Abstract
Phosphate is required for terminal differentiation of hypertrophic chondrocytes during postnatal growth plate maturation. In vitro models of chondrocyte differentiation demonstrate that 7mM phosphate, a concentration analogous to that of the late gestational fetus, activates the mitochondrial apoptotic pathway in hypertrophic chondrocytes. This raises the question as to whether extracellular phosphate modulates chondrocyte differentiation and apoptosis during embryonic endochondral bone formation. To address this question, we performed investigations in the mouse metatarsal culture model that recapitulates in vivo bone development. Metatarsals were cultured for 4, 8 and 12 days with 1.25 mM and 7 mM phosphate. Metatarsals cultured with 7 mM phosphate showed a decrease in proliferation compared to those cultured in 1.25 mM phosphate. This decrease in proliferation was accompanied by an early enhancement in hypertrophic chondrocyte differentiation, associated with an increase in FGF18 expression. By 8 days in culture, an increase caspase-9 activation and apoptosis of hypertrophic chondrocytes was observed in the metatarsals cultured in 7 mM phosphate. Immunohistochemical analyses of embryonic bones demonstrated activation of caspase-9 in hypertrophic chondrocytes, associated with vascular invasion. Thus, these investigations demonstrate that phosphate promotes chondrocyte differentiation during embryonic development and implicate a physiological role for phosphate activation of the mitochondrial apoptotic pathway during embryonic endochondral bone formation.
Keywords: phosphate, embryonic, bone, development
Phosphate is critical for a vast array of cellular processes and, together with calcium, is one of the major components of the skeleton. Extracellular phosphate regulates apoptosis of terminally differentiated hypertrophic chondrocytes during postnatal growth plate maturation. Impaired apoptosis of the late hypertrophic chondrocyte layer is a feature of rachitic hypophosphatemic disorders such as hereditary vitamin D-resistant rickets and X-linked hypophosphatemia [Makras et al., 2008; Sabbagh et al., 2005]. Studies in Hyp mice, a mouse model of X-linked hypophosphatemia, demonstrate that development of hypophosphatemia is associated with a decrease in apoptosis of hypertrophic chondrocytes and expansion of the growth plate, revealing a correlation among serum phosphate levels, programmed cell death of hypertrophic chondrocytes, and the development of rickets [Sabbagh et al., 2005]. The hypothesis that phosphate is a key regulator of hypertrophic chondrocyte apoptosis is supported by investigations in the calcium-sensing receptor knockout mice. Due to impaired parathyroid calcium sensing, these mice exhibit hyperparathyroidism that leads to hypophosphatemia and, despite the presence of concurrent hypercalcemia, their growth plates reveal classical rachitic changes [Tu et al., 2003]. Rendering these mice hypoparathyroid by making them null for the Gcm2 gene, which is required for parathyroid gland development [Gunther et al., 2000], prevents hypophosphatemia and rachitic changes [Tu et al., 2003], confirming the link between circulating phosphorus levels and the development of rickets.
The role of phosphate in endochondral bone development is unknown. Even in states of maternal hypophosphatemia, circulating fetal phosphate is maintained at normal levels [Sabbagh et al., 2005]. It is, therefore, unclear whether changes in extracellular phosphate concentrations impact the fetal skeleton. Endochondral bone formation is a highly regulated process, initiated by the formation of mesenchymal condensations at the sites of future bones [Kronenberg, 2003; Provot and Schipani, 2005]. These mesenchymal cells then differentiate into chondrocytes which undergo progressive maturation from proliferative cells, to hypertrophic cells which ultimately undergo apoptosis. Vascular invasion ensues and the late hypertrophic chondrocytes are replaced by osteoblasts that give rise to the primary spongiosa.
The ex vivo mouse metatarsal culture system has been used for more than a decade to characterize factors that regulate endochondral bone formation and chondrocyte differentiation [Klement and Spooner, 1993; Minkin et al., 1991; Serra et al., 1999; Tao and Minkin, 1994]. Cultures of metatarsals from day 15.5 mouse embryos (dpc) have been shown to maintain the normal pattern of growth and differentiation observed in vivo for up to 15 days [Tao and Minkin, 1994], presumably because of the permissive local environment provided by this organ culture system, in which reciprocal interactions between the periosteum, perichondrium and growing rudiment are maintained. In this culture system, the concentration of phosphate in the medium is 1.25 mM. However, the concentration of phosphate in embryonic plasma is approximately 7 mM [Sabbagh et al., 2005], a phosphate concentration which induces apoptosis of hypertrophic chondrocytes in culture [Mansfield et al., 1999; Mansfield et al., 2001; Sabbagh et al., 2005]. We, therefore, undertook investigations to evaluate the effects of 7 mM phosphate on chondrocyte proliferation, differentiation and apoptosis during endochondral bone development, using the mouse metatarsal organ culture system as a model.
Materials and Methods
Animals
Timed pregnant C57BL/6J mice were sacrificed for isolation of embryonic metatarsals and humeri. All animals were housed in a virus- and parasite-free barrier facility under 12-h light/12-h dark cycles with free access to food and water. All animal procedures were approved by the Massachusetts General Hospital Institutitonal Animal Care and Use Committee.
Metatarsal Cultures
Metatarsals were isolated from embryos of C57BL/6J 15.5 dpc mice, placed in a well of a 24 well plate and cultured with 500 µl of phosphate-free DMEM (Invitrogen/Gibco) supplemented with 0.25 % defined FBS, 0.05 mg/ml ascorbic acid, antibiotic/antimycotic, sodium pyruvate and phosphate at a final concentration of 1.25 mM and 7 mM. Metatarsals were incubated at 37°C, 5%CO2 for 4, 8 and 12 days, with media changes every 4 days. Prior to harvesting, metatarsals were incubated for 4 hours with 0.5 mg/ml 5-bromo-2-deoxy-Uridine (BrdU), to permit evaluation of proliferative cells. Metatarsals were fixed in 4% paraformaldehyde, sequentially incubated in 5% and 30% sucrose, embedded in OCT mounting media and stored at −80 C prior to cryosectioning.
Histology
Serial 5 µm cryosections (Shandon CS Cryotome, Thermo Electron Corporation, Waltham, MA) were employed for in situ hybridization analyses for collagen II, collagen X and osteopontin. These analyses were performed using digoxigenin–UTP (DIG-UTP) labeled (Roche Diagnostic) antisense probes. Hybridization was performed at 65°C for 20 h in 50% formamide, 10 mM Tris (pH7.5), 600 mM NaCl, 1mM EDTA, 0.25% SDS, 1× Denhardt's solution, 10% dextran sulfate and 200 µg/ml yeast tRNA (Gibco). After post-hybridization washes, the samples were incubated with an anti DIG-AP antibody (1:2500, Roche) for 24 h at 4°C in a humidified chamber. mRNA expression was detected using BM Purple AP Substrate (Roche). Toluidine blue O staining (0.1 % Sigma), and Hematoxilin & Eosin (H&E) staining were performed to evaluate morphology. To assess mineralized matrix formation, Von Kossa staining was performed. Sections were incubated in 5% aqueous silver nitrate solution for 15 min under UV light, followed by 30 seconds in 5% sodium thiosulfate and counterstained with Methyl Green.
Evaluation of Apoptosis
Apoptotic cells were identified using a TUNEL-based in situ cell death detection kit (Roche Diagnostics). Sections were incubated with the TUNEL reaction mixture for 1 h at 37°C, rinsed and mounted with Vectashield (Vector Laboratories). To inhibit the mitochondrial apoptotic pathway, the caspase-9 inhibitor Z-LEHD-FMK (Calbiochem) or vehicle were added at the time of initiation of the culture and every 24 hours for 8 days.
Immunohistochemistry
Cleaved caspase-9 and STAT-1 were detected using the TSA biotin kit (PerkinElmer), as recommended by the manufacturer. Samples were incubated with a rabbit polyclonal antibody directed against the active cleaved form of caspase-9 (1:50, Abcam, Cambridge, MA) or against STAT1 (1:250, GenWay, CA) at 4°C in a humidified chamber. Immunoreactive proteins were visualized using a Streptavidin HRP conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or goat-anti-rabbit biotinylated antibody for STAT1 detection (Vector Laboratories) and peroxidase substrate (Vector Laboratories). For evaluation of chondrocyte proliferation, immunohistochemistry was performed using the BrdU staining kit as recommended by the manufacturer(Zymed Laboratories, South San Francisco, CA). The number of BrdU-positive nuclei and total nuclei in the proliferating chondrocyte layer of each metatarsal was analyzed by capturing an image using a Leica microscope and PhotoShop software. The chondrocyte proliferation ratio was defined as the number of BrdU positive nuclei in the proliferative chondrocyte region, divided by the total number of chondrocyte nuclei in that same region.
Real-time PCR
RNA was isolated from triplicates of at least 6 metatarsals for each condition after 24 hours in culture (RNeasy Mini kit, Qiagen). First-Strand cDNA was synthesized, using the SuperScriptTM III First-Strand Synthesis SuperMix (Invitrogen). Oligonucleotides used were as follows: mouse β-actin sense, 5’-CAGGAGGAGCAATGATCTTG-3’, and antisense, 5’- GATGACCCAGATCATGTTTG-3’; mouse FGF18 sense, 5’ ACTGCTGTGCTTCCAGGTTC-3’, and antisense, 5’-CCCAGGACTTGAATGTGCTT-3’; and mouse BMP2 sense 5’-AGCAAGGACGTCGTGGTGCC-3’, and antisense, 5’-ATTATTTCGGTGCTGGAAACTACT-3’.
Real-time PCR was performed with SYBR Green according to the protocol for the LightCycler System (Qiagen), using the Opticon DNA engine system (MJ Research, Waltham, MA). The efficacy of DNase digestion was confirmed by performing analyses in the presence and absence of reverse transcriptase. The mRNA level encoding each gene of interest was normalized for β-actin mRNA in the same sample using the formula of Livak and Schmittgen [Livak and Schmittgen, 2001].
Statistical Analysis
Student's t test was used to analyze significance between two groups. P < 0.05 was considered significant. All analyses were performed with Excel 2001 software.
RESULTS
Metatarsals from 15.5 dpc mouse embryos were isolated and cultured in 7mM phosphate to address whether this concentration of extracellular phosphate, which is analogous to that seen at 18.5 dpc [Sabbagh et al., 2005], affects growth and differentiation of embryonic bones. To evaluate growth, the length of the metatarsals was measured with a micrometer at the time of isolation, as well as at the time of harvest 4, 8 and 12 days later. Metatarsals cultured under control conditions (1.25mM phosphate) increased in length at all time points examined (Figure 1A), demonstrating a 120 ± 6.7 % increase in length over 12 days (Figure 1A). While 7mM phosphate attenuated growth at 4, 8 and 12 days in culture (Figure 1A), there was still a significant increase in metatarsal length over 12 days (70 ± 2.6 % increase, Figure 1A). To determine whether this attenuated growth was a reflection of decreased proliferation, BrdU staining was performed after 4 days of culture. As demonstrated in Figure 1B, 7mM phosphate significantly attenuated chondrocyte proliferation relative to 1.25 mM phosphate (3.7 ± 0.4% versus 5.6 ± 0.4%, respectively, P < 0.0001), demonstrating that decreased proliferation contributes to the impaired growth observed with 7mM phosphate.
Figure 1.
Metatarsal Growth and Proliferation. A. Percentage increase in length of metatarsals cultured with 1.25 mM and 7 mM phosphate for 4, 8 and 12 days. Growth is expressed as a percentage [100 × (length at the end of the culture period-initial length)/initial length)] *P<0.05 versus 7 mM at same day in culture. ** P<0.05 between two samples indicated. B. Percentage of BrdU positive round chondrocytes after 4 days in culture with 1.25 mM and 7 mM phosphate. Data are derived from at least 6 metatarsals for each condition *P<0.0001 versus 7mM.
Morphological evaluation revealed an increase in the hypertrophic chondrocyte zone in metatarsals cultured with 7 mM vs. 1.25 mM phosphate at 4 days (Figure 2A). However, a decrease in the hypertrophic chondrocyte region is observed with 7 mM phosphate at 8 and 12 days in culture compared to 1.25 mM phosphate (Figure 2A).
Figure 2.
Metatarsal Differentiation. Morphology of metatarsals cultured with 1.25 mM and 7 mM phosphate and stained with H&E (A). TB, 0.1% Toluidine blue O stain; VK (Von Kossa) stain and in situ hybridization analysis for Col II, Col X, OP were performed on sections of metatarsals cultured with 1.25 and 7 mM phosphate for 4 days (B), 8 days (C) and 12 days (D). Data are representative of investigations performed on at least 2 sections of each of three metatarsals for each condition.
To characterize this increase in hypertrophic chondrocyte differentiation observed with 7mM phosphate, in situ hybridization analyses were performed to examine markers of chondrocyte differentiation. When cultured under control conditions (1.25 mM phosphate), metatarsals exhibit a progressive decrease in collagen II expressing cells from 4 to 12 days in culture, accompanied by an increase in osteopontin expressing cells, reflecting the normal program of chondrocyte differentiation (Figure 2B, 2C and 2D). Mineralized matrix formation is seen at 12 days in culture under control conditions (Figure 2D). When metatarsals are cultured in 7 mM phosphate, accelerated differentiation is observed, characterized by an earlier appearance of collagen X negative, osteopontin positive hypertrophic chondrocytes at 4 days in culture. However, there is a decrease in osteopontin expressing cells at 8 and 12 days in culture, accompanied by absence of mineralized matrix formation (Figure 2B, 2C and 2D).
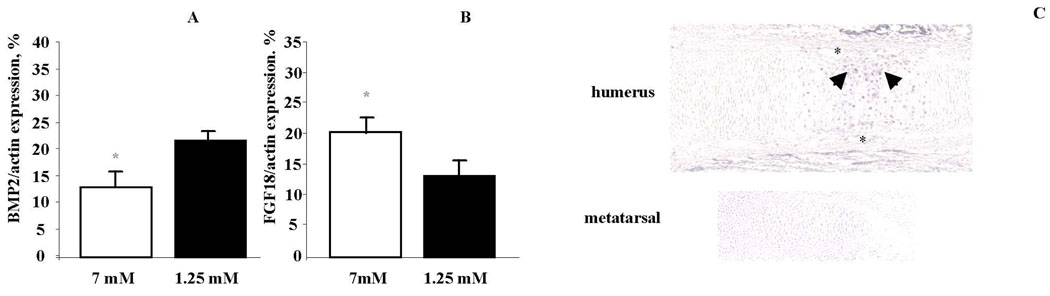
Several signaling pathways regulate chondrocyte proliferation and differentiation. This decrease in proliferation accompanied by an early enchancement in chondrocyte differentiation, is similar to the phenotype observed in mouse embryonic forelimbs [Sahni et al., 2001] and metatarsals [Minina et al., 2002] upon FGF18 activation. We, therefore, addressed whether 7 mM phosphate up-regulated FGF18 expression. RNA was isolated from metatarsals cultured in 1.25 mM and 7 mM phosphate for 24 hours after isolation. Quantitative real-time rt-PCR analyses demonstrated a significant increase in FGF18 mRNA expression, accompanied by a decrease in BMP2 mRNA, in metatarsals cultured with 7mM phosphate vs 1.25 mM phosphate (Figure 3A, 3B). To examine the consequences of this increase in FGF18 expression, immunohistochemistry for STAT1 was performed on e14.5 humeri. STAT-1 immunoreactivity is observed in hypertrophic chondrocytes of e14.5 humeri adjacent to the area of vascular invasion (Figure 3C). The hypertrophic chondrocytes of the metatarsals of the same limb, which have not undergone vascular invasion at this developmental stage, do not exhibit any STAT1 immunoreactivity (Figure 3C).
Figure 3.
Phosphate increases FGF18 expression. FGF18 (A) and BMP2 (B) expression were determined by quantitative real time PCR of RNA isolated from metatarsals cultured with 7mM and 1.25 mM phosphate for 24 hours in culture. Expression levels were normalized to β-actin in each sample. Error bars represent mean ± SEM. *P<0.05. STAT1 immunoreactivity is observed in vivo in hypertrophic, but not proliferative chondrocytes, in the humerus of e14.5 mice. Asterisks point to blood vessels. Arrows point to STAT positive hypertrophic chondrocytes in the humerus. No immunoreactivity is observed in the hypertrophic chondrocytes of metatarsals of e14.5 mice. Data are representative of that obtained from at least 3 independent experiments.
While these changes can account for the decrease in proliferation and early hypertrophic differentiation of the metatarsals cultured with 7 mM phosphate, they cannot explain the progressive decrease in late hypertrophic chondrocytes observed at later time points. To determine whether this reduction in osteopontin-expressing cells was due to increased apoptosis, TUNEL assays were performed. These studies demonstrate an increase in hypertrophic chondrocyte apoptosis in metatarsals cultured in 7 mM phosphate compared to those cultured with 1.25 mM phosphate at 8 days in culture (Figure 4A).
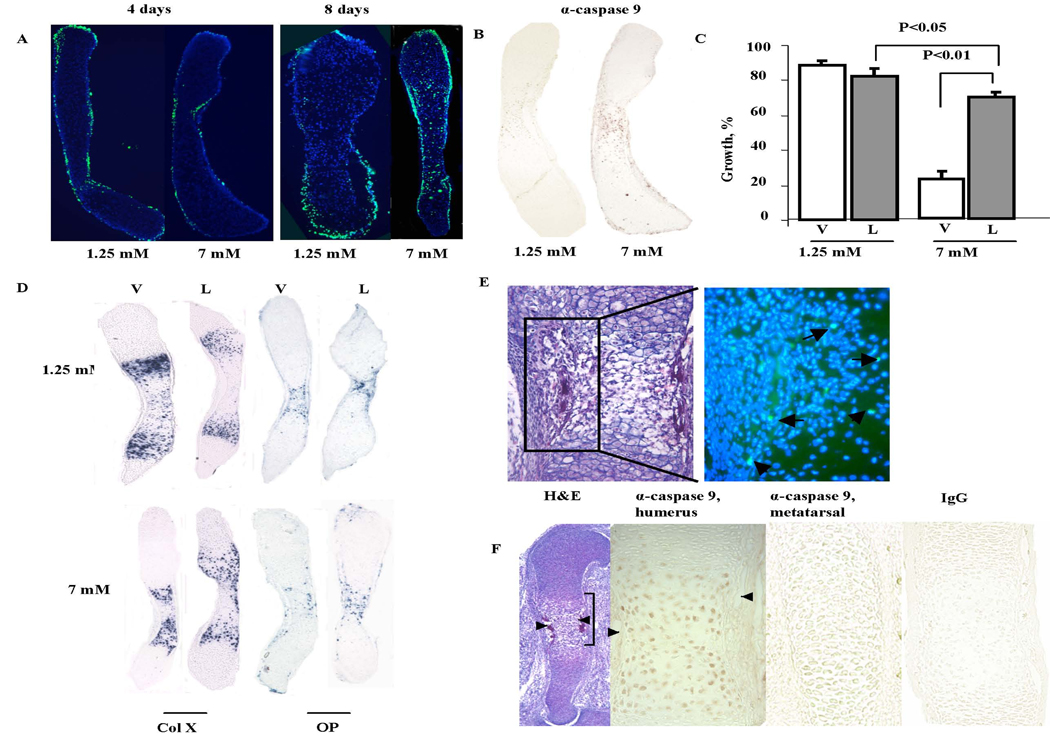
Figure 4.
Evaluation of the mitochondrial apoptotic pathway activation. A. TUNEL assays of metatarsals cultured with 1.25 mM and 7 mM phosphate were performed on sections of metatarsals cultured with 1.25 and 7 mM phosphate for 4 and 8 days. B. Immunohistochemistry with anti-cleaved caspase-9 antibody was performed on sections of metatarsals isolated after 4 days in culture with 1.25 mM and 7 mM phosphate. Non –specific IgG controls confirmed the specificity of the signal (data not shown). C. Percentage increase in the length of metatarsals cultured with 1.25 mM and 7 mM phosphate in the presence of LEHD-FMK (L) or vehicle (V) after 8 days in culture. D. In situ hybridization analyses were performed on sections of metatarsals cultured with 1.25 and 7 mM phosphate for 8 days with 20 µM LEHD-FMK (L) or vehicle (DMSO) (V). E. H&E staining and TUNEL assay of e15.5 humerus. The fluorescent image of the boxed area on the left is shown in the merged DAPI (nuclear stain) and FITC (TUNEL stain) image on the right. Arrows point to TUNEL positive hypertrophic chondrocyte nuclei. F. Cleaved caspase-9 immunoreactivity is observed in vivo in hypertrophic, but not proliferative chondrocytes, in the humerus of e14.5 mice. No immunoreactivity is observed in the hypertrophic chondrocytes of metatarsals of e14.5 mice. Arrows point to blood vessels. Data are representative of that obtained from at least 3 independent experiments.
Phosphate, at a concentration of 7mM, has been shown to activate the mitochondrial apoptotic pathway in cultures of primary chondrocytes [Sabbagh et al., 2005]. To determine whether 7 mM phosphate activates this pathway in developing metatarsals as well, immunohistochemistry was performed, using an antibody against the active (cleaved) form of caspase-9, a key effector of the mitochondrial apoptotic pathway [Green and Kroemer, 2004]. Immunohistochemical analyses revealed cleaved caspase-9 immunoreactivity in the hypertrophic chondrocytes cultured in 7 mM phosphate after 8 days in culture (Figure 4B). To determine if inhibition of caspase-9 activity could, in part, reverse the late phenotype of the metatarsals cultured in 7mM phosphate, cultures were treated with the cell-permeable selective caspase-9 inhibitor Z-Leu-Glu(OMe)-His-Asp(OMe)_Fluoromethyl ketone (Z-LEHD-FMK; 20 µM) for 8 days [Ekert et al., 1999; Nicholson and Thornberry, 1997; Thornberry and Lazebnik, 1998]. TUNEL staining (data not shown) revealed that inhibition of caspase-9 prevented hypertrophic chondrocyte apoptosis in the metatarsals cultured in 7 mM phosphate and partially reversed the growth defect observed under these conditions (Figure 4C). In situ hybridization analyses confirm that inhibition of caspase 9 prevents the dramatic decrease in osteopontin expressing hypertrophic chondrocytes seen in the metatarsals cultured with 7 mM phosphate. Treatment with Z-LEHD-FMK results in similar domains of collagen X and osteopontin expressing cells in the metatarsals cultured with 1.25 mM and 7mM phosphate (Figure 4D). Mineralized matrix was not observed at 8 days under any of these conditions.
Since 7 mM phosphate mimics circulating levels in fetal mice [Sabbagh et al., 2005], studies were performed to address whether there was evidence of apoptosis and caspase 9 activation during embryonic development. As shown in Figure 4E, TUNEL staining shows hypertrophic chondrocyte apoptosis in the humerus of 14.5dpc embryos. Furthermore, cleaved caspase 9 immunoreactivity is observed in hypertrophic, but not proliferative, chondrocytes in the humerus of 14.5dpc embryos, at the time of initiation of vascular invasion. Cleaved caspase 9 immunoreactivity is notably absent from the hypertrophic chondrocytes of the metatarsals, which have not undergone vascular invasion at this developmental stage (Figure 4F).
Discussion
During endochondral bone development, proliferative chondrocytes differentiate into prehypertrophic and then hypertrophic chondrocytes. Vascular invasion follows, leading to the formation of the primary spongiosa and marrow cavity. Although previous investigations have demonstrated that terminal differentiation and apoptosis of late hypertrophic chondrocytes in the developing growth plate is dependent upon the caspase-9-mediated mitochondrial apoptotic pathway [Sabbagh et al., 2005], the role of this pathway during embryonic bone development has not been explored. Investigations directed at addressing the role of phosphate in this process have been hampered by the lack of fetal hypophosphatemic and hyperphosphatemic models. Studies in several genetically altered and physiologically restricted animal models have demonstrated that the placenta possesses a remarkable ability to maintain normal fetal mineral ion levels, in spite of severe disturbances in the maternal circulation. Despite profound maternal hypophosphatemia, hyp fetuses maintain a serum phosphate level of 7.1 ± 0.3 mM at 18.5 days of gestation [Sabbagh et al., 2005], and unlike their hypophosphatemic 3 week old counterparts, demonstrate apoptosis of terminally differentiated hypertrophic chondrocytes similar to that of their wild-type littermates [Sabbagh et al., 2005].
Several pathways regulate chondrocyte proliferation and differentiation during endochondral bone formation [Kronenberg, 2003]. Humans and animals with activating mutations of FGFR3 display achondroplasia, characterized by a decrease in chondrocyte proliferation and accelerated chondrocyte differentiation [Naski et al., 1998, Colvin et al., 1996; Deng et al., 1996]. Activating FGF signaling in vitro in mouse embryonic forelimbs [Sahni et al., 2001] and metatarsals [Minina et al., 2002] also results in a decrease in chondrocyte proliferation, accompanied by an acceleration in differentiation, a phenotype analogous to that observed during the first 4 days in metatarsals cultured with 7mM phosphate. Thus, the increase in FGF18 expression we observed could in part account for the enhanced chondrocyte differentiation observed in metatarsals cultured with 7 mM phosphate.
Studies in both avian and murine chondrocyte cultures demonstrate that 7 mM phosphate causes apoptosis of cultured hypertrophic chondrocytes by activating the mitochondrial apoptotic pathway [Mansfield et al., 1999; Sabbagh et al., 2005]. However, 7 mM phosphate did not activate apoptosis of cultured chondrocytes that had not undergone hypertrophic differentiation, demonstrating a differentiation-dependent specificity of phosphate-induced apoptosis [Sabbagh et al., 2005]. Our data suggest that phosphate activates the mitochondrial apoptotic pathway in hypertrophic chondrocytes in the developing skeleton as well, based on caspase-9 immunoreactivity in the hypertrophic chondrocytes of of e14.5 humerus. The degree of apoptosis that we observed in cultured metatarsals has not been reported in vivo during embryonic bone development. This may be due to the fact that, although the concentration of phosphate employed in our studies reflects that observed in late fetal life, the culture conditions established for metatarsals do not have the same serum concentration as fetal plasma. Additionally, in the metatarsal model, the developing bones are dissected free from adjacent tissues, therefore, may lack exogenous factors that modulate the effects of phosphate on the developing skeleton. An alternative explanation could be that, because the hypertrophic chondrocytes in the developing fetus are avascular and surrounded by connective tissue, they are not directly exposed to the phosphate concentrations observed in fetal plasma. However, vascular invasion would be expected to breach the protected environment of the developing bone, exposing the hypertrophic chondrocytes to 7 mM phosphate and initiating programmed cell death. In support of this latter hypothesis, our studies demonstrate that, at the time of early vascular invasion, in e14.5 humeri there is TUNEL positivity and activation of both caspase 9 and STAT1 in the hypertrophic chondrocytes of the developing humerus. Cleaved caspase-9 and STAT 1 immunoreactivity are notably absent in hypertrophic chondrocytes of skeletal elements that have not yet undergone vascular invasion. Thus, these data suggest that exposure of hypertrophic chondrocytes to normal level of embryonic plasma phosphate (7mM) at the time of vascular invasion is associated with activation of FGF signaling and of mitochondrial apoptotic pathway during embryonic endochondral bone formation.
Thus, our investigations demonstrate that 7 mM phosphate promotes terminal differentiation of hypertrophic chondrocytes in a model of embryonic bone formation. An earlier appearance of osteopontin-positive, collagen X negative chondrocytes is associated with a decrease in BrdU positive proliferating round chondrocytes, suggesting enhanced differentiation of these cells into hypertrophic chondrocytes by 4 days in culture. The relative contribution of decreased proliferation to the growth impairment observed with 7mM phosphate is evidenced by the observation that inhibiting apoptosis of the terminally differentiated hypertrophic chondrocytes results in an incomplete reversal of the impaired growth and phenotype. Thus, our investigations demonstrate that phosphate modulates proliferation, differentiation and apoptosis of embryonic hypertrophic chondrocytes and point to a role for phosphate ions in endochondral bone development.
Acknowledgments
Grants: Contract grant sponsor: NIH; Contract grant numbers: R01DK46974 and T32DK007028
References
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Gunther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, Moseley JM, Martin TJ, Anderson DJ, Karsenty G. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 2000;406:199–203. doi: 10.1038/35018111. [DOI] [PubMed] [Google Scholar]
- Klement BJ, Spooner BS. Embryonic mouse pre-metatarsal development in organ culture. J Exp Zool. 1993;265:285–294. doi: 10.1002/jez.1402650309. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Legeai-Mallet L, Benoist-Lasselin C, Munnich A, Bonaventure J. Overexpression of FGFR3, Stat1, Stat5 and p21Cip1 correlates with phenotypic severity and defective chondrocyte differentiation in FGFR3-related chondrodysplasias. Bone. 2004;34:26–36. doi: 10.1016/j.bone.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Makras P, Hamdy NA, Kant SG, Papapoulos SE. Normal growth and muscle dysfunction in X-linked hypophosphatemic rickets associated with a novel mutation in the PHEX gene. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2007-1296. [DOI] [PubMed] [Google Scholar]
- Mansfield K, Rajpurohit R, Shapiro IM. Extracellular phosphate ions cause apoptosis of terminally differentiated epiphyseal chondrocytes. J Cell Physiol. 1999;179:276–286. doi: 10.1002/(SICI)1097-4652(199906)179:3<276::AID-JCP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mansfield K, Teixeira CC, Adams CS, Shapiro IM. Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism. Bone. 2001;28:1–8. doi: 10.1016/s8756-3282(00)00409-9. [DOI] [PubMed] [Google Scholar]
- Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- Minkin C, St James S, Tao HH, Yu XH, Pockwinse S, MacKay C, Marks SC., Jr Skeletal development and formation of osteoclast-like cells from in situ progenitors in fetal mouse metatarsals cultured in chemically defined medium. Bone Miner. 1991;12:141–155. doi: 10.1016/0169-6009(91)90028-x. [DOI] [PubMed] [Google Scholar]
- Naski MC, Colvin JS, Coffin JD, Ornitz DM. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development. 1998;125:4977–4988. doi: 10.1242/dev.125.24.4977. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci U S A. 2005;102:9637–9642. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni M, Raz R, Coffin JD, Levy D, Basilico C. STAT1 mediates the increased apoptosis and reduced chondrocyte proliferation in mice overexpressing FGF2. Development. 2001;128:2119–2129. doi: 10.1242/dev.128.11.2119. [DOI] [PubMed] [Google Scholar]
- Serra R, Karaplis A, Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J Cell Biol. 1999;145:783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Minkin C. The effects of 1,25-dihydroxyvitamin D3 on osteoclast formation in fetal mouse metatarsal organ cultures. Bone. 1994;15:217–223. doi: 10.1016/8756-3282(94)90711-0. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]