4 affects blood glucose, FFAs, and ketone body levels. Less PDK activity results in greater PDC activity which reduces the levels of lactate, pyruvate, and alanine which attenuates hepatic glucose synthesis and results in lower blood glucose levels. Greater PDC activity results in production of excess acetyl-CoA which the liver converts to ketone bodies. Blood ketone bodies also increase because the increased oxidation of pyruvate inhibits ketone body oxidation by peripheral tissues. It is likely that FFA oxidation is also inhibited in peripheral tissues, but FFAs levels are not greatly elevated, perhaps because lipolysis in adipose tissue is inhibited by the elevated concentrations of ketone bodies [60]. Although complicated, insulin sensitivity may be increased because low PDC activity opposes the action of insulin. Greater PDC activity resulting from reduced PDK activity may facilitate the action of insulin.
Fig. 4.
Effect of inhibition of PDK4 on the metabolic control of carbohydrate and fat. KBs, ketone bodies; FFAs, free fatty acids; Glc, glucose; Lac, lactate; Pyr, pyruvate; CAC, citric acid cycle.
Knocking out PDK4 results in lower blood glucose levels, better glucose tolerance, and greater insulin sensitivity in chow fed mice. The same effects along with reduced hepatic steatosis are observed in PDK4-/- mice fed a high saturated-fat diet. Knocking out both PDK2 and PDK4 lowers blood glucose more effectively and produces even better glucose tolerance and insulin sensitivity in chow fed mice. Based on these findings, we predict that knocking out both PDK2 and PDK4 will have dramatic positive effects on blood glucose, glucose tolerance, and hepatic steatosis in mice fed a high saturated-fat diet. We also anticipate finding greater expression of PGC-1α, more mitochondria, and reduced levels of inflammatory markers in the liver of PDK2/PDK4 DKO mice. Such findings would provide further support for our contention that the PDKs may be good therapeutic targets for the treatment of type 2 diabetes.
ACKNOWLEDGMENTS
This study was supported by funding from the WCU program through the National Research Foundation of Korea (N. H. J., R. A. H.) and a Merit Award from the Department of Veterans Affairs (R. A. H.) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0008815) (N. H. J.)
References
- 1.Harris RA, Popov KM, Zhao Y, Kedishvili NY, Shimomura Y, Crabb DW. A new family of protein kinases: the mitochondrial protein kinases. Adv Enzyme Regul. 1995;35:147–162. doi: 10.1016/0065-2571(94)00020-4. [DOI] [PubMed] [Google Scholar]
- 2.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34(Pt 2):217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 3.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem. 1998;273:17680–17688. doi: 10.1074/jbc.273.28.17680. [DOI] [PubMed] [Google Scholar]
- 5.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–28114. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998;329(Pt 1):197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999;48:1593–1599. doi: 10.2337/diabetes.48.8.1593. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000;381:1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- 11.Sugden MC, Kraus A, Harris RA, Holness MJ. Fibre-type specific modification of the activity and regulation of skeletal muscle pyruvate dehydrogenase kinase (PDK) by prolonged starvation and refeeding is associated with targeted regulation of PDK isoenzyme 4 expression. Biochem J. 2000;346(Pt 3):651–657. [PMC free article] [PubMed] [Google Scholar]
- 12.Peters SJ, Harris RA, Heigenhauser GJ, Spriet LL. Muscle fiber type comparison of PDH kinase activity and isoform expression in fed and fasted rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R661–R668. doi: 10.1152/ajpregu.2001.280.3.R661. [DOI] [PubMed] [Google Scholar]
- 13.Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol Genet Metab. 1998;65:181–186. doi: 10.1006/mgme.1998.2748. [DOI] [PubMed] [Google Scholar]
- 14.Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes. 2002;51:276–283. doi: 10.2337/diabetes.51.2.276. [DOI] [PubMed] [Google Scholar]
- 15.Abbot EL, McCormack JG, Reynet C, Hassall DG, Buchan KW, Yeaman SJ. Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells. FEBS J. 2005;272:3004–3014. doi: 10.1111/j.1742-4658.2005.04713.x. [DOI] [PubMed] [Google Scholar]
- 16.Sugden MC, Bulmer K, Gibbons GF, Holness MJ. Role of peroxisome proliferator-activated receptor-alpha in the mechanism underlying changes in renal pyruvate dehydrogenase kinase isoform 4 protein expression in starvation and after refeeding. Arch Biochem Biophys. 2001;395:246–252. doi: 10.1006/abbi.2001.2586. [DOI] [PubMed] [Google Scholar]
- 17.Burgess SC, Iizuka K, Jeoung NH, Harris RA, Kashiwaya Y, Veech RL, Kitazume T, Uyeda K. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem. 2008;283:1670–1678. doi: 10.1074/jbc.M706540200. [DOI] [PubMed] [Google Scholar]
- 18.Ravindran S, Radke GA, Guest JR, Roche TE. Lipoyl domain-based mechanism for the integrated feedback control of the pyruvate dehydrogenase complex by enhancement of pyruvate dehydrogenase kinase activity. J Biol Chem. 1996;271:653–662. doi: 10.1074/jbc.271.2.653. [DOI] [PubMed] [Google Scholar]
- 19.Baker JC, Yan X, Peng T, Kasten S, Roche TE. Marked differences between two isoforms of human pyruvate dehydrogenase kinase. J Biol Chem. 2000;275:15773–15781. doi: 10.1074/jbc.M909488199. [DOI] [PubMed] [Google Scholar]
- 20.Behal RH, Buxton DB, Robertson JG, Olson MS. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu Rev Nutr. 1993;13:497–520. doi: 10.1146/annurev.nu.13.070193.002433. [DOI] [PubMed] [Google Scholar]
- 21.Holness MJ, Kraus A, Harris RA, Sugden MC. Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes. 2000;49:775–781. doi: 10.2337/diabetes.49.5.775. [DOI] [PubMed] [Google Scholar]
- 22.Rosa G, Di Rocco P, Manco M, Greco AV, Castagneto M, Vidal H, Mingrone G. Reduced PDK4 expression associates with increased insulin sensitivity in postobese patients. Obes Res. 2003;11:176–182. doi: 10.1038/oby.2003.28. [DOI] [PubMed] [Google Scholar]
- 23.Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D. Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting. J Appl Physiol. 2004;96:2082–2087. doi: 10.1152/japplphysiol.01318.2003. [DOI] [PubMed] [Google Scholar]
- 24.Bajotto G, Murakami T, Nagasaki M, Tamura T, Tamura N, Harris RA, Shimomura Y, Sato Y. Downregulation of the skeletal muscle pyruvate dehydrogenase complex in the Otsuka Long-Evans Tokushima Fatty rat both before and after the onset of diabetes mellitus. Life Sci. 2004;75:2117–2130. doi: 10.1016/j.lfs.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Chokkalingam K, Jewell K, Norton L, Littlewood J, van Loon LJ, Mansell P, Macdonald IA, Tsintzas K. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: An important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab. 2007;92:284–292. doi: 10.1210/jc.2006-1592. [DOI] [PubMed] [Google Scholar]
- 26.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watt MJ, Hevener AL. Fluxing the mitochondria to insulin resistance. Cell Metab. 2008;7:5–6. doi: 10.1016/j.cmet.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J. 2006;397:417–425. doi: 10.1042/BJ20060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E46–E54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 33.Crabb DW, Yount EA, Harris RA. The metabolic effects of dichloroacetate. Metabolism. 1981;30:1024–1039. doi: 10.1016/0026-0495(81)90105-0. [DOI] [PubMed] [Google Scholar]
- 34.Clark AS, Mitch WE, Goodman MN, Fagan JM, Goheer MA, Curnow RT. Dichloroacetate inhibits glycolysis and augments insulin-stimulated glycogen synthesis in rat muscle. J Clin Invest. 1987;79:588–594. doi: 10.1172/JCI112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yount EA, Felten SY, O'Connor BL, Peterson RG, Powell RS, Yum MN, Harris RA. Comparison of the metabolic and toxic effects of 2-chloropropionate and dichloroacetate. J Pharmacol Exp Ther. 1982;222:501–508. [PubMed] [Google Scholar]
- 36.Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res. 2004;38:1083–1092. doi: 10.1080/10715760400004168. [DOI] [PubMed] [Google Scholar]
- 37.Walgren JL, Amani Z, McMillan JM, Locher M, Buse MG. Effect of R(+)alpha-lipoic acid on pyruvate metabolism and fatty acid oxidation in rat hepatocytes. Metabolism. 2004;53:165–173. doi: 10.1016/j.metabol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med. 1999;27:309–314. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 39.Konrad T, Vicini P, Kusterer K, Hoflich A, Assadkhani A, Bohles HJ, Sewell A, Tritschler HJ, Cobelli C, Usadel KH. Alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care. 1999;22:280–287. doi: 10.2337/diacare.22.2.280. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal SA. Inhibition of gluconeogenesis in rat liver by lipoic acid. Evidence for more than one site of action. Biochem J. 1984;219:773–780. doi: 10.1042/bj2190773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–2494. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- 42.Bebernitz GR, Aicher TD, Stanton JL, Gao J, Shetty SS, Knorr DC, Strohschein RJ, Tan J, Brand LJ, Liu C, Wang WH, Vinluan CC, Kaplan EL, Dragland CJ, DelGrande D, Islam A, Lozito RJ, Liu X, Maniara WM, Mann WR. Anilides of (R)-trifluoro-2-hydroxy-2-methylpropionic acid as inhibitors of pyruvate dehydrogenase kinase. J Med Chem. 2000;43:2248–2257. doi: 10.1021/jm0000923. [DOI] [PubMed] [Google Scholar]
- 43.Morrell JA, Orme J, Butlin RJ, Roche TE, Mayers RM, Kilgour E. AZD7545 is a selective inhibitor of pyruvate dehydrogenase kinase 2. Biochem Soc Trans. 2003;31(Pt 6):1168–1170. doi: 10.1042/bst0311168. [DOI] [PubMed] [Google Scholar]
- 44.Kato M, Li J, Chuang JL, Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayers RM, Leighton B, Kilgour E. PDH kinase inhibitors: a novel therapy for Type II diabetes? Biochem Soc Trans. 2005;33(Pt 2):367–370. doi: 10.1042/BST0330367. [DOI] [PubMed] [Google Scholar]
- 46.Aicher TD, Damon RE, Koletar J, Vinluan CC, Brand LJ, Gao J, Shetty SS, Kaplan EL, Mann WR. Triterpene and diterpene inhibitors of pyruvate dehydrogenase kinase (PDK) Bioorg Med Chem Lett. 1999;9:2223–2228. doi: 10.1016/s0960-894x(99)00380-7. [DOI] [PubMed] [Google Scholar]
- 47.Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes. 2008;57:2272–2279. doi: 10.2337/db08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 49.Olswang Y, Cohen H, Papo O, Cassuto H, Croniger CM, Hakimi P, Tilghman SM, Hanson RW, Reshef L. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci U S A. 2002;99:625–630. doi: 10.1073/pnas.022616299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franckhauser S, Munoz S, Elias I, Ferre T, Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes. 2006;55:273–280. doi: 10.2337/diabetes.55.02.06.db05-0482. [DOI] [PubMed] [Google Scholar]
- 51.Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalhan SC, Bugianesi E, McCullough AJ, Hanson RW, Kelley DE. Estimates of hepatic glyceroneogenesis in type 2 diabetes mellitus in humans. Metabolism. 2008;57:305–312. doi: 10.1016/j.metabol.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang B, Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J. 2009;423:243–252. doi: 10.1042/BJ20090390. [DOI] [PubMed] [Google Scholar]
- 54.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958–1966. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 55.Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall'Agata M, Trevisani F, Guerrieri F, Bernardi M, Altomare E. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology. 2001;33:808–815. doi: 10.1053/jhep.2001.23060. [DOI] [PubMed] [Google Scholar]
- 56.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 57.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:15439–15450. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 60.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]