Abstract
Articular cartilage composition and structure are maintained and remodeled by chondrocytes under the influence of loading. Exercise-induced changes in the composition, structure, mechanical properties and tissue integrity of growing and aging hamster articular cartilage were investigated. Articular cartilage samples (n = 191) were harvested from the proximal tibiae of hamsters aged 1, 3, 6, 12 and 15 months. The hamsters were divided into runners and controls. The runners had free access to a running wheel between 1 and 3 months (runner groups 3-, 12- and 15-month-old hamsters) or 1 and 6 months (runner group 6-month-old hamsters) of age. Control animals were subjected to a sedentary lifestyle. Mechanical indentation tests and depth-wise compositional and structural analyses were performed for the cartilage samples. Furthermore, the integrity of articular cartilage was assessed using histological osteoarthritis grading. Exercise affected the collagen network organization after a 5-month exercise period, especially in the middle and deep zones. However, no effect on the mechanical properties was detected after exercise. Before the age of 12 months, the runners showed less osteoarthritis than the controls, whereas at 15 months of age the situation was reversed. It is concluded that, in hamsters, physical exercise at a young age enhances cartilage maturation and alters the depth-wise cartilage structure and composition. This may be considered beneficial. However, exercise at a young age demonstrated adverse effects on cartilage at a later age with a significant increase in the incidence of osteoarthritis.
Keywords: articular cartilage, biomechanics, collagen, osteoarthritis, physical exercise
Introduction
The mechanical function of articular cartilage is determined by the organization and distribution of the collagen fibril network, proteoglycans (PGs) and interstitial water (Parsons & Black, 1987; Soltz & Ateshian, 1998; Wayne et al. 2003). Articular cartilage is repeatedly subjected to high impact forces during daily locomotion. The articulating joint surfaces are protected from potentially harmful excessive stresses by their exceptional mechanical properties. The integrity and function of articular cartilage are seriously jeopardized by the degenerative joint disease, osteoarthritis (OA) (Buckwalter & Martin, 1995). Minor changes in tissue integrity have been reported to alter tissue mechanical properties (Saarakkala et al. 2003). The mechanical properties of cartilage become inferior and the tissue may not be capable of bearing normal weights, transmitting forces and absorbing shocks over the articular surfaces as the OA progresses. This makes the tissue susceptible to further damage and may result in the advancement of OA. As a consequence, joint function deteriorates and mobility decreases (Buckwalter & Martin, 1995).
The composition of articular cartilage is heterogeneous over tissue depth with a high fraction of water and low concentration of PGs in the superficial zone; the water fraction decreases, whereas the PG content increases towards the deep cartilage zone (Linn & Sokoloff, 1965; Jones et al. 1977; Shapiro et al. 2001). The PGs are entrapped within the collagen fibril network that exhibits a characteristic depth-dependent architecture. The postnatal collagen network lacks the organized collagen structure, and the characteristic collagen network develops over a long period of time during skeletal maturation (Grunder, 2006; Hunziker et al. 2007; Julkunen et al. 2009; Rieppo et al. 2009). It has been proposed that the maturation of the articular cartilage collagen network is guided by the external loading conditions or results of physical exercise (Helminen et al. 2000; Grunder, 2006; Hunziker et al. 2007; Brama et al. 2009a). Several recent studies have supported these speculations in different animal models (Grunder, 2006; Hunziker et al. 2007; Brama et al. 2009a; Hyttinen et al. 2009; Julkunen et al. 2009; Rieppo et al. 2009). The postnatal collagen network is gradually reassembled from an unorganized structure into a highly organized collagen network. The maturation process takes a long time and multiple phenotypes of cartilage can be seen (e.g. unorganized cartilage, preferential collagen fibril direction parallel to the cartilage surface, multiple laminar structures and finally a classical Benninghoff-type trilaminar cartilage) (Benninghoff, 1925; Helminen et al. 2000; Hyttinen et al. 2001; Grunder, 2006; Hunziker et al. 2007; Brama et al. 2009a; Julkunen et al. 2009; Rieppo et al. 2009). The articular cartilage composition changes in parallel with the structural changes, and it has been shown that the biochemical composition changes during tissue maturation (Williamson et al. 2001; Klein et al. 2007; Julkunen et al. 2009). The collagen content increases especially in the deep tissue and the amount of PGs increases simultaneously (Klein et al. 2007; Julkunen et al. 2009; Rieppo et al. 2009). At an early age the articular cartilage demonstrates a rather homogeneous composition and structure, indicating that fibril network adaptation to loading and growth has not yet taken place (Grunder, 2006; Julkunen et al. 2009). The mature structure of articular cartilage may result from the division and activity of the superficial zone stem cells with the concomitant production of extracellular matrix molecules, PGs and collagen, with simultaneous tissue resorption and structural neoformation deeper in the tissue (Grunder, 2006; Hunziker et al. 2007). At this stage, the cartilage matrix turnover is still high. In adult articular cartilage, the biomechanical properties, together with the tissue composition and structure of the collagen network, vary topographically over different joint surfaces (Grunder, 2006; Brama et al. 2009b; Julkunen et al. 2009). It is known that, in mature cartilage, the matrix turnover is low, collagen turnover being much slower than the turnover of PGs (Maroudas, 1980; Bank et al. 1998; Helminen et al. 2000).
It has been shown that joint loading induces specific changes in the collagen network architecture by increasing the parallelism of collagen fibrils and affecting the depth-wise fibril orientation (Brama et al. 2009a). In particular, changes in the superficial collagen network have been reported (Hyttinen et al. 2001). The modification of cartilage composition on account of exercise could protect the joint from OA during later periods of life (Otterness et al. 1998; Helminen et al. 2000). Furthermore, it has been suggested that early exercise would advance the maturation of the PGs and collagen (Säämänen et al. 1987; van Weeren et al. 2008). Life-long moderate, although enforced, running of mice has been shown to increase the incidence and severity of OA (Lapveteläinen et al. 1995).
The aim of the present study was to assess the mechanical, compositional and structural changes that would be induced by physical exercise in hamster articular cartilage, and to distinguish them from changes resulting from growth and maturation. Furthermore, we assessed whether the observed exercise-induced changes are long-lasting or transient. In addition, we aimed to evaluate whether the exercise is beneficial for articular cartilage integrity in older age. We hypothesized that voluntary physical exercise in the running wheel at a young age improves tissue properties, and strengthens the collagen network, improving the tissue resistance against osteoarthritic degeneration later in life (Helminen et al. 2000).
Materials and methods
Samples
Female Syrian golden hamsters (purchased from Harlan, The Netherlands) were used (n = 191). The animals were kept in individual cages (365 mm length × 207 mm width × 140 mm height). Half of the cages had running wheels. The development of articular cartilage was examined in specimens of the tibial medial plateaus, at the ages of 1, 3, 6, 12 and 15 months, from sedentary hamsters and from hamsters that had access to running wheels from 1 to 3 months or from 1 to 6 months of age (Fig. 1). The activity of the runner animals on the running wheel was recorded with infrared sensors connected to a computer (Lapveteläinen et al. 1997). The daily and total running distances of the runners from 1 to 3 months were 13.2 ± 2.3 km day−1 and 832 ± 160 km, respectively, whereas the distances of the animals running from 1 to 6 months of age were 12.2 ± 1.0 km day−1 and 1886 ± 155 km, respectively. Sedentary control animals had no access to running wheels and were not subjected to any high-speed or high-force work; however, they were allowed to move freely in their cages. The animals were weighed at 1 month of age, then monthly, and at killing. The hamster were made to sleep with carbon dioxide inhalation and then killed by cervical dislocation. Permission to conduct the experiments was obtained from the Animal Care and Use Committee of the University of Eastern Finland, Kuopio, Finland. The samples from the right tibial medial plateaus were prepared for microscopic and spectroscopic analyses, and those from the left tibial medial plateaus were prepared for mechanical testing.
Fig. 1.
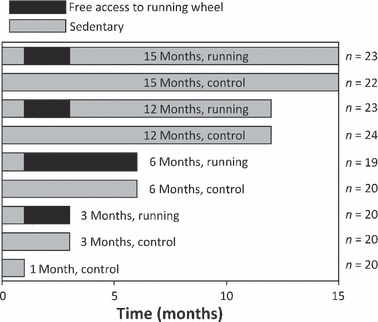
Duration of physical exercise of the hamsters in different age-groups. Control hamster groups are indicated with fully gray bars, whereas the black bars indicate the time and duration of the access of hamsters to running wheels. The number of animals in each group is presented next to the bars.
Mechanical moduli
The intact left medial tibial condyles, also including the underlying bone, were prepared by cleaning the surface and adjusting the articular surface perpendicular to the indenter at the contact site on the medial plateau. The samples underwent mechanical indentation under stress relaxation in four steps (step-wise strain of 7% from the prevailing sample thickness). An impermeable, plane-ended indenter with a diameter of 0.210 mm was used. A mechanical testing device equipped with a precision motion controller (PM500-C, Newport, Irvine, CA, USA) and a 50-g load-cell (resolution 0.01 g, Sensotec, Columbus, OH, USA) was used to perform the mechanical testing. Before the experiments, the samples were equilibrated under 0.15 g preload. Instantaneous loading was conducted with a ramp compression rate of 10 mm s−1 and the relaxation time after each compression was 300 s. The thickness of the uncalcified cartilage of the samples was measured by using the needle technique (Morpho Insect Pins, 000/0.25 mm) (Hoch et al. 1983; Jurvelin et al. 1995).
From the mechanical test, the isotropic equilibrium modulus was determined using the equilibrium points of each step-relaxation, whereas the instantaneous modulus was determined using the ramp response at the second step-compression. The modulus values were calculated using Hayes’ solution (Hayes et al. 1972), which accounts for the indenter diameter and shape, as well as the sample thickness and Poisson's ratio. Poisson's ratios of 0.15 and 0.5 were assumed when calculating the equilibrium and instantaneous modulus, respectively (Mak et al. 1987; Jurvelin et al. 1997).
Proteoglycan content distribution
Digital densitometry was used for the semiquantitative determination of spatial PG content (assessed by optical density) (Kiviranta et al. 1985). Microscopic sections (3 μm thick) were cut, at right angles with the cartilage surface, from formalin-fixed and paraffin-embedded cartilage specimens and stained with Safranin O. Paraffin was dissolved with xylene from the sections prior to staining. Five parallel sections for each cartilage sample were analyzed, averaged and normalized with the pixel area. In the depth-wise analysis, samples were investigated in 100 layers. Detailed descriptions of the densitometry technique and Safranin O staining were published previously (Kiviranta et al. 1985; Király et al. 1996).
Collagen orientation and parallelism index
The depth-wise collagen fibril orientation and parallelism index (PI) from the samples described above were analyzed with the enhanced polarized light microscopy system (Ortholux II POL, Leitz Wetzlar, Wetzlar, Germany) using 7-μm-thick unstained sections. The enhanced polarized light microscopy data were measured by capturing a series of eight background corrected images (0°, 15°, 30°, 45°, 60°, 75°, 90° and 90° + phase shifter). Crossed polarizers were rotated between images and the light intensity was measured with a charged coupled device camera for each pixel separately. Images were used for the calculation of collagen network parallelism and orientation as described earlier (Rieppo et al. 2008). Multiple sections were measured for each specimen and the measurements were averaged before the final calculations. Subsequently, image pixels were horizontally averaged to obtain depth-wise collagen orientation profiles. The PI characterizes the degree of collagen fibril parallelism. A high PI at a specific pixel indicates that a majority of the collagen fibrils in the pixel run in the same direction. Correspondingly, a low PI represents tissue with more randomly arranged collagen fibrils. The orientation angle of collagen fibrils was defined as the angle between the joint surface and the measured average length-wise orientation of collagen fibrils. In the depth-wise analysis, the samples were investigated in 100 equally thick layers. A detailed description of the technique for the image analysis of the collagen PI and orientation in cartilage was published previously (Rieppo et al. 2008).
Collagen content distribution
For the evaluation of collagen content, microscopic unstained 5-μm-thick sections were prepared for Fourier transform infrared (FTIR) imaging spectroscopy measurements. Sections were placed on BaF2 windows for the measurements. Measurements were conducted using the Spectrum Spotlight 300 imaging system (Perkin Elmer, Shelton, CO, USA). The spatial pixel resolution was 6.25 μm, spectral resolution was set to 8 cm−1, and a spectral region of 670–2000 cm−1 was investigated. The depth-wise collagen content of the samples was evaluated in 100 layers by measuring the integrated absorbance of the amide I peak (1585–1720 cm−1) from the infrared absorption spectrum (Camacho et al. 2001; Boskey & Pleshko Camacho, 2007; Saarakkala et al. 2010).
Tissue integrity
Histological grading was used to determine the tissue integrity and OA. Grading was performed in a blind manner by two authors. The five grades (Grades 0–4) were as follows (Lapveteläinen et al. 1995). Grade 0: the cartilage was intact and articular surfaces were smooth. Grade 1: superficial fibrillation was detected. Grade 2: lesion extending into the deep zone was detected. Grade 3: lesion surpassing the tidemark and affecting the calcified cartilage was detected. Further, the loss of cells in the superficial zone and formation of clones in the deep zone were observed. Grade 4: no cartilage tissue was left between the two adjacent bone ends. Therefore, no mechanical or compositional analyses were performed on samples with Grade 4 OA.
Statistical analyses
Age-dependent changes in the depth-wise composition and structure as well as mechanical parameters, tissue thickness and animal weight gain were analyzed using one-way anova with least significant difference adjustment between the consecutive age-groups. This was performed separately for the runner and control groups, with the exception that the 3-month age-group was compared with both the 6-month and 12-month age-groups. However, due to differences in the exercise duration, the 6-month age-group was not compared with the 12-month age-group (Fig. 1). Exercise- and maturation-induced changes in tissue integrity (OA grade) were tested using a Mann–Whitney U-test. Statistical analyses for mechanical tests, tissue thickness and animal weights were performed with SPSS (version 16, SPSS Inc., Chicago, IL, USA). The mechanical properties, tissue thickness and animal weight-gain were presented as mean ± SD. The threshold level for statistical significance was set to P < 0.05.
The structural and compositional profiles were analyzed depth-wise, and the profiles were resampled to 100 depth-wise points over the tissue thickness. The absolute tissue depth used in the comparisons was dependent on the individual thickness of the sample in question. The statistical significance of the differences in the depth-wise profiles between the groups was interpreted and presented using the 95% confidence intervals for the differences. The difference between the groups was interpreted as significant (P < 0.05) if the confidence interval did not cross zero difference. The depth-wise comparisons were conducted with Matlab (version 2007b, MathWorks Inc., Natick, MA, USA).
Results
Thickness, weight gain and mechanical properties
No significant changes were observed in the mechanical properties between the controls and runners at any time-point. The instantaneous modulus displayed the highest values (P < 0.05) at the age of 3 months, whereas the equilibrium modulus reached the values of the more mature animals at this time-point (Fig. 2A,B). The instantaneous modulus reached the value of adult animals at 6 months of age (Fig. 2B). With growth and maturation, the tissue thickness decreased significantly (P < 0.05) and continued to become thinner up to the age of 12 months (Fig. 2C). Overall, the tissue thickness was only slightly affected by the physical exercise. However, at 15 months of age, the cartilage thickness of the runners was significantly greater (P < 0.05) than that of the controls. The weight gain of the animals during growth and maturation was significantly (P < 0.05) higher in the runners than in the controls (Fig. 2D). However, the weight difference disappeared after the exercise training had ended, and no significant difference was observed at the age of 15 months between the runners and controls. A significant decrease in weight (P < 0.05) was recorded at the age of 15 months in the runners.
Fig. 2.
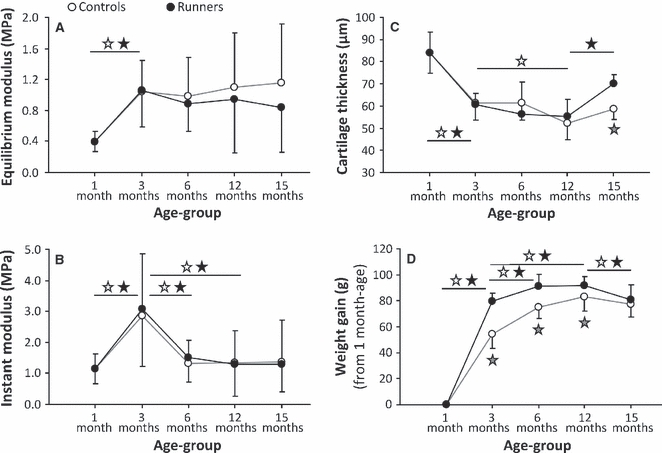
Mechanical (equilibrium and instant) moduli (A,B), cartilage thickness (C) and weight gain (D) as a function of age. Group-wise mean ± SD values are presented. White and black stars denote significant (P < 0.05) differences between the controls (○) and runners (•) of consecutive groups, respectively. Gray stars indicate a significant (P < 0.05) difference between the controls and runners within a certain age-group. Note that the age-scale is not linear.
Proteoglycan content
Between the ages of 1 and 3 months, the PG content of articular cartilage increased significantly (P < 0.01) over almost the entire depth of the tissue in both the runner and control groups (Figs 3A and 4A). The PG content was observed to decrease significantly (P < 0.05) in the deep zone between the ages of 3 and 6 months in both groups (Fig. 4B). In the control group, a significant (P < 0.05) decrease was observed in the PG content in the deep zone between the ages of 3 and 12 months (Fig. 4C), whereas a clear decrease (P < 0.05) was observed in the PG content between the ages of 12 and 15 months over the entire tissue depth (Fig. 4D). Only a slight, but significant (P < 0.05), decrease in the PG content was observed above the deep zone of the control group between the ages of 12 and 15 months. Within the age-groups, only minor differences were observed between the runners and controls. Significant (P < 0.05) differences between these groups were observed in the superficial zone at the age of 3 and 6 months, and in the deep zone at the age of 12 months (Fig. 4E–H).
Fig. 3.
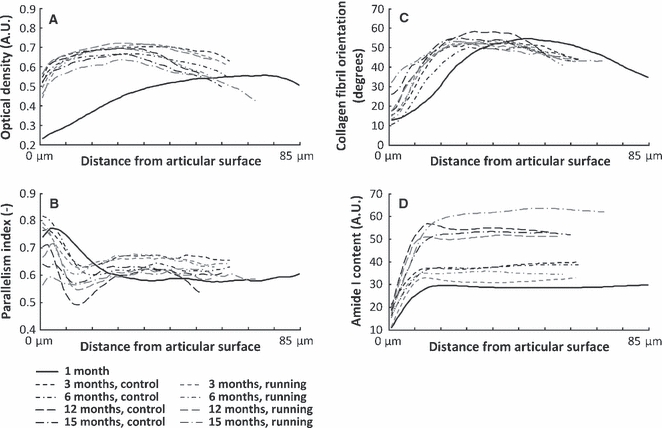
Depth-wise profiles for proteoglycan content (A), quantified as optical density, parallelism index (B), collagen fibril orientation angle (C) and amide I content (D), are presented as group-wise mean profiles for different age-groups, and separately for the sedentary controls and runners. Statistical comparison of the depth-wise profiles between the groups is presented in Figs 4–7. A.U., absorption unit.
Fig. 4.
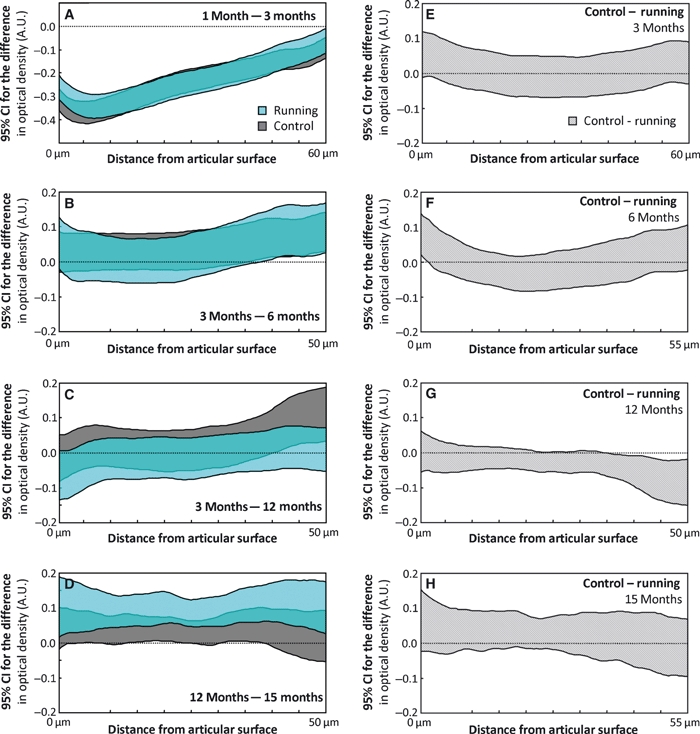
Maturation-dependent changes in the proteoglycan (PG) content, quantified as optical density, are presented as 95% confidence intervals (CIs) of the difference between consecutive age-groups for both the control and runner groups (A–D). Similarly, the exercise-dependent differences between the controls and runners are presented between ages (E–H). The CIs are presented as a function of tissue depth. The maximum distance from the articular surface depends on the thickness of the specimen within the compared groups. A difference below the value of 0 indicates that the older age-group (A–D) or runner group (E–H) has a higher PG content. Due to differences in the exercise modes, the 12-month age-group is not compared with the 6-month age-group, but with the 3-month age-group with similar exercise mode. A.U., absorption unit. The differences in each panel should be interpreted according to the title of the panel, e.g. the content at 1 month of age minus the content at 3 months of age (A).
Collagen fibril orientation and parallelism index
The PI of both runners and controls increased significantly (P < 0.05) in the deep zone and decreased significantly (P < 0.05) in the middle zone between the ages of 1 and 3 months (Figs 3B and 5A). Between 3 and 12 months, the PI decreased (P < 0.05) at and below the middle zone of the runners and controls (Fig. 5C). Between the ages of 12 and 15 months, the PI decreased (P < 0.05) in the superficial and middle zones of both the controls and runners, whereas in the upper deep zone an increase (P < 0.05) in the PI was observed in the controls (Fig. 5D).
Fig. 5.
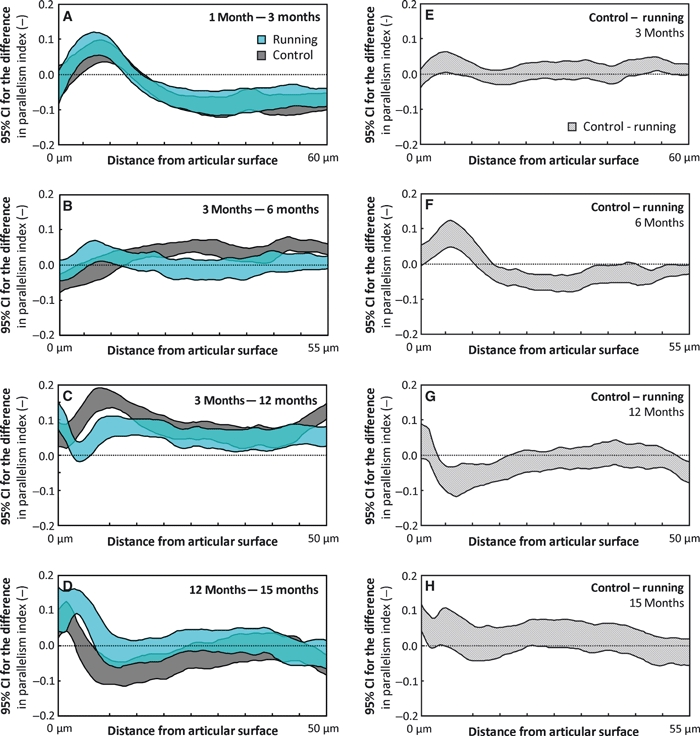
Maturation-dependent changes in the parallelism index (PI) are presented as 95% confidence intervals (CIs) of the difference between consecutive age-groups for both the control and runner groups (A–D). Similarly, the exercise-dependent differences between the controls and runners are presented between ages (E–H). The CIs are presented as a function of tissue depth. The maximum distance from the articular surface depends on the thickness of the specimen within the compared groups. A difference below the value of 0 indicates that the older age-group (A–D) or runner group (E–H) has a higher PI value. Due to differences in the exercise modes, the 12-month age-group is not compared with the 6-month age-group, but with the 3-month age-group with similar exercise mode. The differences in each panel should be interpreted according to the title of the panel, e.g. the PI at 1 month of age minus the PI at 3 months of age (A).
Significant effects of physical exercise were observed in the PI from the age of 6 to 15 months (Fig. 5F–H). No significant exercise-induced differences were observed at the age of 3 months (Fig. 5E). At the age of 6 months, when the runners had run for 5 months, i.e. 3 months more than in the other runner groups, the PI was significantly (P < 0.05) higher in the upper deep zone of the runners and significantly (P < 0.05) lower in the middle zone (Fig. 5F). However, in the 12-month-old runners, the PI was significantly (P < 0.05) higher in the middle zone only (Fig. 5G).
Between the ages of 1 and 3 months, in both the controls and runners, the collagen fibrils oriented more perpendicularly to the surface in the middle zone (P < 0.05, Figs 3C and 6A). Between the ages of 3 and 6 months, no significant differences were observed in the collagen orientation angle of the runners (Fig. 6B), whereas a significant (P < 0.05) reorientation towards a more parallel-to-surface orientation was observed in the superficial and middle zones of the controls. Between the ages of 3 and 12 months, the fibril orientation became more perpendicular to the surface below the superficial zone of the controls (P < 0.05) and no significant changes were observed in the runners (Fig. 6C). From 12 to 15 months of age, the collagen fibril orientation became significantly (P < 0.05) more perpendicular to the surface in the superficial zone (Fig. 6D).
Fig. 6.
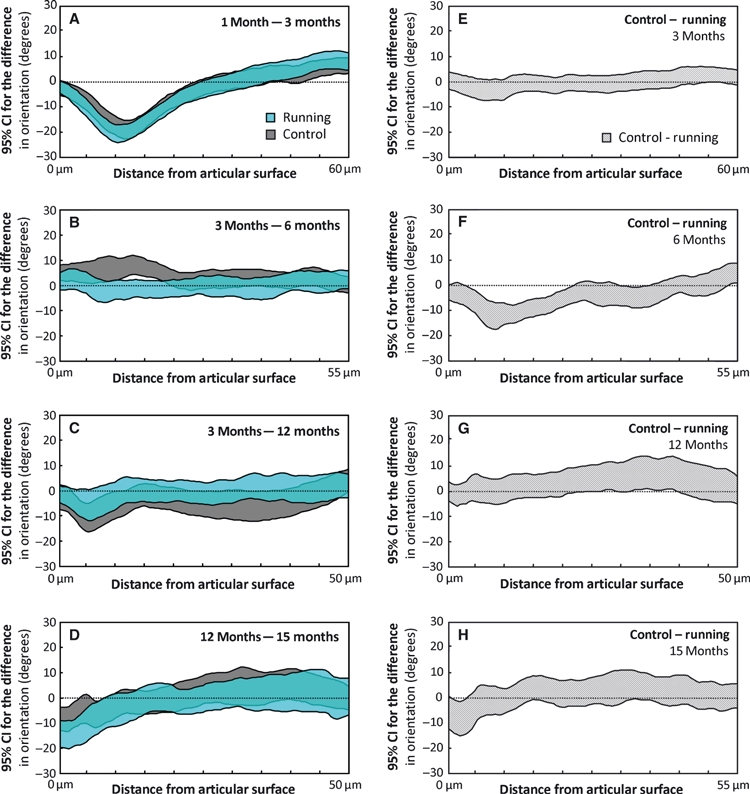
Maturation-dependent changes in the collagen fibril orientation are presented as 95% confidence intervals (CIs) of the difference between consecutive age-groups for both the control and runner groups (A–D). Similarly, the exercise-dependent differences between the controls and runners are presented between ages (E–H). The CIs are presented as a function of tissue depth. The maximum distance from the articular surface depends on the thickness of the specimen within the compared groups. A difference below the value of 0 indicates that the older age-group (A–D) or runner group (E–H) has more collagen fibrils with a perpendicular-to-surface orientation. Due to differences in the exercise modes, the 12-month age-group is not compared with the 6-month age-group, but with the 3-month age-group with similar exercise mode. The differences in each panel should be interpreted according to the title of the panel, e.g. the orientation angle at 1 month of age minus the orientation angle at 3 months of age (A).
Although running exercise had a negligible effect on the collagen fibril orientation at 3 months of age (Fig. 6E), at 6 months of age the fibril orientation was more perpendicular to the surface in the middle zone of the runners (P < 0.05, Fig. 6F). Furthermore, the collagen fibril orientation of the running hamsters was significantly (P < 0.05) more perpendicular to the surface in the superficial zone at the age of 15 months compared with the sedentary hamsters (Fig. 6H).
Collagen content
Between the ages of 1 and 3 months, the collagen content in the control animals increased over the entire tissue depth (P < 0.05), whereas, in the runners, a significant (P < 0.05) increase in collagen content was observed only in the middle zone (Figs 3D and 7A). Between the ages of 3 and 12 months, a clear increase (P < 0.05) in collagen content was observed in both groups (Fig. 7B). The controls had a significantly (P < 0.05) higher collagen content in the superficial and deep zones (Fig. 7C) compared with the runners in the 3-month-old age-group.
Fig. 7.
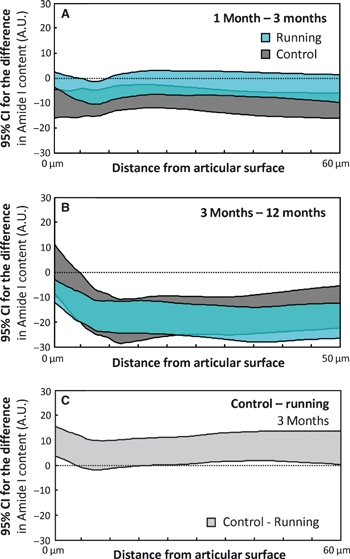
Maturation-dependent changes in the collagen content, as quantified by FTIR-derived amide I content, are presented as 95% confidence intervals (CIs) of the difference between consecutive age-groups [1 month and 3 months (A), and 3 months and 12 months (B)] for both the control and runner groups. Similarly, the exercise-dependent differences between the controls and runners are presented at the age of 3 months (C). The CIs are presented as a function of tissue depth. A difference below the value of 0 indicates that the older age-group (A,B) or runner group (C) has the higher collagen content. Due to differences in the exercise modes, the 12-month age-group is not compared with the 6-month age-group, but with the 3-month age-group with similar exercise mode. A.U., absorption unit. The differences in each panel should be interpreted according to the title of the panel, e.g. the content at 1 month of age minus the content at 3 months of age (A).
Tissue degradation
When comparing the OA grading of the different age-groups, as well as the effect of physical exercise on the OA grade, it was observed that the OA grades overall became higher with age (Fig. 8). In the 15-month-old hamsters, the runners exhibited significantly higher (P < 0.05) OA grades (Fig. 8), even though in the younger groups runners showed a trend to less OA.
Fig. 8.
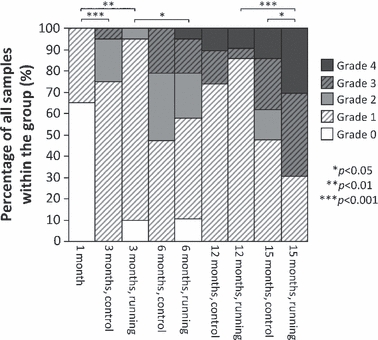
Osteoarthritis (OA) grading in different age-groups and separately in running and control groups. Percentage of each OA grade (0–4) of all samples within each group is presented. Furthermore, significant differences between the overall OA grades within the groups are indicated above the bar-plot.
Discussion
Growth, maturation and aging of the hamsters, as well as physical exercise, were able to bring about significant alterations in the structure and properties of articular cartilage. For example, an effect of physical exercise on the weight gain and cartilage thickness at the age of 15 months was demonstrated up until 12 months after the exercise had ended. The mechanical properties of the full-thickness articular cartilage specimens did not seem to be affected by the voluntary physical exercise. However, when the samples were analysed spatially throughout the cartilage depth, significant changes in the cartilage structure and composition were observed to have been induced by the physical exercise. Some of these changes were immediate, whereas some appeared gradually with time.
In particular, the properties of the superficial zone, middle zone and the upper part of the deep zone displayed major alterations during growth, aging and physical exercise (Fig. 3). We found that the surface PG content was significantly (P < 0.05) lower in the runners than in the controls after the 5-month exercise period at the age of 6 months (Fig. 4F). A similar, but non-significant, trend was observed at the age of 3 months after a 2-month exercise period (Fig. 4E). Similar findings have been reported at the same joint site in beagle dogs after strenuous running exercise (Kiviranta et al. 1992; Arokoski et al. 1993). A decrease in the surface PG content has commonly been associated with a deterioration of tissue characteristics in OA. However, the decrease could actually be the result of the adaptation of tissue properties to altered functional demands and this may explain some of the findings of the present study. Physical exercise was previously demonstrated to enhance the maturation and aging of the PG matrix of rabbit articular cartilage (Säämänen et al. 1987). In the present study, the hamsters were active runners, covering a distance of on average more than 12 km day−1 on the running wheel.
Importantly, the collagen architecture was modified significantly by the long-term exercise, as was observed in the 6-month age-group (Figs 5F and 6F), and supported by an earlier study (Arokoski et al. 1996). However, such differences were not observed in the 3-month-old runner group. Remodeling of the collagen architecture could reflect an attempt of the tissue to reach optimal functional properties, concomitant with altered cellular biosynthesis, and occurring slowly over a longer period of time. This could explain why the changes in the PI due to exercise or weight gain were observed in the 12-month-old group but not in the 3-month-old animals (Fig. 5E,G).
One limitation in our testing protocol is that the amount of running activity was not controlled within the runner groups but instead the exercise was voluntary. However, the coefficient of variation of the actual workload per animal in the runner groups was < 20%. Nevertheless, this variation may have left potential compositional and structural changes out of reach with the present methods. Also, there was a significant difference (P < 0.01) in the daily running activity between the animals running between the ages of 1 and 3 months (13.2 ± 2.3 km day−1) compared with those running between the ages of 1 and 6 months (12.2 ± 1.0 km day−1). Therefore, the rate of running activity seemed to decrease with age, as has been observed previously (Gattermann et al. 2004). However, the difference in the running activity of the 6-month animals exerted no major influence on the results on the effects of exercise.
The voluntary running wheel exercise performed by the runner group hamsters was considered to be equal to the amount of activity that they would have encountered in their natural environment. This assumption was supported by the similar activity levels shown in laboratory and wild-type hamsters of similar age during circadian rhythm activity studies (Weinert et al. 2001; Gattermann et al. 2002). We observed that the hamsters were most active during the night. However, in a previous study, it was shown that the hamsters are nocturnal only in captivity (laboratory environment), being crepuscular (most active at dawn and dusk) in their natural environment (Gattermann et al. 2008). It is understandable that the hamsters are exposed to different types of stresses in the laboratory environment compared with their natural environment. In this study, the living arrangements were similar for both the sedentary and runner animals, with the exception of the running wheels. Therefore, the possible laboratory stress factors similarly affected both the sedentary and runner animals. However, the potential stress caused by limiting the ability to undertake voluntary exercise cannot be excluded in the present study.
The development of the mechanical properties, such as instantaneous modulus, until musculoskeletal maturity of hamster articular cartilage (Fig. 2) was found to be similar to that of rabbit articular cartilage (Julkunen et al. 2009). The instantaneous modulus apparently increased as a result of the remodeling of the collagen network structure simultaneously with the advancement of the endochondral ossification front from the secondary ossification center of the epiphysis (Figs 2B, 3, 5–7). Such a change was not observed in the equilibrium modulus (Fig. 2A), possibly because the equilibrium modulus depends more strongly on the PGs of the tissue (Fig. 4). However, the determination of the mechanical properties in small animals is rather challenging due to the low forces and small deformations in the low-thickness cartilage. Hence, in the present study the lack of observed exercise-induced changes in the mechanical tissue properties may be explained by the high variance in the data among the groups within which the changes disappear. In the present study, we investigated cartilage of the medial tibial plateau. Therefore, we may not have been able to observe clear extra laminae in the deep zone, as has been visualized in, e.g. the femoral cartilage of the sheep or rabbit (Grunder, 2006; Julkunen et al. 2009). During maturation, the collagen and PG contents increase (Hyttinen et al. 2009; Julkunen et al. 2009; Rieppo et al. 2009), as was also observed in the present study (Figs 3, 4 and 7).
The effects of two different modes of physical exercise were investigated. Most of the runners had free access to running wheel over a 2-month period (age-groups 3, 12 and 15 months), whereas one runner group had free access to a running wheel over a 5-month period (6-month age-group). It is possible that physical exercise speeds up the remodeling of the collagen fibril network during maturation. This hypothesis is supported by the findings with the 6-month-old animals because: (i) the PI of the runners was elevated in the deep zone (Fig. 5F) and (ii) the orientation of the collagen fibrils was more perpendicular to the surface at and below the middle zone as compared with the controls (Fig. 6F) (Grunder, 2006; Shinar & Navon, 2006; Rieppo et al. 2009; Julkunen et al. 2010).
Immediate changes in the composition and structure were observed between the controls and runners. The most profound changes occurred after a longer period of exercise, i.e. in 6-month-old runners. These included changes in the collagen PI and orientation. In addition, the superficial PG content decreased due to physical exercise (Fig. 4). After a shorter period of exercise (2 months), only minor differences were observed between the controls and runners: (i) the collagen content and (ii) the PI in the deep zone were decreased due to physical exercise (Figs 5 and 7). Such effects were not observed in the depth-wise composition after exercise for 5 months. Thus, the long-term effects of the short exercise period were less clear. However, an increase in the middle and upper deep zone PI at 12 months and a decrease in the superficial zone PI at 15 months were observed and were possibly caused by the physical exercise at an early age (Fig. 5G,H). This may be related to the reduced cartilage integrity and potential fibrillation of the tissue in the runners.
Exercise of the hamsters at 3 months of age reduced changes typical of OA, corroborating earlier findings (Otterness et al. 1998). A similar trend was noticed in the 6-month-old and 12-month-old animals, but with a less obvious reduction of OA changes (Fig. 8). However, at a later age (15 months), the runners demonstrated significantly reduced (P < 0.05) cartilage integrity as compared with the control hamsters. It is possible that the active physical exercise at a young age resulted in subtle alterations of the cartilage surface, resulting in more OA changes later in life. In addition, the body weights of the runners were higher than those of the control animals. High body weight is known to contribute to a higher rate of OA. The biosynthesis of the cartilage matrix by chondrocytes may also be altered in the long term due to physical exercise at a young age, suggesting that a continued higher stimulus level is required to maintain an optimal level of biosynthesis. However, the balance of the anabolic and catabolic activity of the chondrocytes may be disturbed by the lack of adequate cell stimulation via physical exercise. This can ultimately result in earlier impairment of tissue integrity. Similarly, with life-long moderate non-voluntary exercise, OA scoring revealed that the controls had better tissue integrity at an older age compared with running mice (Lapveteläinen et al. 1995).
It has been suggested that cartilage maturation is advanced due to physical exercise (van Weeren et al. 2008), which was also suggested by the present study. It can even be hypothesized that, with time, cartilage becomes more prone to injuries due to the advancement of the maturation process (van Weeren et al. 2008). After reaching maturity, the remodeling process of the collagen network and PGs slows down significantly (Helminen et al. 2000), causing the tissue remodeling only to occur as a result of external loading or lack of physical exercise. Slow turnover can also decrease the ability of the tissue to show repair. This could explain our observation of decreased tissue integrity in old age, as a result of reduced physical exercise at a younger age (Fig. 8).
The body weight of the runner animals was higher than that in the control group from 3 to 12 months of age (Fig. 2D). This was probably due to the greater muscle mass of the runners (Borer et al. 1979). It is possible that, in hamsters, exercise at a young age protects against OA. At an older age the difference between the sedentary and runner animals disappears (Fig. 8), which was also observed as a thickening of the cartilage in runners (Fig. 2C). Similar indications have been observed in humans (Slemenda et al. 1997).
Hamsters can be considered skeletally mature at the age of 6 months, whereas they are sexually mature at about 4–6 weeks of age (Otterness et al. 1994). Syrian hamsters have a lifespan of 2–3 years, indicating that the oldest investigated group in this study probably represents middle-aged or slightly older animals. Compared with humans, the cartilage of hamsters is significantly thinner, possibly meaning that the shear stresses within the cartilage matrix are higher than in humans. This has been suggested in hamsters by the frequent separation of the cartilage at the tidemark. This probably contributes to the increased incidence of OA (Meachim & Illman, 1967; Otterness et al. 1999). As this is more common in small animals than in large mammals, the hamsters are probably more prone to OA than humans. However, the wheel-running exercise of hamsters at an intensity of 12 km day−1 for 11 days did not increase tidemark separation (Otterness et al. 1999). Furthermore, in contrast to other species, like humans, hamsters gain weight during the exercise (Gattermann et al. 2004). Overall, it is difficult to directly translate the findings of the present study to humans, probably except for the early months of growth and maturation.
In conclusion, modification of the depth-wise structure and composition of PGs and collagen probably has a significant impact on the integrity, durability and maintenance of articular cartilage, as well as on its functional properties at a later age. The functional changes of the mechanical properties of cartilage after physical exercise were minor as compared with the effects of maturation but appear to be significant in relation to the long-term integrity of the tissue. Together, the aforementioned exercise-related modifications of the tissue seemed to improve the integrity of articular cartilage at an early age. It was noteworthy that, in comparison to the life-long sedentary way of living, active running exercise at a young age did not protect articular cartilage from poor tissue integrity at an older age (Fig. 8).
Acknowledgments
Funding from the Ministry of Education, Finland to the University of Eastern Finland (project 5765) and Academy of Finland (projects 110595 and 113112) is acknowledged.
References
- Arokoski J, Kiviranta I, Jurvelin J, et al. Long-distance running causes site-dependent decrease of cartilage glycosaminoglycan content in the knee joints of beagle dogs. Arthritis Rheum. 1993;36:1451–1459. doi: 10.1002/art.1780361018. [DOI] [PubMed] [Google Scholar]
- Arokoski JP, Hyttinen MM, Lapveteläinen T, et al. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann Rheum Dis. 1996;55:253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank RA, Bayliss MT, Lafeber FP, et al. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Function. Zeitschrift für Zellforschung. 1925;2:783–862. [Google Scholar]
- Borer KT, Hallfrisch J, Tsai AC, et al. The effect of exercise and dietary protein levels on somatic growth, body composition, and serum lipid levels in adult hamsters. J Nutr. 1979;109:222–228. doi: 10.1093/jn/109.2.222. [DOI] [PubMed] [Google Scholar]
- Boskey A, Pleshko Camacho N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28:2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brama PA, Holopainen J, van Weeren PR, et al. Effect of loading on the organization of the collagen fibril network in juvenile equine articular cartilage. J Orthop Res. 2009a;27:1226–1234. doi: 10.1002/jor.20866. [DOI] [PubMed] [Google Scholar]
- Brama PA, Holopainen J, van Weeren PR, et al. Influence of exercise and joint topography on depth-related spatial distribution of proteoglycan and collagen content in immature equine articular cartilage. Equine Vet J. 2009b;41:557–563. doi: 10.2746/042516409x424162. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Martin J. Degenerative joint disease. Clin Symp. 1995;47:1–32. [PubMed] [Google Scholar]
- Camacho NP, West P, Torzilli PA, et al. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62:1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Weinandy R, et al. Comparative studies of body mass, body measurements and organ weights of wild-derived and laboratory golden hamsters (Mesocricetus auratus) Lab Anim. 2002;36:445–454. doi: 10.1258/002367702320389125. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Weinandy R, Fritzsche P. Running-wheel activity and body composition in golden hamsters (Mesocricetus auratus) Physiol Behav. 2004;82:541–544. doi: 10.1016/j.physbeh.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Johnston RE, Yigit N, et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder W. MRI assessment of cartilage ultrastructure. NMR Biomed. 2006;19:855–876. doi: 10.1002/nbm.1092. [DOI] [PubMed] [Google Scholar]
- Hayes WC, Keer LM, Herrmann G, et al. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- Helminen HJ, Hyttinen MM, Lammi MJ, et al. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. J Bone Miner Metab. 2000;18:245–257. doi: 10.1007/pl00010638. [DOI] [PubMed] [Google Scholar]
- Hoch DH, Grodzinsky AJ, Koob TJ, et al. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1:4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hyttinen MM, Arokoski JP, Parkkinen JJ, et al. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarthritis Cartilage. 2001;9:694–701. doi: 10.1053/joca.2001.0466. [DOI] [PubMed] [Google Scholar]
- Hyttinen MM, Holopainen J, Rene van Weeren P, et al. Changes in collagen fibril network organization and proteoglycan distribution in equine articular cartilage during maturation and growth. J Anat. 2009;215:584–591. doi: 10.1111/j.1469-7580.2009.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IL, Larsson SE, Lemperg R. The glycosaminoglycans of human articular cartilage: concentration and distribution in different layers in the adult individual. Clin Orthop Relat Res. 1977;127:257–264. [PubMed] [Google Scholar]
- Julkunen P, Harjula T, Iivarinen J, et al. Biomechanical, biochemical and structural correlations in immature and mature rabbit articular cartilage. Osteoarthritis Cartilage. 2009;17:1628–1638. doi: 10.1016/j.joca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Iivarinen J, Brama PA, et al. Maturation of collagen fibril network structure in tibial and femoral cartilage of rabbits. Osteoarthritis Cartilage. 2010;18:406–415. doi: 10.1016/j.joca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Räsänen T, Kolmonen P, et al. Comparison of optical, needle probe and ultrasonic techniques for the measurement of articular cartilage thickness. J Biomech. 1995;28:231–235. doi: 10.1016/0021-9290(94)00060-h. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of Poisson's ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30:235–241. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- Király K, Lapveteläinen T, Arokoski J, et al. Application of selected cationic dyes for the semiquantitative estimation of glycosaminoglycans in histological sections of articular cartilage by microspectrophotometry. Histochem J. 1996;28:577–590. doi: 10.1007/BF02331378. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Jurvelin J, Tammi M, et al. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82:249–255. doi: 10.1007/BF00501401. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Tammi M, Jurvelin J, et al. Articular cartilage thickness and glycosaminoglycan distribution in the canine knee joint after strenuous running exercise. Clin Orthop Relat Res. 1992;283:302–308. [PubMed] [Google Scholar]
- Klein TJ, Chaudhry M, Bae WC, et al. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech. 2007;40:182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Lapveteläinen T, Nevalainen T, Parkkinen JJ, et al. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec. 1995;242:159–165. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- Lapveteläinen T, Tiihonen A, Koskela P, et al. Training a large number of laboratory mice using running wheels and analyzing running behavior by use of a computer-assisted system. Lab Anim Sci. 1997;47:172–179. [PubMed] [Google Scholar]
- Linn FC, Sokoloff L. Movement and composition of interstitial fluid of cartilage. Arthritis Rheum. 1965;8:481–494. doi: 10.1002/art.1780080402. [DOI] [PubMed] [Google Scholar]
- Mak AF, Lai WM, Mow VC. Biphasic indentation of articular cartilage. I. Theoretical analysis. J Biomech. 1987;20:703–714. doi: 10.1016/0021-9290(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Metabolism of cartilaginous tissues: a quantitative approach. In: Maroudas A, Holborow EJ, editors. Studies in Joint Disease. Vol. 1. Tunbridge Wells: Pitman Medical; 1980. pp. 59–86. [Google Scholar]
- Meachim G, Illman O. Articular cartilage degeneration in hamsters and in pigs. Z Versuchstierkd. 1967;9:33–46. [Google Scholar]
- Otterness IG, Bliven ML, Milici AJ, et al. Comparison of mobility changes with histological and biochemical changes during lipopolysaccharide-induced arthritis in the hamster. Am J Pathol. 1994;144:1098–1108. [PMC free article] [PubMed] [Google Scholar]
- Otterness IG, Eskra JD, Bliven ML, et al. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41:2068–2076. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Otterness IG, Chang M, Burkhardt JE, et al. Histology and tissue chemistry of tidemark separation in hamsters. Vet Pathol. 1999;36:138–145. doi: 10.1354/vp.36-2-138. [DOI] [PubMed] [Google Scholar]
- Parsons JR, Black J. Mechanical behavior of articular cartilage quantitative changes with enzymatic alteration of the proteoglycan fraction. Bull Hosp Jt Dis Orthop Inst. 1987;47:13–30. [PubMed] [Google Scholar]
- Rieppo J, Hallikainen J, Jurvelin JS, et al. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc Res Tech. 2008;71:279–287. doi: 10.1002/jemt.20551. [DOI] [PubMed] [Google Scholar]
- Rieppo J, Hyttinen MM, Halmesmäki E, et al. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthritis Cartilage. 2009;17:448–455. doi: 10.1016/j.joca.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Säämänen AM, Tammi M, Kiviranta I, et al. Maturation of proteoglycan matrix in articular cartilage under increased and decreased joint loading. A study in young rabbits. Connect Tissue Res. 1987;16:163–175. doi: 10.3109/03008208709002004. [DOI] [PubMed] [Google Scholar]
- Saarakkala S, Laasanen MS, Jurvelin JS, et al. Ultrasound indentation of normal and spontaneously degenerated bovine articular cartilage. Osteoarthritis Cartilage. 2003;11:697–705. doi: 10.1016/s1063-4584(03)00154-7. [DOI] [PubMed] [Google Scholar]
- Saarakkala S, Julkunen P, Kiviranta P, et al. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage. 2010;18:73–81. doi: 10.1016/j.joca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Shapiro EM, Borthakur A, Kaufman JH, et al. Water distribution patterns inside bovine articular cartilage as visualized by 1H magnetic resonance imaging. Osteoarthritis Cartilage. 2001;9:533–538. doi: 10.1053/joca.2001.0428. [DOI] [PubMed] [Google Scholar]
- Shinar H, Navon G. Multinuclear NMR and microscopic MRI studies of the articular cartilage nanostructure. NMR Biomed. 2006;19:877–893. doi: 10.1002/nbm.1068. [DOI] [PubMed] [Google Scholar]
- Slemenda C, Brandt KD, Heilman DK, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Wayne JS, Kraft KA, Shields KJ, et al. MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology. 2003;228:493–499. doi: 10.1148/radiol.2282012012. [DOI] [PubMed] [Google Scholar]
- van Weeren PR, Firth EC, Brommer B, et al. Early exercise advances the maturation of glycosaminoglycans and collagen in the extracellular matrix of articular cartilage in the horse. Equine Vet J. 2008;40:128–135. doi: 10.2746/042516408X253091. [DOI] [PubMed] [Google Scholar]
- Weinert D, Fritzsche P, Gattermann R. Activity rhythms of wild and laboratory golden hamsters (Mesocricetus auratus) under entrained and free-running conditions. Chronobiol Int. 2001;18:921–932. doi: 10.1081/cbi-100107968. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]


