Abstract
It has been speculated that the mandibular condyle develops via the differentiation of the fibroblast-like cells covering the condyle into chondrocytes; however, the developmental mechanisms behind this process have not been revealed. We used laser-capture microdissection and cDNA microarray analysis to elucidate the genes that are highly expressed in these fibroblast-like cells. Among these genes, the transcription of Ten-m/Odz3 was significantly increased in the fibroblast-like cells compared with other cartilage tissues. For the first time, we describe the temporal and spatial expression of Ten-m/Odz3 mRNA in relation to the expression of type I, II, and X collagen mRNA, as determined by in-situ hybridization in mouse mandibular condylar cartilage and mouse femoral cartilage during the early stages of development. Ten-m/Odz3 was expressed in the fibrous layer and the proliferating and mature chondrocyte layers, which expressed type I and II collagen, respectively, but was not detected in the hypertrophic chondrocyte layer. Furthermore, we evaluated the in-vitro expression of Ten-m/Odz3 using ATDC5 cells, a mouse chondrogenic cell line. Ten-m/Odz3 was expressed during the early stage of the differentiation of mesenchymal cells into chondrocytes. These findings suggest that Ten-m/Odz3 is involved in the differentiation of chondrocytes and that it acts as a regulatory factor in the early stages of the development of mandibular condylar cartilage.
Keywords: cartilage, chondrocyte, gene expression, odd Oz, tenascin-like molecule major
Introduction
Mandibular condylar cartilage, unlike other cartilage, has a unique structure. It plays dual roles as articular cartilage responding to biomechanical stress, such as that produced by jaw movement during mastication, and as the growth plate cartilage of long bones during growth (Symons, 1965). The growth plate cartilage of long bones only functions during development and elongation, which occur via the proliferation and differentiation of chondrocytes. The growth plate cartilage then disappears during the late stage of development (Silbermann & Frommer, 1972; Mizoguchi et al. 1990). However, articular cartilage is present throughout postnatal life, and its morphological and biosynthetic features remain unchanged (Roberts & Hartsfield, 2004). In contrast, for mandibular cartilage it has been speculated that fibroblast-like cells covering the surface of the mandibular condyle differentiate into chondrocytes, which then actively proliferate (Fukada et al. 1999; Shibata et al. 2006) and that the mandibular condyle subsequently develops by endochondral ossification during growth (Weiss et al. 1990; Blackwood, 1996; Inoue et al. 2002). Finally, after development, mandibular condylar cartilage remains as fibrous cartilage and functions as articular cartilage (Silbermann & Frommer, 1972; Mizoguchi et al. 1990). Thus, during postnatal growth, fibroblast-like cells in the superficial layer of mandibular condylar cartilage may function as a source of undifferentiated mesenchymal cells that subsequently differentiate into chondrocytes and are stress loaded during physiological jaw movement.
Two novel molecules were discovered and named Ten-a (tenascin-like molecule accessory) (Bumgartner & Chiquet-Ehrismann, 1993) and Ten-m (tenascin-like molecule major) (Baumgartner et al. 1994) while investigating the function of glycoprotein tenascin-C. Ten-m was originally identified as the Drosophila pair-rule gene because of its odd pair-rule mutant phenotype and was renamed odd Oz (Odz) (Baumgartner et al. 1994; Levine et al. 1994). To date, four members of the Ten-m/Odz protein family have been identified in several species. The Ten-m/Odz gene contains a series of tenascin-type epidermal growth factor-like repeat domains between its putative transmembrane and putative signaling peptide domains (Ben-Zur et al. 2000). Its transcript produces a type II transmembrane protein in the form of a homodimer and is involved in signal transduction on the cell surface (Levine et al. 1994, 1997; Feng et al. 2002). In vertebrates, Ten-m/Odz mRNA is expressed in the central nervous system (CNS) of zebrafish (Mieda et al. 1999), chick (Minet et al. 1999; Rubin et al. 1999), mouse (Wang et al. 1998), and human (Minet et al. 1999; Minet & Chiquet-Ehrismann, 2000) embryos. Additionally, in a recent study, Ten-m/Odz mRNA expression was also reported in various tissues, such as the pharyngeal arches and some somites (Tucker et al. 2001) and limb buds (Tucker & Chiquet-Ehrismann, 2006). However, the role of Ten-m/Odz in mandibular condylar cartilage has not been investigated.
The advent of cDNA microarray allows global comparison of gene expression. In the present study, we distinguished the fibroblast-like cells (the ‘fibrous layer’) from the ‘cartilage layer’, which showed metachromasia as determined by toluidine blue (TB) staining, in mouse mandibular condylar cartilage and used laser-capture microdissection (LCM) and cDNA microarray to investigate new factors that dominate the differentiation of mesenchymal cells in mandibular condylar cartilage in growing postnatal mice. As a result, we identified Ten-m/Odz3, which was expressed intensely in fibroblast-like cells on the superficial layer of the mandibular condylar cartilage. We then performed reverse transcription-polymerase chain reaction analysis to investigate Ten-m/Odz3 expression. The present study also investigated the temporal and spatial expression of Ten-m/Odz3 mRNA in mouse mandibular condylar and femoral cartilage after birth, as well as in ATDC5 cells, a mouse chondrogenic cell line. Our findings suggest that Ten-m/Odz3 is involved in the differentiation of the fibroblast-like cells in the superficial layer of mandibular condylar cartilage into chondrocytes.
Materials and methods
Tissue preparation and histology
Institute of Cancer Research mice (CLEA Japan, Inc., Tokyo, Japan) of 1 day (n = 5), 1 week (n = 5), and 3 weeks (n = 5) of age were used. The experiment was approved by the animal committee of Okayama University Graduate School. Under inhalation anesthesia with ether (nacalai tesque, Kyoto, Japan), the animals were perfused with 4% paraformaldehyde in 0.1 m sodium phosphate buffer and then their mandibles were dissected. After dissection, the specimens were perfused in a similar manner for 12 h. The dissected mandibles were then decalcified with 20% ethylenediaminetetra-acetate for 2 weeks, processed by passage through graded ethanols and toluene, and embedded in paraffin wax, before 7-μm-thick longitudinal sections were cut and mounted on triethoxyaminopropylsilane-coated slides. The resultant slides were stored at 4 °C until they were stained with hematoxylin and eosin for histological examination, and TB staining was also carried out to identify the cartilage matrix.
Procurement of fibrous and cartilage layers using laser-capture microdissection and total RNA extraction
One-day-old mice were used. We distinguished the fibrous layer of the condyle from the cartilage layer, which showed metachromasia staining. Cryostat sections (7 μm) were quickly fixed in 100% methanol for 3 min and then stained with 1% TB. The LCM processing system CLONIS (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to procure target fibrous and cartilage layers (Fig. 1). Figure 1 shows the division of mandibular condylar cartilage for LCM (f shows the ‘fibrous layer’ not showing metachromasia and c shows the ‘cartilage layer’ showing metachromasia by TB staining). The fibrous and cartilage layers bound on an ultrathin transparent supporter membrane were dissected with a diode infrared laser. The target cell layer was then collected and total RNA was isolated using the acid-guanidinium thiocyanate-phenol-chloroform method (Chomczynski & Sacchi, 1987). The isolated fibrous and cartilage layers were homogenized by vortexing in denaturation guanidinium isothiocyanate-based buffer containing 25 mm sodium citrate, 0.5% sarcosyl and 0.1 m 2-mercaptoethanol. RNA was isolated by the sequential addition of 2 m sodium acetate, pH 4.0, chloroform-isoamyl alcohol mixture, and water-saturated phenol. Samples were vortexed and placed on ice for 15 min before centrifugation. The aqueous phase was transferred to a new microtube and an equal volume of isopropanol and 10 g mL−1 glycogen was added. The precipitated RNA was obtained by incubation at −80 °C for 30 min followed by centrifugation. After precipitation, the RNA pellet was dissolved in 10 μL of DNase, RNase-free water (Eppendorf AG, Hamburg, Germany) and kept at −80 °C until processing.
Fig. 1.
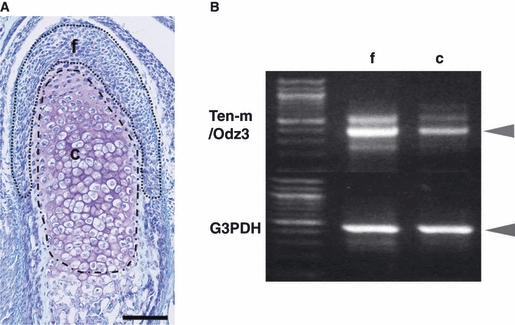
(A) Sagittal view of the mandibular condylar cartilage of a 1-day-old mouse stained with toluidine blue (TB). The two enclosed areas (f and c) show the division of the mandibular condylar cartilage. [This division was also used for laser-capture microdissection (LCM) and cDNA microarray analysis in our preliminary research.] f is the ‘fibrous layer’ not showing metachromasia, and c is the ‘cartilage layer’ showing metachromasia, as shown by TB staining. Bar = 100 μm. (B) Reverse transcription-polymerase chain reaction (RT-PCR) analysis of the expression of Ten-m/Odz3 in the two layers. RT-PCR was performed with total RNA samples from the fibrous (f) and cartilage (c) layers using LCM. The upper row shows Ten-m/Odz3 (396 bp), and the lower layer represents glyceraldehyde-3-phosphate dehydrogenase (G3PDH), a housekeeping gene. Ten-m/Odz3 expression was normalized to the expression of G3PDH. Results are representative of five independent experiments.
cDNA microarray analysis
To identify gene expression differences in fibrous and cartilage layers of mouse mandibular condylar cartilage, LCM and high-density oligonucleotide array analysis were performed for screening. The target fibrous and cartilage layers in mouse mandibular condylar cartilage were harvested by LCM (Fig. 1). Total RNA was mixed with T7-oligo (dT) promoter primer, reverse-transcribed to cDNA, and synthesized to double-stranded cDNA with a GeneChip® Expression 3′-Amplification Reagents Two-Cycle cDNA Synthesis Kit (Affymetrix, Santa Clara, CA, USA). The double-stranded cDNA was purified and served as a template in the subsequent in-vitro transcription using a GeneChip®in-vitro transcription labeling kit (Affymetrix). The biotinylated cRNA target was then fragmented and hybridized to the array (GeneChip® Mouse Genome 430A 2.0 Array; Affymetrix), which contains 39 000 known genes. Immediately following hybridization, the array was washed and stained by streptavidin-phycoerythrin (Molecular Probes, Eugene, OR, USA). Each probe array was scanned and an average of the two images calculated; the probe cells were defined and the intensity was computed for each cell. Finally, the results were analyzed using Microarray Suite Expression Analysis (Affymetrix) and Genespring software.
Probe preparation and in-situ hybridization
Digoxigenin-11-UTP-labeled single-stranded RNA probes were prepared with a digoxigenin RNA Labeling kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. A 0.39-kb Ten-m/Odz3 fragment (NM_011857, located between 8103 and 8499), a 0.24-kb type I collagen fragment (Brandsten et al. 1999), a 0.30-kb type II collagen fragment (Cheah et al. 1991), and a 0.44-kb type X collagen fragment (Marks et al. 2000) were used to generate sense and antisense probes. Briefly, the paraffin sections were dewaxed, and endogenous peroxidase activity was inhibited by treating the sections with 0.3% (v/v) H2O2 in methanol solution for 30 min. After treatment with proteinase K (2.0 g mL−1) for 15 min at 37 °C, the sections were postfixed with 0.1 m phosphate buffer (PB) (pH 7.4) containing 4% (v/v) paraformaldehyde, washed with 0.1 m PB, treated with 0.2 N HCl for 20 min, and rewashed with 0.1 m PB at 24 °C. Finally, the sections were rinsed twice in 4 × saline sodium citrate (SSC) (1 × SSC is 150 mm NaCl and 15 mm sodium citrate) for 10 min at 24 °C, before being incubated with 50% (v/v) formamide for 3 h at 42 °C. Hybridization was performed at 43 °C using the denatured antisense probe in hybridization solution [50% (v/v) formamide, 0.6 m NaCl, and 1 × Denhardt's solution (10 mm Tris-HCl, 1.0 mm EDTA, 10% (w/v) dextran, and 200 μg mL−1 yeast tRNA)] overnight. The controls were hybridized with sense probes (data not shown). After hybridization, the sections were washed in 5 × SSC at 45 °C for 20 min, before being rinsed three times in 2 × SSC containing 50% (v/v) formamide for 20 min at 45 °C. Unhybridized probes were removed by treating the sections with 20 μg μL−1 ribonuclease A (Sigma, St Louis, MO, USA) at 37 °C for 30 min and then washing them sequentially in 2 × SSC and 0.2 × SSC at 45 °C for 20 min. The Tyramide Signal Amplification Biotin System (NEN Life Science Products, Boston, MA, USA) was then used for blocking, incorporation of horseradish peroxidase, and Tyramide Signal Amplification Biotin System amplification and visualization, according to the manufacturer's instructions. Relative expression levels were assessed as follows. We evaluated three levels of expression strength: ‘strong’, ‘moderate’, and ‘weak’. The strength of expression intensity was arbitrarily assigned visually by four independent observers. The average expression scores in the tables were calculated from independently assigned values.
Total RNA isolation from ATDC5 cells and reverse transcription-polymerase chain reaction
The ATDC5 cells were cultured in Dulbecco's Modified Eagle Medium/F-12 1:1 Mixture (Invitrogen, Carlsbad, CA,USA) containing 5% fetal bovine serum and maintained at 37 °C in 5% CO2. The inoculum size of the cells was 2 × 104 cells well−1 in a 24-well plate. After 5 days’ culture, the cells reached confluence and then Insulin-Transferrin-Selenium Supplement (Invitrogen) was added to induce chondrogenic differentiation and maturation according to a previously reported method (Shukunami et al. 1996). The cells were harvested on days 5, 8, 11, 14, 17, 20, 23, and 26 of the culture period using Trizol (Invitrogen), and total RNA was isolated from them using Trizol, according to the manufacturer's protocol. Total RNA was reverse transcribed into cDNA using M-MLV Reverse Trancriptase (Invitrogen), and polymerase chain reactions were performed with the primers listed below. The reactions were carried out for 14–29 cycles of 15 s at 95 °C and 1 min at 60 °C with the last cycle involving 5 min extension at 72 °C. The resultant polymerase chain reaction products were subjected to electrophoresis on 1.5% agarose gels (Invitrogen) in Tris acetate–EDTA buffer (Sigma). The primers used were as follows: Ten-m/Odz3, 5′-TGGGAAAAGAGGACTGCCGTTTTG-3′ (sense), 5′-TGCCACCACTTCAGCGTTTTTAGTC -3′ (antisense); type I collagen, 5′-ATGAGGAGACGGGCAGCTT-3′ (sense), 5′- GGCAGGCGAGATGGCTTAT-3′ (antisense); type II collagen, 5′-AGGGACTGATGGTATTCCTGGAGC-3′ (sense), 5′-AGCACCTCGTTTGCCTTCTTCAC-3′ (antisense); type X collagen, 5′-TTTCTGGGATGCCGCTTGTCAG-3′ (sense), 5′-TCACATGGGAGCCACTAGGAATCC-3′(antisense); and hypoxanthine phosphoribosyltransferase, 5′-GCGTCGTGATTAGCGATGATGA-3′(sense), 5′-GTCAAGGGCATATCCAACAACA-3′ (antisense).
Results
Changes in the gene expression of Ten-m/Odz3 and type I, II, and X collagen in the mandibular condylar cartilage of 1-day-, 1-week-, and 3-week-old mice
Toluidine blue staining was used to identify the areas of the fibrous layer not showing metachromasia and the cartilage layers showing metachromasia (Fig. 1A). RNA was collected from the fibrous and cartilage layers using LCM, and the reverse transcription-polymerase chain reaction was performed (Fig. 1B). Ten-m/Odz3 showed higher expression in the fibrous layer than in the cartilage layer (Fig. 1B).
The horizontal width of the condyle increased as it grew from 1 day to 3 weeks (Fig. 2A–C). Although the width of the fibrous layer in the condylar cartilage decreased, the amount of bone tissue was increased by endochondral ossification (Fig. 2A–C). At all ages, a fibrous layer that did not demonstrate metachromasia was found on the condyle surface. Immediately below the fibrous layer, a cartilage layer showing metachromasia was present (Fig. 2D,I,N).
Fig. 2.
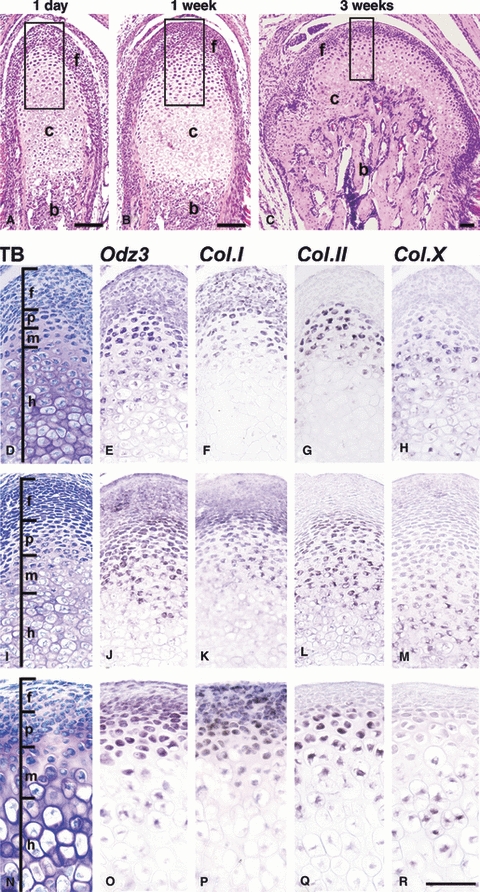
Expression of Ten-m/Odz3, and type I, type II, and type X collagen mRNA in 1-day-old (E–H), 1-week-old (J–M) and 3-week-old (O–R) mouse mandibular condylar cartilage. (A–C) Histology of sagittal sections of mandibular condylar cartilage at 1 day, 1 week, and 3 weeks with hematoxylin and eosin staining. The small rectangles in A, B, and C indicate the areas enlarged in D–H, I–M, and N–R, respectively. Serial sections were stained with toluidine blue (TB) (D,I,N) and hybridized with RNA probes for Ten-m/Odz3 (E,J,O), type I collagen (F,K,P) (Col.I), type II collagen (G,L,Q) (Col.II), and type X collagen (H,M,R) (Col.X). Type I collagen mRNA was strongly expressed in the fibrous layer and recognized in the proliferating chondrocyte layer (F,K,P). Type II collagen showed characteristic expression in the proliferating and mature chondrocyte layers (G,L,Q). Type X collagen was found in the hypertrophic chondrocyte layer (H,M,R), but its expression area decreased as the mandible grew. Ten-m/Odz3 mRNA was expressed in the fibrous and proliferating chondrocyte layers, showed weak expression in the mature chondrocyte layer, and was not detected in the hypertrophic chondrocyte layer (E,J,O). f, fibrous layer; p, proliferating chondrocyte layer; m, mature chondrocyte layer; h, hypertrophic chondrocyte layer; c, cartilage layer; b, subchondral bone. Bar = 100 μm.
On day 1, type I collagen mRNA was expressed in the fibrous and proliferating chondrocyte layers (Fig. 2F). Type II collagen mRNA showed specific expression in both the proliferating and mature chondrocyte layers (Fig. 2G). Type X collagen mRNA showed high expression in the hypertrophic chondrocyte layer and was also found in the mature chondrocyte layer (Fig. 2H). Ten-m/Odz3 mRNA was strongly expressed in the fibrous and proliferating chondrocyte layers, in which type I collagen mRNA was expressed, and weakly in the mature chondrocyte layer, in which type II collagen mRNA was expressed, but was not expressed in the hypertrophic chondrocyte layer, in which type X collagen mRNA was expressed (Fig. 2E).
At 1 week, type I, II, and X collagen mRNA were expressed in the fibrous and proliferating chondrocyte layers, the proliferating and mature chondrocyte layers, and the hypertrophic chondrocyte layer, respectively, as observed on day 1 (Fig. 2K,L,M). However, the areas of the type II collagen mRNA-positive proliferating and mature chondrocyte layers were increased compared with those observed on day 1. Ten-m/Odz3 mRNA was also strongly expressed in the fibrous and proliferating chondrocyte layers and moderately expressed in the mature chondrocyte layer, as observed on day 1 (Fig. 2J).
At 3 weeks, type I, II, and X collagen mRNA were expressed in the fibrous and proliferating chondrocyte layers, the proliferating and mature chondrocyte layers, and the hypertrophic chondrocyte layer, respectively, as shown on day 1 and at 1 week, although the thickness of the type X collagen-positive hypertrophic chondrocyte layer was increased compared with those at 1 day and 1 week (Fig. 2F–H,K–M,P-R). However, Ten-m/Odz3 mRNA showed a different expression pattern. It was highly expressed in the lower region of the fibrous layer and the proliferating and mature chondrocyte layers, but was scarcely detectable in the upper region of the fibrous layer (Fig. 2O).
The sections hybridized with sense probes demonstrated no hybridization (data not shown). The distribution of all gene expression in the mandibular condyle is summarized in Table 1 (n = 5).
Table 1.
Gene expression of Odz3 and collagen type I (Col. I), II (Col. II) and X (Col. X) in the mandibular condylar cartilage of mice.
| Odz3 | Col. I | Col. II | Col. X | ||
|---|---|---|---|---|---|
| 1-day-old mice (n = 5) | |||||
| Fibroblast-like cells in fibrous layer | Upper side | + | + | − | − |
| Lower side | + | + | − | − | |
| Proliferating chondrocytes | + + | + + | + + | ± | |
| Mature chondrocytes | + | ± | + + | + | |
| Hypertrophic chondrocytes | − | − | − | + | |
| 1-week-old mice (n = 5) | |||||
| Fibroblast-like cells in fibrous layer | Upper side | ± | + | − | − |
| Lower side | + | + | − | − | |
| Proliferating chondrocytes | + + | + + | + + | ± | |
| Mature chondrocytes | + | ± | + + | + | |
| Hypertrophic chondrocytes | − | − | − | + | |
| 3-week-old mice (n = 5) | |||||
| Fibroblast-like cells in fibrous layer | Upper side | − | + | − | − |
| Lower side | + + | + | − | − | |
| Proliferating chondrocytes | + + | + + | + + | ± | |
| Mature chondrocytes | + | ± | + + | + | |
| Hypertrophic chondrocytes | − | − | − | + | |
−, no expression; ±, weak expression; +, modern expression; ++, strong expression.
Gene expression of Ten-m/Odz3 and type I, II, and X collagen in the femoral cartilage of 1-day-old mice
The femoral cartilage was covered with perichondrium, which showed no metachromasia, as determined by TB, and the cartilage layers showed proliferating, mature, and hypertrophic chondrocyte layers at 1 day after birth (Fig. 3A). Thus, the femoral cartilage was morphologically similar to the mandibular condylar cartilage at 1 day. Type I collagen mRNA was sparsely localized in the perichondrium of the femoral articular cartilage, although in the mandibular condylar cartilage type I collagen mRNA was distributed in the fibrous layer and the proliferating and mature chondrocyte layers (Fig. 3D,I). Type II collagen mRNA was expressed in the proliferating and mature chondrocyte layers (Fig. 3E,J), and type X collagen mRNA was mainly found in the hypertrophic chondrocyte layer (Fig. 3K). Ten-m/Odz3 mRNA was strongly expressed in the perichondrium and proliferating chondrocyte layer and was moderately expressed in the mature chondrocyte layer (Fig. 3C,H). Ten-m/Odz3 and collagen type I, II, and X mRNA showed slightly different but almost identical expression patterns between the mandibular condylar cartilage and femoral cartilage. The gene expression pattern in the femoral cartilage is summarized in Table 2 (n = 5).
Fig. 3.
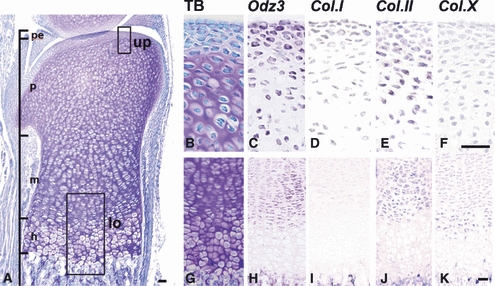
Expression of Ten-m/Odz3, type I, type II, and type X collagen mRNA in the femoral cartilage of a 1-day-old mouse. (A) Histology of a sagittal section of the femoral cartilage of a 1-day-old mouse stained with toluidine blue (TB). The small rectangles marked as up (upper side) and lo (lower side) in A indicate the areas enlarged in B–F and G–K, respectively. Serial sections were stained with TB (B,G) and hybridized with RNA probes for Ten-m/Odz3 (C,H), type I collagen (D,I) (Col.I), type II collagen (E,J) (Col.II), and type X collagen (F,K) (Col.X). Type I collagen mRNA was weakly expressed in the perichondrium (D,I). Type II collagen mRNA showed characteristic expression in the proliferating and mature chondrocyte layers (E,J). Type X collagen mRNA was mainly found in the hypertrophic chondrocyte layer (F,K). Ten-m/Odz3 mRNA was strongly expressed in the perichondrium and proliferating chondrocyte layer (C,H). pe, perichondrium; p, proliferating chondrocyte layer; m, mature chondrocyte layer; h, hypertrophic chondrocyte layer. Bar = 50 μm.
Table 2.
Gene expression of Odz3 and collagen type I (Col. I), II (Col. II), and X (Col. X) in the femoral cartilage of 1-day-old mice (n = 5).
| Odz3 | Col. I | Col. II | Col. X | |
|---|---|---|---|---|
| Upper femoral cartilage | ||||
| Perichondrium | + + | ± | ± | − |
| Proliferating chondrocytes | + + | − | + + | − |
| Lower femoral cartilage | ||||
| Mature chondrocytes | + | − | + | ± |
| Hypertrophic chondrocytes | − | − | − | + |
−, no expression; ±, weak expression; +, modern expression; ++, strong expression.
Ten-m/Odz3 expression during chondrogenic maturation in ATDC5 cells
It has been reported that ATDC5 cells, a mouse chondrogenic cell line, undergo chondrogenic differentiation and maturation involving sequential phenotype transitions in their mRNA levels of type I, II, and X collagen, which are phenotypic markers of prechondrogenic mesenchymal cells, chondrocytes, and hypertrophic chondrocytes, respectively (Shukunami et al. 1996; Akiyama et al. 1997). ATDC5 cells were found to express type I collagen on days 5, 8, 11, and 14 (Fig. 4B). The expression of type II collagen was undetectable on days 5 and 8 but became detectable from day 11 onwards (Fig. 4C). The expression of type X collagen was detectable on day 17, and its expression level increased in the following experimental period (Fig. 4D). Ten-m/Odz3 was highly expressed on days 5, 8, and 11 (Fig. 4A). Ten-m/Odz3 was strongly expressed in ATDC5 cells during the early stage of chondrogenesis in culture, which was similar to the expression pattern of type I collagen.
Fig. 4.
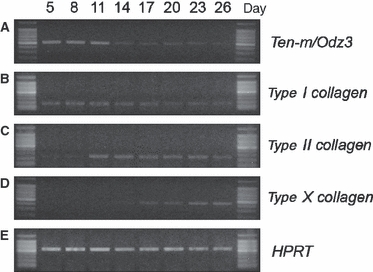
Expression of Ten-m/Odz3 in ATDC5 cells. To assess the expression pattern of Ten-m/Odz3 during chondrogenic differentiation and maturation in vitro, total RNA was isolated from ATDC5 cells on days 5, 8, 11, 14, 17, 20, 23, and 26 during the culture period and subjected to reverse transcription. The expression levels of Ten-m/Odz3 (A), type I collagen (B), type II collagen (C), and type X collagen (D) were analyzed by reverse transcription-polymerase chain reaction. Hypoxanthine phosphoribosyltransferase (E) was amplified as a positive control. Ten-m/Odz3 was strongly expressed during the early stage of chondrogenic differentiation in the ATDC5 cell culture.
Discussion
Cartilage tissue is classified as primary cartilage or secondary cartilage (Shen & Darendeliler, 2005); the former contains various types of articular cartilage such as femoral cartilage, but the latter only includes mandibular condylar cartilage. Fetal condylar cartilage begins to form later than the articular cartilage of tubular bone. Whereas postnatal condylar cartilage is covered with fibrous mesenchymal tissue, the articular cartilage of tubular bone is covered with a thin perichondrium. The fibrous mesenchymal tissues of mandibular condylar cartilage could be important for the growth of condylar cartilage. It is thought that fibroblast-like cells in the fibrous layer of the mandibular condyle differentiate into chondrocytes, which then actively proliferate (Fukada et al. 1999; Shibata et al. 2006), before the mandibular condyle develops by endochondral ossification (Weiss et al. 1990; Blackwood, 1996; Inoue et al. 2002). In the early stage of the differentiation of mesenchymal stem cells into chondrocytes, Sox5, 6, and 9 mainly function (de Crombrugghe et al. 2000; Lefebvre et al. 2001). Fibroblast growth factor, insulin-like growth factor, transforming growth factor, proliferating cell nuclear antigen, bone morphogenic protein, and wingless-related MMTV integration site family (Wnt) regulate the proliferation of the mesenchyme and chondrocytes (Rudnicki & Brown, 1997; Tsurimoto, 1999; Ogawa et al. 2003; Tuli et al. 2003; Ueno et al. 2003; Yang et al. 2003). Parathyroid hormone related protein/Indian hedgehog, core binding factor alpha 1/Runt related transcription factor 2, and Wnt act in the maturation and differentiation of chondrocytes (Rudnicki & Brown, 1997; Alvarez et al. 2002; Rabie et al. 2004). However, the mechanism behind the regulation of the differentiation of undifferentiated mesenchymal cells into chondrocytes in the mandibular condylar cartilage has not been fully elucidated.
In the present study, we isolated the fibrous and cartilage layers of mandibular condylar cartilage precisely using LCM to analyze the fibrous tissue including undifferentiated mesenchymal cells. Next, we used cDNA microarray (GeneChip®) and found 579 genes that were upregulated in the fibrous layer compared with the cartilage layer (our unpublished data). Of all the genes, Ten-m/Odz3 showed high expression in the fibrous layer. Ten-m/Odz3 is known to be mainly expressed in the CNS and limb buds in the embryonic period, but not in cartilage tissues, especially after birth (Wang et al. 1998; Mieda et al. 1999; Minet et al. 1999; Rubin et al. 1999; Minet & Chiquet-Ehrismann, 2000; Tucker et al. 2001; Tucker & Chiquet-Ehrismann, 2006). Therefore, we evaluated the distribution patterns of Ten-m/Odz3 mRNA expression in fibrous and cartilage layers in vivo using in-situ hybridization and performed an in-vitro study using ATDC5 cells. In the present study, we clearly elucidated the spatial and temporal distribution of Ten-m/Odz3 in the mandibular condyle and found that it was expressed especially strongly in the fibrous layer during postnatal development. Moreover, Ten-m/Odz3 was also expressed in the femoral cartilage. Additionally, the present study was also the first to show the expression of Ten-m/Odz3 in a mouse chondrogenic cell line in vitro.
Several reports have suggested an association between cartilaginous tissue and neuropeptides, such as substance P, a calcitonin gene-related peptide in vitro (Halliday et al. 1993; Edoff & Hildebrand, 2003). In addition, we have reported the expression of brain-derived neurotrophic factor in bone and cartilage tissues (Yamashiro et al. 2001). In vertebrates, Ten-m/Odz mRNA is expressed in the CNS of zebrafish (Mieda et al. 1999), chick (Minet et al. 1999; Rubin et al. 1999), mouse (Wang et al. 1998), and human (Minet et al. 1999; Minet & Chiquet-Ehrismann, 2000) embryos. These findings suggest that a factor expressed in neurons might play a role in cartilage and bone.
To identify the cells expressing Ten-m/Odz3, we analyzed the coexpression pattern of type I, II, and X collagen mRNA in Ten-m/Odz3-positive chondrocytes. Ten-m/Odz3 expression was revealed in the upper and lower regions of the fibrous layer, which expressed type I collagen, and in the proliferating layer, which expressed type I and II collagen at 1 day and 1 week. However, at 3 weeks Ten-m/Odz3 showed weak expression in the upper region of the fibrous layer compared with that at 1 day and 1 week. Usually, normal mice progress through the lactation period (from birth to approximately 1 week old), weaning period (around 3 weeks old), and mastication period (from approximately 3 weeks onward after birth). Therefore, at 3 weeks during the weaning period, the condyle began to be stress loaded during physiological jaw movement such as mastication. In this study, we observed that the mandibular condyle in 3-week-old mice showed remarked morphological changes with increasing horizontal width and thinning fibrous layer compared with 1-day-old and 1-week-old mice. Taken together, the change in Ten-m/Odz3 expression in the fibrous layer from 1-day-old and 1-week-old to 3-week-old might be related to the transition from lactation to weaning and/or the morphological change of mandibular condylar cartilage in mice.
Using ATDC5 cells, we also found that Ten-m/Odz3 mRNA was strongly expressed in the early stage of the culture period and that its expression pattern was similar to that of type I collagen mRNA. It has been reported that ATDC5 cells undergo chondrogenic differentiation and maturation, during which they show changes in the marker genes that they express (Shukunami et al. 1996; Akiyama et al. 1997). According to these previous studies, undifferentiated ATDC5 cells express type I collagen and, after chondrogenesis, they express chondrocyte markers such as type II collagen (Shukunami et al. 1996). Furthermore, after hypertrophic maturation, ATDC5 cells express hypertrophic chondrocyte markers such as type X collagen (Akiyama et al. 1997). These results suggest that Ten-m/Odz3 is expressed in prechondrogenic mesenchymal cells and may be directly or indirectly involved in the early stage of chondrogenic differentiation.
Previously, it has been reported that tenascin-C was expressed in the synovial membrane and fibrocartilaginous disc of the temporomandibular joint, and the expression in synovial fibroblasts was modulated by hypoxic conditions with inflammation (Tojyo et al. 2008). Moreover, tenascin-C expression was enhanced by mechanical loading in the myotendinous junction, bone and muscle (Webb et al. 1997; Fluck et al. 2000; Jarvinen et al. 2003). The Ten-m/Odz3 gene contains a series of tenascin-type epidermal growth factor-like repeat domains and was discovered in investigations of the function of glycoprotein tenascin-C (Baumgartner et al. 1994; Ben-Zur et al. 2000). Therefore, inflammatory and/or mechanical stress may also modulate Ten-m/Odz3 expression in synobial fibroblasts in the temporomandibular joint.
Concluding remarks
We investigated Ten-m/Odz3 expression in the mandibular condylar cartilage, femoral articular cartilage, and ATDC5 cells. As a result, it was found that Ten-m/Odz3 was expressed during the early stage of the differentiation of mesenchymal cells into chondrocytes. The function of Ten-m/Odz3 in the fibrous and cartilage layers of mandibular condylar cartilage remains unclear. However, it is conceivable that Ten-m/Odz3 acts, at least in part, as a regulatory factor in the early development of chondrocytes and may modulate the differentiation of mesenchymal cells into chondrocytes in the mandibular condylar cartilage.
Acknowledgments
We thank Dr Mana Ichimura for her cooperation with LCM and Ms Asayo Imaoka for her excellent help with the cDNA microarray analysis. This study was supported by Grants-In-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Akiyama H, Shigeno C, Hiraki Y, et al. Cloning of a mouse smoothened cDNA and expression patterns of hedgehog signaling molecules during chondrogenesis and cartilage differentiation in clonal mouse EC cells, ATDC5. Biochem Biophys Res Commun. 1997;235:142–147. doi: 10.1006/bbrc.1997.6750. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Sohn P, Zeng X, et al. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- Baumgartner S, Martin D, Hagios C, et al. Tenm, a Drosophila gene related to tenascin, is a new pair-rule gene. EMBO J. 1994;13:3728–3740. doi: 10.1002/j.1460-2075.1994.tb06682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zur T, Feige E, Motro B, et al. The mammalian Odz gene family: homologs of Drosophila pair-rile gene with expression implying distinct yet overlapping developmental roles. Dev Biol. 2000;217:107–120. doi: 10.1006/dbio.1999.9532. [DOI] [PubMed] [Google Scholar]
- Blackwood HJ. Growth of the mandibular condyle of the rat studied with tritiated thymidine. Arch Oral Biol. 1996;11:493–500. doi: 10.1016/0003-9969(66)90155-5. [DOI] [PubMed] [Google Scholar]
- Brandsten C, Lundmark C, Christersson C, et al. Expression of collagen alpha1(I) mRNA variants during tooth and bone formation in the rat. J Dent Res. 1999;78:11–19. doi: 10.1177/00220345990780010101. [DOI] [PubMed] [Google Scholar]
- Bumgartner S, Chiquet-Ehrismann R. Ten-a, a Drosophila gene related to tenascin, shows selective transcript localization. Mech Dev. 1993;40:165–176. doi: 10.1016/0925-4773(93)90074-8. [DOI] [PubMed] [Google Scholar]
- Cheah KS, Au PK, Lau ET, et al. The mouse Col2a-1 gene is highly conserved and is linked to Int-1 on chromosome 15. Mamm Genome. 1991;1:171–183. doi: 10.1007/BF00351064. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocynate-phenol-choloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B, Lefebvre V, Behringer RP, et al. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Edoff K, Hildebrand C. Neuropeptide effects on rat chondrocytes and perichondrial cells in vitro. Neuropeptides. 2003;37:316–318. doi: 10.1016/j.npep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Feng K, Zhou XH, Oohashi T, et al. All four members of the Ten-m/Odz family of transmembrane proteins form dimers. J Biol Chem. 2002;277:26128–26135. doi: 10.1074/jbc.M203722200. [DOI] [PubMed] [Google Scholar]
- Fluck M, Tunc-Civelek V, Chiquet M. Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by loading of skeletal muscle. J Cell Sci. 2000;113:3583–3591. doi: 10.1242/jcs.113.20.3583. [DOI] [PubMed] [Google Scholar]
- Fukada K, Shibata S, Suzuki S, et al. In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J Anat. 1999;195:321–329. doi: 10.1046/j.1469-7580.1999.19530321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DA, McNeil JD, Betts WH, et al. The substance P fragment SP-(7-11) increases prostaglandin E2, intracellular Ca2+ and collagenase production in bovine articular chondrocytes. Biochem J. 1993;292:57–62. doi: 10.1042/bj2920057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hiraki Y, Nawa T, et al. Phenotypic switching of in vitro mandibular condylar cartilage during matrix mineralization. Anat Sci Int. 2002;7:237–246. doi: 10.1046/j.0022-7722.2002.00031.x. [DOI] [PubMed] [Google Scholar]
- Jarvinen TAH, Jozsa L, Kannus P, et al. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci. 2003;116:857–866. doi: 10.1242/jcs.00303. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Behringer RP, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9:S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Levine A, Bashan-Ahrend A, Budai-Hadrian O, et al. Odd Oz: a novel Drosophila pair rule gene. Cell. 1994;77:587–598. doi: 10.1016/0092-8674(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Levine A, Gartenberg D, Yakov R, et al. The genetics and molecular structure of the Drosophila pair-rule gene odd Oz (odz) Gene. 1997;200:59–74. doi: 10.1016/s0378-1119(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Marks SC, Jr, Lundmark C, Christersson C, et al. Endochondral bone formation in toothless (osteopetrotic) rats: failures of chondrocyte patterning and type X collagen expression. Int J Dev Biol. 2000;44:309–316. [PubMed] [Google Scholar]
- Mieda M, Kikuchi Y, Hirate Y, et al. Compartmentalized expression of zebrafish ten-m3 and ten-m4, homologues of the Drosophila ten(m)/odd Oz gene, in the central nervous system. Mech Dev. 1999;87:223–227. doi: 10.1016/s0925-4773(99)00155-0. [DOI] [PubMed] [Google Scholar]
- Minet AD, Chiquet-Ehrismann R. Phylogenetic analysis of teneurin genes and comparison to the rearrangement hot spot elements of E. coli. Gene. 2000;257:87–97. doi: 10.1016/s0378-1119(00)00388-7. [DOI] [PubMed] [Google Scholar]
- Minet AD, Rubin BP, Tucker RP, et al. Teneurin-1, a vertebrate homologue of the Drosophila pair-rule gene ten-m, is a neuronal protein with a novel type of heparin-binding domain. J Cell Sci. 1999;112:2019–2032. doi: 10.1242/jcs.112.12.2019. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Nakamura M, Takahashi I, et al. An immunohistochemical study of localization of type I and type II collagens in mandibular condylar cartilage compared with tibial growth plate. Histochemistry. 1990;93:593–599. doi: 10.1007/BF00272201. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Shimokawa H, Fukada K, et al. Localization and inhibitory effect of basic fibroblast growth factor on chondrogenesis in cultured mouse mandibular condyle. J Bone Miner Metab. 2003;21:145–153. doi: 10.1007/s007740300023. [DOI] [PubMed] [Google Scholar]
- Rabie AB, Tan GH, Hägg U. Cbfa1 couples chondrocytes maturation and endochondral ossification in rat mandibular condylar cartilage. Arch Oral Biol. 2004;49:109–118. doi: 10.1016/j.archoralbio.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Roberts WE, Hartsfield JK., Jr Bone development and function: genetic and environmental mechanism. Semin Orthod. 2004;10:100–122. [Google Scholar]
- Rubin BP, Tucker RP, Martin D, et al. Teneurins: a novel family of neuronal cell surface proteins in vertebrates, homologous to the Drosophila pair-rule gene product Ten-m. Dev Biol. 1999;216:195–209. doi: 10.1006/dbio.1999.9503. [DOI] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Shen G, Darendeliler A. The adaptive remodeling of condylar cartilage – a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- Shibata S, Suda N, Suzuki S, et al. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–177. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukunami C, Shigeno C, Atsumi T, et al. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133:457–468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbermann M, Frommer J. The nature of endochondral ossification in the mandibular condyle of the mouse. Anat Rec. 1972;172:659–668. doi: 10.1002/ar.1091720406. [DOI] [PubMed] [Google Scholar]
- Symons NB. A histochemical study of secondary cartilage of the mandibular condyle in the rat. Arch Oral Biol. 1965;10:579–584. doi: 10.1016/0003-9969(65)90003-8. [DOI] [PubMed] [Google Scholar]
- Tojyo I, Yamaguchi A, Nitta T, et al. Effect of hypoxia and interleukin-1b on expression of tenascin-C in temporomandibular joint. Oral Dis. 2008;14:45–50. doi: 10.1111/j.1601-0825.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T. PCNA binding proteins. Front Biosci. 1999;4:D849–D858. doi: 10.2741/tsurimoto. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R. Teneurins: a conserved family of transmembrane proteins involved in intercellular signaling during development. Dev Biol. 2006;290:237–245. doi: 10.1016/j.ydbio.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R, Chevron MP, et al. Teneurin-2 is expressed in tissues that regulate limb and somite pattern formation and is induced in vitro and in situ by FGF8. Dev Dyn. 2001;220:27–39. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1084>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- Ueno T, Kagawa T, Kanou M, et al. Immunohistochemical observations of cellular differentiation and proliferation in endochondral bone formation from grafted periosteum: expression and localization of BMP-2 and -4 in the grafted periosteum. J Craniomaxillofac Surg. 2003;31:356–361. doi: 10.1016/j.jcms.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Kuroda M, Sok J, et al. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CM, Zaman G, Mosley JR, et al. Expression of tenascin-C in bones responding to mechanical load. J Bone Miner Res. 1997;12:52–58. doi: 10.1359/jbmr.1997.12.1.52. [DOI] [PubMed] [Google Scholar]
- Weiss A, Livne E, Silbermann M. Growth and differentiation of murine cartilage cells in vitro following a short-term exposure to triamcinolone acetonide. Cell Tissue Res. 1990;260:513–520. doi: 10.1007/BF00297231. [DOI] [PubMed] [Google Scholar]
- Yamashiro T, Fukunaga T, Yamashita K, et al. Gene and protein expression of brain-derived neurotrophic factor and TrkB in bone and cartilage. Bone. 2001;28:404–409. doi: 10.1016/s8756-3282(01)00405-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Topol L, Lee H, et al. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]


