Abstract
This study was designed to provide more detailed information on the subcellular sites of binding of the porphycene, termed 9-capronyloxytetrakis (methoxyethyl) porphycene (CPO), with a fluorescence resonance energy transfer (FRET) technique. The proximity of CPO to two fluorescent probes was determined: nonyl acridine orange (NAO), a dye with specific affinity for the mitochondrial lipid cardiolipin, and dihexaoxacarbocyanine iodide (DiOC6), an agent that labels the endoplasmic reticulum (ER). FRET spectra indicated energy transfer between DiOC6 and CPO but no significant transfer between NAO and CPO. These results confirm data obtained by fluorescence microscopy, suggesting a similar pattern of subcellular localization by CPO and DiOC6 but not by CPO and NAO. However, when cells containing CPO were irradiated and then loaded with NAO, FRET between the two fluorophores was observed. Hence, a relocalization of CPO can occur during irradiation. These data provide an explanation for recent studies on CPO-catalyzed photodamage to both ER and mitochondrial Bcl-2.
INTRODUCTION
In a recent report (1), we examined sites of photodamage in murine leukemia L1210 cells using the porphycene termed 9-capronyloxytetrakis (methoxyethyl) porphycene (CPO) (2,3) as the photosensitizing agent. Colocalization studies indicated that CPO was predominantly localized in the endoplasmic reticulum (ER). Moreover, Bcl-2 associated with the ER was a target for photodamage. At an increased light dose, however, we also observed photodamage to mitochondrial Bcl-2. One explanation is that a minor fraction of CPO was bound to mitochondria, but the degree of binding was insufficient for detection by fluorescence microscopy. Another possibility is that CPO relocalized during irradiation.
In this study, we used fluorescence resonance energy transfer (FRET) techniques to examine the proximity of CPO to two fluorescent probes, 3,3′-dihexyloxacarbocyanine iodide (DiOC6) and nonyl acridine orange (NAO), which are commonly used in fluorescence microscopy to identify the ER and mitochondria, respectively (4,5). The data obtained are consistent with CPO being initially associated with the ER but, during subsequent irradiation, some relocalization to the mitochondria occurs, rendering that organelle sensitive to photodamage.
MATERIALS AND METHODS
Synthetic procedures
CPO was prepared essentially as described by Richert et al. (2) with minor modifications. Characterization of intermediates was in agreement with data provided in this reference.
Cell culture procedures
Murine L1210 cells were maintained in suspension culture in an approximation of Fisher’s growth medium. This was obtained by supplementing α-MEM (GIBCO-BRL, Grand Island, NY) with MgCl2 (45 mg/L), methionine (75 mg/L), phenylalanine (30 mg/L), valine (30 mg/L) and folic acid (9 mg/L). Additional components were 10% horse serum, 1 mM glutathione, 1 mM mercaptoethanol and gentamicin.
Spectral studies
The absorbance spectrum of CPO was acquired in ethanolic solution with a Shimadzu BioSpec model 1602 dual-beam spectrophotometer. Fluorescence excitation and emission spectra of CPO, NAO and DiOC6 were obtained with an SLM 48000 fluorometer as modified by ISS (Champaign, IL). To obtain complete emission spectra of NAO and DiOC6, excitation at 488 nm was employed. Excitation spectra were obtained with 540 nm emission. All FRET studies involved excitation at 488 nm (bandwidth = nm). This wavelength does not elicit CPO fluorescence, so any long-wavelength emission from cells containing CPO + NAO or DiOC6 could arise only from an energy transfer phenomenon. The CPO fluorescence emission at 650 nm was determined with 550 nm excitation, whereas the excitation spectrum in the vicinity of 550 nm was determined with 650 nm emission.
Fluorescence microscopy
Fluorescence images were acquired with a SenSys CCD camera (Photometrics, Tucson, AZ) fit with a Uniblitz shutter (Vincent Associates, Rochester, NY) and MetaMorph software (Universal Imaging, Downingtown, PA). All studies were carried out on live cells with a microscope stage thermoelectrically cooled to 15°C to prevent metabolic changes during image acquisition. CPO was excited at 350–450 nm with fluorescence detected at wavelengths >600 nm; NAO and DiOC6 were excited at 450–490 nm with fluorescence detected at 515–535 nm. To minimize photobleaching the Uniblitz shutter was configured to open and close with the camera shutter, limiting exposure of the samples to exciting light for <300 ms.
FRET acquisition
L1210 cells (7 mg/mL, 2 × 106 cells/mL) in FHS medium (Fischer’s medium containing 10% horse serum with the NaHCO3 replaced by 10 mM HEPES buffer, pH 7.4) were singly incubated with 2 µM CPO, 300 nM NAO or 800 nM DiOC6 or with combinations of CPO + NAO and CPO + DiOC6. The procedure involved incubation with CPO for 30 min at 37°C, with the fluorescent probe added for the final 5 min. When cells were labeled with only the probes, total incubation time was 5 min. The cells were washed with 0.9% NaCl and suspended in a modified FHS buffer at 15°C. This formulation lacked the serum, vitamins and amino acids present in the growth medium. The final density was 7 × 105 cells/mL. Fluorescence emission spectra showing FRET were acquired with 488 ± 2 nm excitation. In another series of studies, cells were loaded with 2 µM CPO for 30 min at 37°C, washed and resuspended in fresh medium at 10°C and irradiated at a light dose of 180 mJ/cm2. This corresponds to the conditions that elicit loss of both ER and mitochondrial Bcl-2 (1). The fluorescent probes were added after irradiation, and the cells were then used for FRET analysis carried out at 15°C.
The light source was a 700 watt quartz-halogen lamp filtered with 10 cm of water to remove excess infrared radiation. The transmission wavelength was confined to 550–700 nm with band-pass filters (Oriel, Stratford, CT) corresponding to the three CPO Q-bands (Fig. 1).
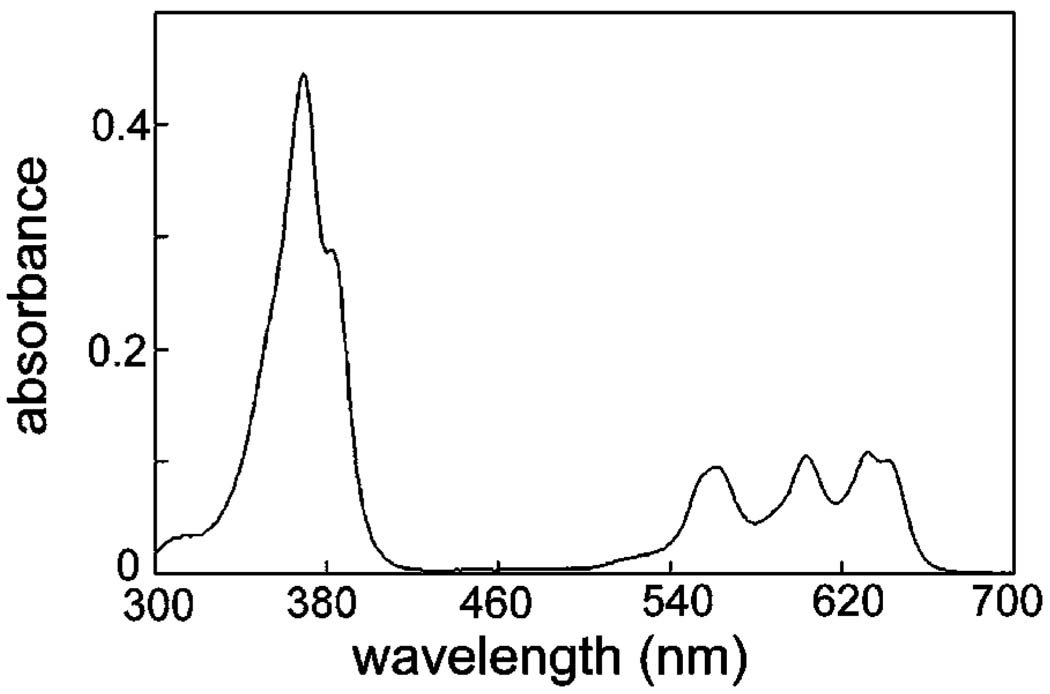
Figure 1.
Absorbance spectrum of CPO (3 µM in ethanol).
RESULTS AND DISCUSSION
Absorbance spectra
The absorbance spectrum of CPO is shown in Fig. 1. A soret band at 369 nm was detected along with Q bands at 560, 603 and 632 nm. The appearance of an absorbance band in the green indicated that FRET between CPO and fluorescence probes for specific organelles was feasible because both DiOC6 and NAO have fluorescence emission bands in this region of the spectrum.
Fluorescence excitation and emission spectra
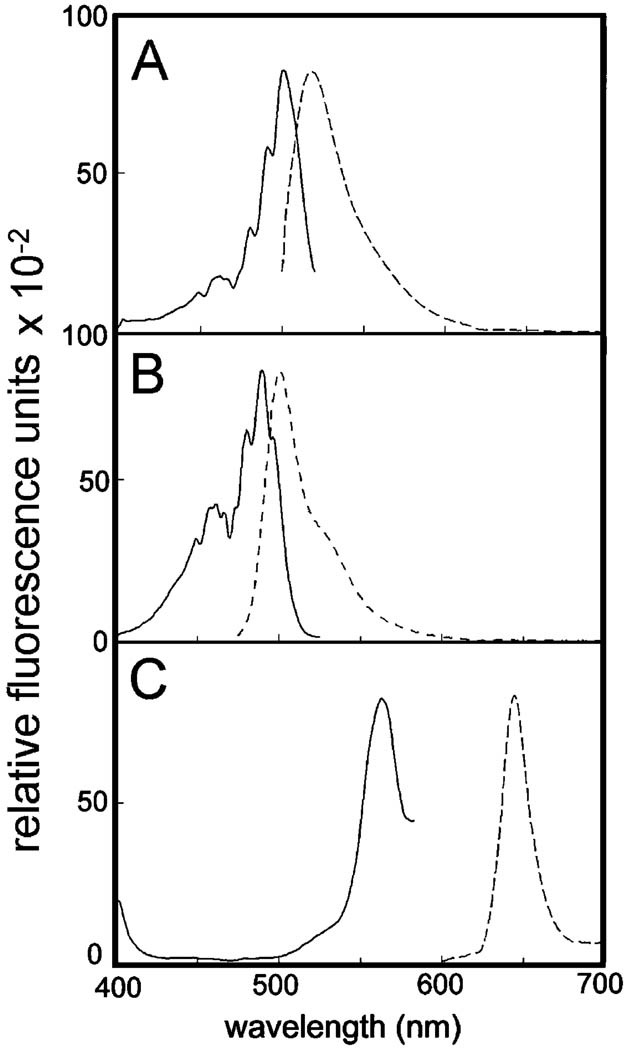
NAO showed an excitation optimum of 501 nm with an emission peak at 518 nm (Fig. 2A). DiOC6 exhibited excitation and emission optima at 490 and 501 nm, respectively (Fig. 2B). In contrast, the excitation and emission optima of CPO at 563 and 644 nm, respectively, were markedly different from those of NAO or DiOC6 (compare Fig. 2C with A and B). In principal, because the emission spectra of both NAO and DiOC6 overlapped with the excitation spectrum of CPO, FRET should be observed with the NAO/CPO and DiOC6/CPO pairs, providing the two agents are in sufficiently close proximity.
Figure 2.
Fluorescence emission and excitation spectra of NAO (A) DiOC6 (B) and CPO (C) in ethanolic solution. Solid lines = excitation spectra, dashed lines = emission spectra.
Fluorescence microscopy
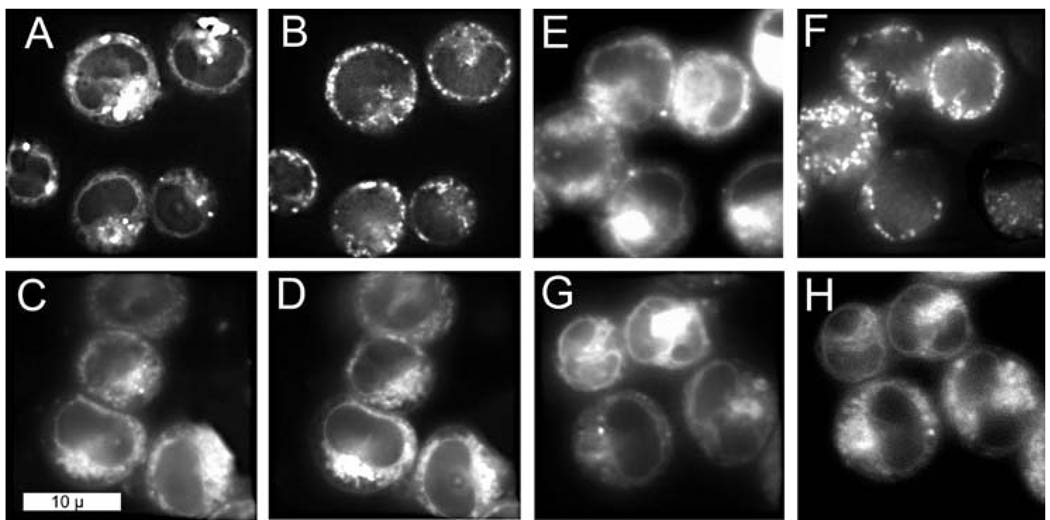
Fluorescence microscopy demonstrated that CPO and NAO did not colocalize in L1210 cells (compare Fig. 3A with B). In contrast, there was a pronounced colocalization of CPO with the ER probe DiOC6 (compare patterns in Fig. 3C with D). Additional images were acquired after cells were loaded with CPO and irradiated. The CPO signal became somewhat more diffuse after irradiation, but there were no significant changes in the DiOC6 and NAO images.
Figure 3.
Fluorescence images of cells simultaneously loaded with two fluorophores. A–D. Before irradiation; E–H. After irradiation. A, E = CPO; B, F = NAO; C, G = CPO; D, H = DiOC6. Fluorescence emission signals were acquired at 600–700 nm (CPO) and 515–535 nm (NAO and DiOC6).
FRET analysis
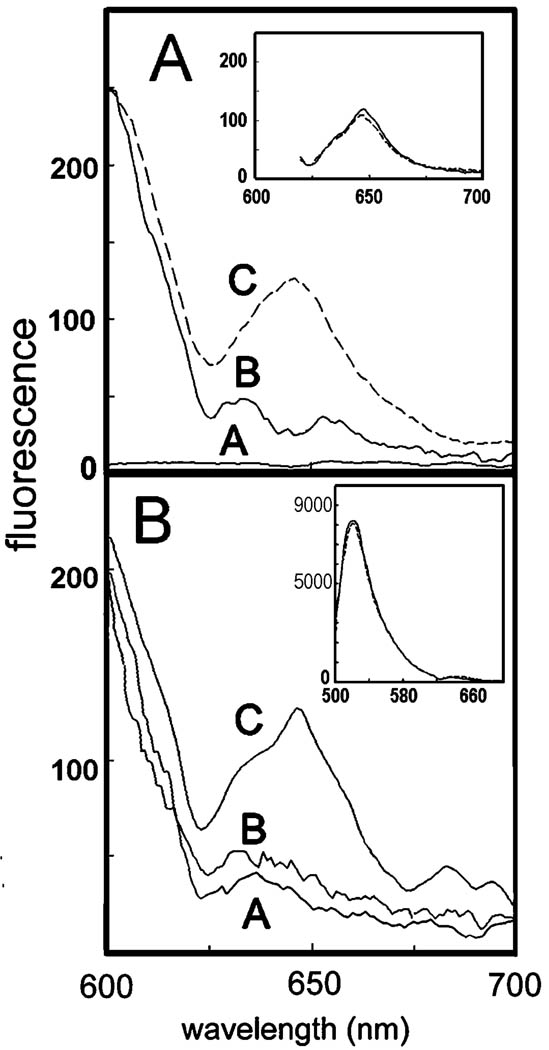
Irradiation of cells containing CPO at 488 nm yielded no detectable fluorescence (Fig. 4A, trace A). This wavelength was therefore appropriate for FRET studies because fluorescence excitation of NAO or DiOC6 could occur without eliciting any response from CPO in the absence of FRET.
Figure 4.
Fluorescence emission spectrum of L1210 cell suspensions showing FRET between CPO and DiOC6 (A) and between CPO and NAO (B). In all cases, the excitation wavelength was 488 ± 2 nm. Top: Trace A = CPO, trace B = DiOC6, trace C = CPO + DiOC6. Inset: FRET between DiOC6 and CPO before (dashed line) and after (solid line) irradiation. Bottom: Trace A = NAO alone, trace B = NAO + CPO (no activation of CPO prior to NAO addition), trace C = NAO + CPO (NAO added after PDT). Inset: The complete fluorescence emission spectrum for NAO in this experiment before (solid line) and after (dashed line) irradiation.
A faint fluorescence signal was obtained at wavelengths above 620 nm when cells containing only DiOC6 were excited at 488 nm (Fig. 4A, trace B). A greater signal, indicative of FRET, was obtained when both DiOC6 and CPO were present during excitation (Fig. 4A, trace C). In an inset to Fig. 4A, we show the FRET signal from DiOC6 + CPO before (solid line) and after (dashed line) irradiation. In the latter study, the probe was added after irradiation to avoid possible artifacts caused by DiOC6 photooxidation. No significant change was observed, indicating that the proximity of CPO and the fluorescent probe were not altered by irradiation.
When cells contained only NAO, excitation at 488 nm resulted in a weak fluorescence emission signal at wavelengths >620 nm (Fig. 4B, trace A). This signal was not significantly affected when CPO was also present (Fig. 4B, trace B). Hence, there was essentially no FRET in cells that had been coloaded with NAO and CPO. This situation changed if cultures were first loaded with CPO and irradiated at 550–700 nm, resulting in a photodynamic therapy (PDT) effect, then treated with NAO and excited at 488 nm to specifically excite NAO. In such a protocol, FRET between NAO and CPO occurred, and an enhanced CPO emission signal was seen at ~650 nm (Fig. 4B, trace C).
It is common to note a decrease in donor fluorescence when FRET occurs. In these experiments, the magnitude of the NAO and DiOC6 fluorescence was much greater than the FRET signal, so it was difficult to distinguish this effect. An inset in Fig. 4B shows the NAO signal before and after irradiation. No significant alteration in the fluorescence emission spectrum was detected after irradiation.
CONCLUSIONS
On the basis of the CPO absorbance spectrum (Fig. 1) and the fluorescence excitation spectra of NAO and DiOC6 (Fig. 2), we determined that FRET could be used to obtain information on the proximity of the photosensitizer CPO to NAO and DiOC6, two fluorescent probes useful for subcellular localization. The latter dye was initially developed as a fluorescent label for the ER (4), whereas NAO is a specific probe for cardiolipin, a lipid found only in the mitochondrial inner membrane (5). NAO had previously been used in a FRET study that identified mitochondrial cardiolipin as a site of binding of the phthalocyanine photosensitizer Pc 4 (6). In this study, we have not carried out calculations on probable distances between CPO and the fluorescence probes as was done by Oleinick’s group (6) because some of the required parameters are unknown.
Results from fluorescence microscopy described here (Fig. 3) and elsewhere (1) suggested that the porphycene CPO is initially bound to the ER. Because these data are only qualitative, it is difficult to assign definitive localization patterns solely on the basis of such studies. This study demonstrates the potential value of FRET for delineating colocalization phenomena in which fluorescence microscopy is insufficiently sensitive. The small cytoplasmic compartment of L1210 cells causes considerable overlap between cellular organelles confined to that region. We have now shown that FRET between CPO and the mitochondrial probe NAO was essentially undetectable, whereas FRET between CPO and the ER probe DiOC6 was readily apparent (Fig. 4A). These data enable us to provide a more definitive indication of subcellular CPO binding sites.
On irradiation of CPO-sensitized L1210 cells, loss of Bcl-2 from both the ER and mitochondria was observed, although the effect was greatest for Bcl-2 located in the ER (1). One possible explanation for this result is the partial relocalization of CPO during irradiation, with mitochondria being among the new sites of affinity. Although DiOC6 can also label mitochondria (7), the lack of FRET between NAO and CPO indicates that the FRET signal obtained with DiOC6 solely derives from the ER localization of this probe.
In this study, we show that CPO did not have sufficient affinity to mitochondria to elicit FRET with the cardiolpin probe NAO, but energy transfer was observed after PDT (Fig. 4B). Relocalization of photosensitizing agents during irradiation has been reported before. Moan’s group (8) described a PDT-induced movement of a photosensitizing agent from lysosomes to the cytosol, and we provided another example involving the migration of a cationic sensitizer from the plasma membrane to cytosolic proteins during irradiation (9). This study documents a third such example. It appears that the initial site(s) of binding of a photosensitizer might be only one determinant of the ultimate loci of subcellular photodamage.
Acknowledgements
This work was supported by grants CA 92618 and CA 23378 from the National Institutes of Health. We thank Ann Marie Santiago and Nakaiya Adjeley Okan-Mensah for excellent technical assistance.
Abbreviations
- CPO
9-capronyloxytetrakis (methoxyethyl) porphycene
- DiOC6
3,3′-dihexyloxacarbocyanine iodide
- ER
endoplasmic reticulum
- FHS
Fischer’s medium containing 10% horse serum with the NaHCO3 replaced by 10 mM HEPES buffer pH 7.4
- FRET
fluorescence resonance energy transfer
- NAO
nonyl acridine orange
- PDT
photodynamic therapy
Footnotes
Posted on the website on 3 March 2005
REFERENCES
- 1.Kessel D, Castelli M, Reiners JJ., Jr Ruthenium red-mediated suppression of Bcl-2 loss and Ca2+ release initiated by photodamage to the endoplasmic reticulum: scavenging of reactive oxygen species. Cell Death Differ. 2005 doi: 10.1038/sj.cdd.4401579. doi: 10.1038/sj.cdd.4401579. [DOI] [PubMed] [Google Scholar]
- 2.Richert C, Wessels JM, Muller M, Kisters M, Benninghaus T, Goetz AE. Photodynamic antitumor agents: beta-methoxyethyl groups give access to functionalized porphycenes and enhance cellular uptake and activity. J. Med. Chem. 1994;37:2797–2807. doi: 10.1021/jm00043a019. [DOI] [PubMed] [Google Scholar]
- 3.Toledano H, Edrei R, Kimel S. Photodynamic damage by liposome-bound porphycenes: comparison between in vitro and in vivo models. J. Photochem. Photobiol. B. 1998;42:20–27. doi: 10.1016/S1011-1344(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 4.Terasaki M, Song J, Wong JR, Weiss MJ, Chen JB. Localization of endoplasmic reticulum in living and glutaraldehyde fixed cells with fluorescent dyes. Cell. 1984;38:101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- 5.Petit JM, Maftah A, Ratinaud MH, Julien R. 10N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur. J. Biochem. 1992;209:267–273. doi: 10.1111/j.1432-1033.1992.tb17285.x. [DOI] [PubMed] [Google Scholar]
- 6.Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney MF, Samia AC, Burda C, Oleinick NL. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194–5197. [PubMed] [Google Scholar]
- 7.Lee C, Wu SS, Chen LB. Photosensitization by 3,3–-dihexyloxacarbocyanine iodide: specific disruption of microtubules and inactivation of organelle motility. Cancer Res. 1995;55:2063–2069. [PubMed] [Google Scholar]
- 8.Berg K, Madslien K, Bommer JC, Oftebro R, Winkelman JW, Moan J. Light induced relocalization of sulfonated mesotetraphenylporphines in NHIK 3025 cells and effects of dose fractionation. Photochem Photobiol. 1991;53:203–210. doi: 10.1111/j.1751-1097.1991.tb03924.x. [DOI] [PubMed] [Google Scholar]
- 9.Kessel D. Relocalization of cationic porphyrins during photodynamic therapy. Photochem. Photobiol. Sci. 2002;1:837–840. doi: 10.1039/b206046a. [DOI] [PubMed] [Google Scholar]