Abstract
Background
The passenger leukocytes in the intestine have a lineage profile that predisposes to graft-versus-host disease (GVHD) in some animal models and have inferior tolerogenic qualities compared with the leukocytes in the liver, other solid organs, and bone marrow. Elimination by ex vivo irradiation of mature lymphoid elements from the bowel allografts is known to eliminate the GVHD risk. We hypothesized that infusion of donor bone marrow cells (BMC) in recipients of irradiated intestine would improve tolerogenesis without increasing the risk of GVHD.
Methods
Orthotopic small intestine transplantation was performed with the GVHD-prone Lewis (LEW)-to-Brown Norway (BN) combination and the reverse GVHD-resistant BN-to-LEW model under a short course of tacrolimus treatment (1 mg/kg/day, days 0–13, 20, 27). Grafts were irradiated ex vivo, using a 137Cs source. In selected experimental groups, donor BMC (2.5×l08) were infused on the day of small intestine transplantation.
Results
The unmodified LEW intestine remained intact, whether transplanted alone or with adjunct donor BMC infusion, but all of the BN recipients died of GVHD after approximately 2 months. Intestinal graft irradiation (10 Gy) effectively prevented the GVHD and prolonged survival to 92.5 days, but all of the BN recipients died with chronic rejection of the LEW grafts, which was prevented by infusion of adjunct donor BMC without causing GVHD. In the GVHD-resistant reverse strain direction (BN → LEW), all intestinal recipients treated for 27 days with tacrolimus survived ≥150 days without regard for graft irradiation or adjunct BMC, but chronic rejection was severe in the irradiated intestine, moderate in the unaltered graft, and least in the irradiated intestine transplanted with adjunct BMC. Mild arteritis in the 150 day allografts of both strain combinations (i.e., LEW → BN and BN → LEW) may have been irradiation associated, but this was prevented when weekly doses of tacrolimus were continued for the duration of the experiment rather than being stopped at 27 days.
Conclusions
Recipients are protected from GVHD by irradiating intestinal allografts, but the resulting leukocyte depletion leads to chronic rejection of the transplanted bowel. The chronic rejection is prevented with adjunct donor BMC without causing GVHD. Although application of the strategy may be limited by the possibility of radiation injury, the results are consistent with the paradigm that we have proposed to explain organ-induced graft acceptance, tolerance, and chronic rejection.
Recent evidence has shown that passenger leukocytes that are normal constituents of all organs migrate after transplantation and are replaced by recipient cells of the same hematolymphopoietic lineages (1–5). With engraftment, the “accepted” organ and the recipient become genetic composites in that a trace population of the donor leukocytes survives peripherally in lymphoid and nonlymphoid recipient tissues (microchimerism) as well as in the allograft. Acute contemporaneous graft-versus-host (GVH) and host-versus-graft (HVG) immune reactions and the subsequent persistence of multilineage microchimerism have been proposed to be essential for successful organ transplantation and the variable development of donor-specific nonreactivity (tolerance).
The quantity and lineage profiles of the passenger leukocytes contained in different kinds of organ grafts strongly influences the quantity and lineages of the chimerism found in the recipients, the relative strengths of the interactive HVG and GVH reactions, graft survival, and graft function (5–7). The passenger leukocytes of the liver and bone marrow cells (BMC), both of which include large numbers of immature leukocytes and cells of myeloid origin, have been shown to be more tolerogenic with a lower risk of graft-versus-host disease (GVHD) than the intestinal passenger leukocytes, which are rich in mature lymphocytes (5).
In the experiments reported here, the hypothesis was tested that modification of the intestinal passenger leukocytes with ex vivo irradiation and the adjunct infusion of donor BMC would improve the control of rejection in a fully allogeneic small intestinal transplantation (SITx) model without causing GVHD. The irradiation doses were designed to eliminate or substantially reduce the mature lymphoid elements of the passenger leukocytes, without destroying precursor hematopoietic cells and without causing irradiation injury to the intestinal epithelial components. The irradiated intestinal grafts were transplanted alone or simultaneously with intravenously infused donor BMC.
The efficacy of these treatments was assessed by recipient and graft survival, recipient weight maintenance, graft histopathology, and the quality and quantity of donor leukocyte microchimerism in Lewis (LEW)-to-Brown Norway (BN) and BN-to-LEW rat strain combinations. We have previously shown that the former combination was GVHD prone in that recipient animals developed fatal GVHD after SITx under a short course of immunosuppression (5–7). Although the lymphocyte migration patterns are similar in both strain combinations, BN-to-LEW transplantation is not complicated by GVHD. Thus, we were able to examine the effect of treatment strategies on the GVH and HVG immune responses in GVHD-prone and GVHD-resistant settings.
MATERIALS AND METHODS
Animals
Inbred male LEW (RT11) and BN (RT1n) rats (200–250 g) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and maintained in laminar-flow, specific pathogen-free animal facilities at the University of Pittsburgh. All procedures in this study were performed according to the guidelines of the Council on Animal Care at the University of Pittsburgh.
Surgical Procedures
Under methoxyflurane anesthesia, orthotopic SITx with caval drainage was performed from LEW-to-BN and BN-to-LEW strains, with previously described methods (6–8). The entire small intestine, from the ligament of Trietz to the ileocecal valve, was harvested on a vascular pedicle consisting of the superior mesenteric artery with a piece of aorta and portal vein. The excised intestinal graft, stored in cold lactated Ringer’s solution in a 50-ml Falcon tube, was then ex vivo irradiated from a 137Cs source. The total time to achieve the irradiation dose of 10 Gy was 2.5 min. End-to-side vascular anastomoses between the graft aorta and the recipient infrarenal aorta, and between the graft portal vein and recipient vena cava, were performed with 10-0 Novafil suture. The recipient small bowel was removed except for a few centimeters of proximal jejunum and distal ileum. Enteric continuity was restored by proximal and distal end-to-end intestinal anastomoses.
Donor BMC were obtained as reported before (5). The medulla of the tibias and femurs were flushed with RPMI, supplemented with 50 µg/ml gentamicin (Life Technologies, Grand Island, NY). A total of 2.5×108 cells, determined to be viable by trypan blue exclusion, was intravenously infused into recipients on the day of SITx. The dose of BMC (2.5×108) was selected because it corresponded to that obtained from a single donor rat. We previously showed that infusion on the day of transplantation with this dose of BMC augmented chimerism and improved heart allograft outcome (9, 10). We have previously shown that infusion of syngenic (BN) BMC does not have any effect on allograft outcome (9).
Animals were given prophylactic cefamandole nafate (20 mg/day) for 3 postoperative days. Tacrolimus (FK506; Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan) was intramuscularly administered to recipients at a daily dosage of 1.0 mg/kg on days 0–13, 20, and 27. All animals were followed for at least 150 days after SITx and some were kept as long as 203 days before sacrifice to establish the permanence of graft acceptance. No animals that lived 150 days died subsequently for reasons other than sacrifice for study purposes. Clinical signs of rejection were defined as continuous or intermittent diarrhea and weight loss. GVHD was clinically defined by cutaneous erythema, and by hair and weight loss. Both diagnoses were confirmed by histopathological analysis.
Routine and Immunohistopathological Analyses
Histopathological analysis was performed on all animals including those surviving ≥150 days and those that died prematurely. Graft jejunum and ileum including Peyer’s patches (PP), mesenteric lymph nodes (MLN), and recipient tissues were fixed in formalin, embedded, cut in 6-µm sections, and stained with hematoxylin and eosin.
The histopathological changes of the intestinal grafts and native intestines were evaluated in a blinded fashion. The degree of epithelial injury was determined by conventional histopathologic criteria and by the frequency of apoptosis in crypt epithelial cells. The extent and nature of inflammation in the intestine and the presence of mesenteric fibrosis in MLN were separately recorded as either present or absent. The number of germinal centers in the intestine was counted and expressed as the number per section. Severity of mesenteric arteriopathy was semiquantitatively graded as 0 (none), 1 (mild), and 2 (severe).
Another portion of each sample was snap-frozen in OCT compound and sectioned at 4 µm for immunohistochemical stain using a routine indirect avidin-biotin complex method as previously described (9). Biotinylated monoclonal antibody (mAb) L21-6 (mouse IgG1), which is specific for MHC class II antigens on LEW but not BN (11), was applied to differentiate donor MHC class II+ cells in the tissue obtained after LEW-to-BN SITx.
Flow Cytometry
Recipient peripheral blood
Percentages of donor and recipient hematolymphoid cells were sequentially determined by flow cytometry in recipient peripheral blood after lysis of red blood cells. LEW and BN cells were identified with affinity-purified biotinylated rat mAbs 163 (rat IgG2b) and 42 (rat IgG2a) (from Dr. Heinz Kunz, Department of Pathology, University of Pittsburgh), which are specific for the RT1.A1 (MHC class I) antigen on LEW and the RT1.An antigen on BN, respectively (12). Phycoerythrin-conjugated streptavidin (PharMingen, San Diego, CA) was used as a secondary antibody. Cells were analyzed using Coulter Elite ESP (Coulter Corp., Miami, FL).
Allografts
MLN lymphocytes were isolated by injecting RPMI into MLN and by filtration through nylon mesh. Lymphocytes in the PP were obtained as previously described (6). After the mucosal layer of the intestine was disrupted, PP were excised, cut into small pieces, digested in a water bath for 30 min at 37°C with RPMI containing 0.05% collagenase (type B; Boehringer Mannheim, Mannheim, Germany), 10 mM HEPES (Life Technologies), 50 µg/ml gentamicin, and 2% fetal bovine serum (Life Technologies), and then passed through nylon mesh to separate the remaining connective tissue fragments from the lymphocyte fraction. Isolated cells were washed twice with RPMI containing 5% fetal bovine serum, 10 mM HEPES, and 50 µg/ml gentamicin, and further purified by centrifugation over Ficoll-Paque (s.g. 1.077; Biotech AB, Uppsala, Sweden). The interface was collected and washed twice with Hanks’ balanced salt solution containing 1% bovine serum albumin and 0.1% sodium azide (Sigma, St. Louis, MO).
Phenotypes and lineages of lymphocytes in the graft MLN and PP were determined with two-color flow cytometry using mAbs 163 and 42; and fluorescein-conjugated lineage-specific mAbs, including R7.3 (αβTCR), W3/25 (CD4), OX8 (CD8), 0×33 (B cells), and 3.2.3 (natural killer cells) (all from PharMingen). Isotype-matched nonspecific antibodies were substituted for the primary reagents in the negative controls.
Statistical Analyses
Comparison of animal survival days between groups was made with Wilcoxon rank-sum test. Data in histopathology and flow cytometry were shown as mean ± SD and analyzed by one-way analysis of variance, Fisher’s PLSD test, and Student’s t test. Statistical significance was established at P<0.05.
RESULTS
Effects of Different Irradiation Doses in Syngenic SITx
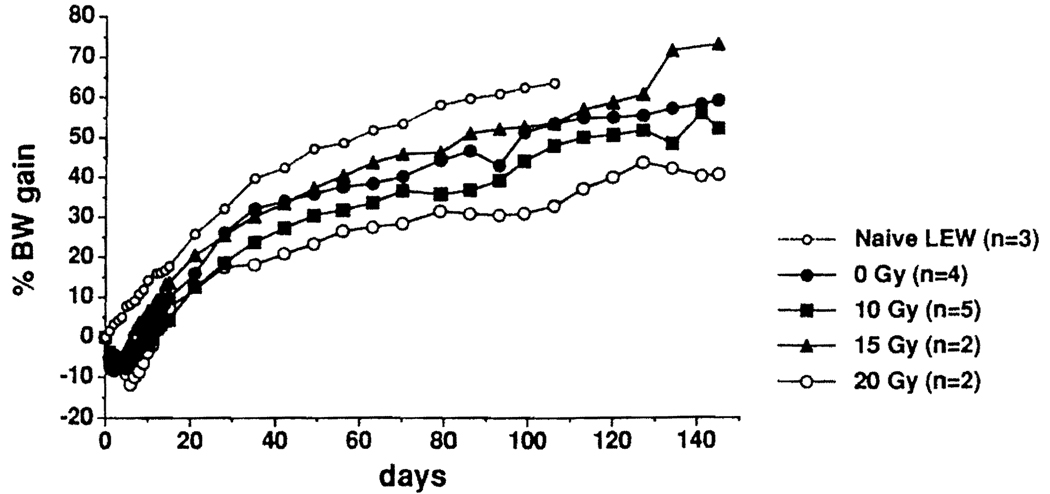
LEW recipients received syngenic LEW intestine after variable doses of graft irradiation. Long-term adverse effects were assessed by animal growth (i.e., body weight) and graft histopathology. Using escalating doses from 0 to 15 Gy ex vivo graft irradiation, rates of body weight gain after SITx were no different from nonirradiated controls. There was evidence of a malnutrition, however, in animals whose grafts were exposed to 20 Gy (Fig. 1).
FIGURE 1.
Effect of different dosages of ex vivo graft irradiation on mean body weight gain of LEW recipients of syngeneic intestinal grafts compared with recipients of nonirradiated grafts and nonoperated controls. Growth retardation was seen only after 20 Gy (2000 rad).
No specific histopathological changes attributable to radiation were found in the intestine from rats sacrificed between 150 and 300 days after transplantation. Epithelia and crypts were intact in all animals, with only rare apoptotic bodies (0–2 cells per 10 crypts), even in intestine subjected to 20 Gy. Arterial changes including inflammation, atherosclerosis, or thrombosis were not seen in grafts irradiated with 10 or 15 Gy. Vacuolization of smooth muscle in the mesenteric arteries was minimally increased in grafts subjected to 10 and 15 Gy, but this also was found in nonirradiated grafts. When grafts were irradiated with 20 Gy, the smooth muscle vacuolization was noticeably increased, and one animal had mild atherosclerosis and fibrosis in the arterial media. Based on these data, ex vivo irradiation at a dose of 10 Gy was selected for the following allotransplantation experiments.
Animal Survival, Graft Histopathology, and Chimerism in Allogenic SITx
LEW-to-BN transplantation
Survival
Untreated BN recipients rejected LEW allografts after a median of 11.5 days (group 1, Table 1). As previously reported (5–7), all of the tacrolimus-treated control recipients died of GVHD with a median survival of 51 days (group 2). Death of some animals in this group was associated with pneumonia. Tacrolimus-treated recipients of unmodified intestinal grafts who were given adjunct donor BMC (group 3) also died of GVHD after a slightly longer survival (median 63 days), but the difference from group 2 was not statistically significant (P=0.068).
TABLE 1.
Recipient survival after LEW-to-BN and BN-to-LEW intestinal transplantation
| Group | Graft irradiation (Gy) | Donor BMC (×108) | Tacrolimusa | N | Survival (days) | Median (days) |
|---|---|---|---|---|---|---|
| LEW-to-BN | ||||||
| 1 | 0 | 0 | No | 6 | 11, 11, 12, 12, 13, 13 | 11.5 |
| 2 | 0 | 0 | Yes | 5 | 41, 46, 51, 55, 55, | 51.0 |
| 3 | 0 | 2.5 | Yes | 5 | 48, 52, 63, 65, 70 | 63.0 |
| 4 | 10 | 0 | Yes | 6 | 70, 84, 92, 93, 98, 134 | 92.5 |
| 5 | 10 | 2.5 | Yes | 6 | 135, >150×5 | >150 |
| BN-to-LEW | ||||||
| 6 | 0 | 0 | No | 6 | 9, 9, 10, 11, 12, 14 | 10.5 |
| 7 | 0 | 0 | Yes | 6 | >150 ×6 | >150 |
| 8 | 10 | 0 | Yes | 5 | >150 ×5 | >150 |
| 9 | 10 | 2.5 | Yes | 5 | >150 ×5 | >150 |
Tacrolimus; 1.0 mg/kg/day on days 0–13, 20, and 27.
Bold number: severe and fatal GVHD.
Graft irradiation (10 Gy) prevented GVHD in all rats of group 4 and prolonged median survival to 92.5 days (range 70 to 134), but all died of nutritional failure. In contrast, five of six recipients of irradiated intestine plus adjunct donor BMC (group 5) survived >150 days without GVHD (Table 1); the exceptional animal began to lose weight at 100 days and died at 135 days.
Nutrition
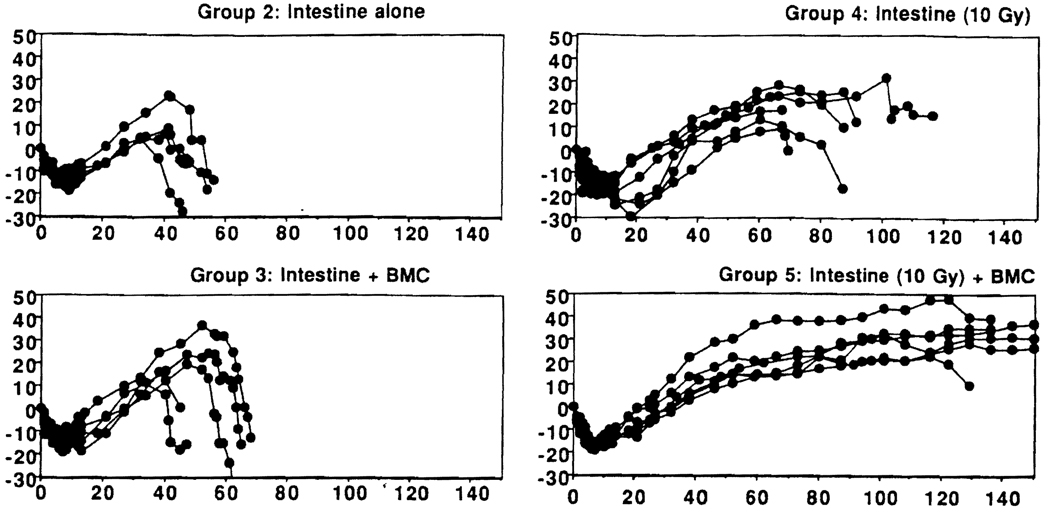
The body weight changes correlated well with survival (Fig. 2). Weight gain of tacrolimus-treated BN recipients of nonirradiated (group 2) as well as nonirradiated allografts with adjunct donor BMC (group 3) was interrupted with the development of GVHD, followed by rapid weight loss and death. Recipients of irradiated intestine (group 4) steadily gained weight for about 2 months, but they developed periodic diarrhea thereafter and lost body weight until death. In contrast, four of the six BN recipients of irradiated intestine and adjunct donor BMC (group 5) maintained normal weight for 150 days.
FIGURE 2.
Body weight changes (expressed in %, pretransplantation weight) of BN recipients of LEW intestinal allografts transplanted alone (groups 2 and 4) or with adjunct BMC. Each line represents a single animal.
Histopathology
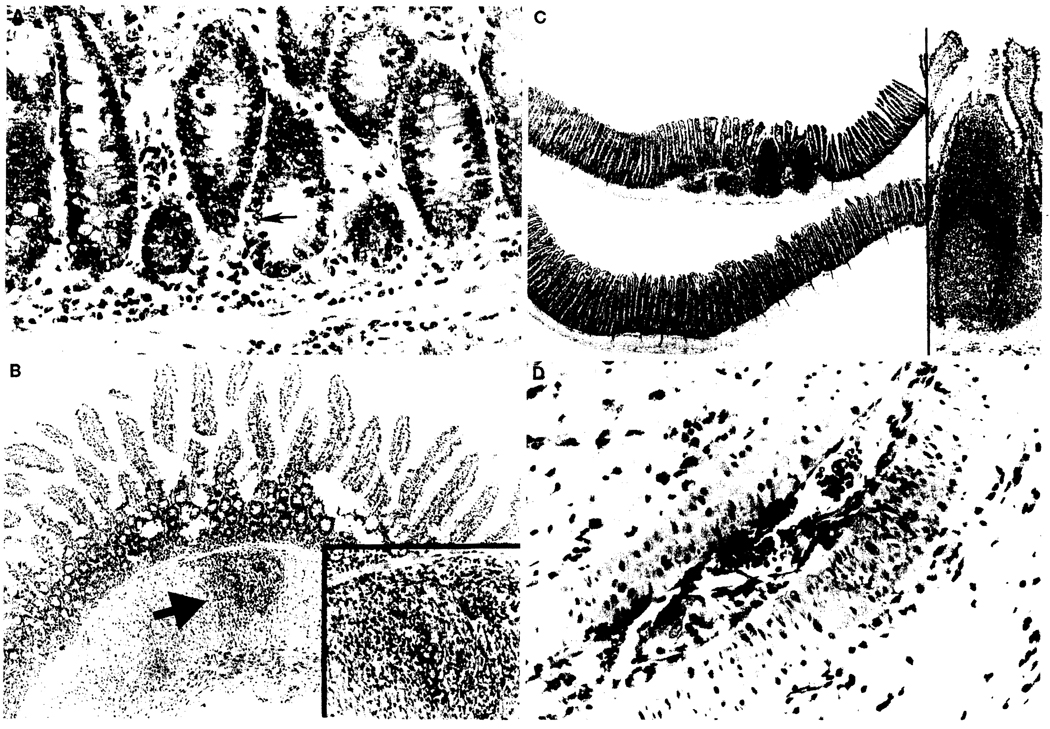
Low-grade apoptosis, ranging from 0 to 5 cells per 10 crypts, was found in all LEW intestinal allografts (Table 2 and Fig. 3A). The allografts were intact in the group 2 and 3 animals that died from GVHD after 41–70 days. One of the five intestinal allografts in group 4 and three of the six in group 5 contained significant foci of mural inflammation (Table 2 and Fig. 3B [arrow]), comprised mostly of histiocytes and numerous eosinophils. These foci were located primarily in the muscularis propria and seemed to follow penetrating branches of vessels. The intestinal inflammation was unlike that seen in uncontrolled acute rejection. It also was found to a lesser degree in the remnants of recipient bowel.
TABLE 2.
Histopathological findings of allograft intestine and MLN
| Group | Graft irradiation (Gy) | Donor BMC (×108) | Intestine |
MLN |
|||
|---|---|---|---|---|---|---|---|
| Epithelial apoptosisa | Mural inflammationc |
Population of germinal center |
Fibrosisc | Arteritis | |||
| LEW-to-BN | |||||||
| 2 | 0 | 0 | 0–5 | 0 | 2 | 0 | 0 |
| 4 | 10 | 0 | 0–5 | 1/5 | 0.71±0.42 | 4/4 | 1.60±0.89 |
| 5 | 10 | 2.5 | 0–3 | 3/6 | 1.27±1.49 | 4/6 | 0.83±0.68 |
| 5Ab | 10 | 2.5 | 0–1 | 1/5 | 2.20±0.88 | 0 | 0 |
| BN-to-LEW | |||||||
| 7 | 0 | 0 | 0–1 | 0 | 0.60±0.62 | 1/4 | 0.38±0.25 |
| 8 | 10 | 0 | 0–1 | 0 | 0.05±0.11 | 4/5 | 1.50±0.71 |
| 9 | 10 | 2.5 | 0–1 | 0 | 0.99±0.96 | 1/5 | 1.00±0.61 |
Apoptosis per 10 crypts.
5A: extra group of BN recipients (n=9) received 10 Gy irradiated LEW grafts and BMC. They were treated continuously with tacrolimus and sacrificed at 60 (n=4) and 150 days (n=5). Results from animals sacrificed at 150 days were shown here and were not significantly different compared with those at 60 days.
Number of animals with intestinal mural inflammation or MLN fibrosis.
FIGURE 3.
Irradiated LEW allografts (all 10 Gy) in different experimental groups. (A) Group 4 (postoperative day [POD] 84), without adjunct BMC: lower crypts. Occasional crypt cell apoptosis was seen (arrow) (original magnification ×20). (B) Group 5 (POD 157), with adjunct BMC: the mucosal architecture is unremarkable, but focal mural inflammation is evident (arrow) without obvious infectious etiology. A less severe similar lesion was seen in the residual recipient bowel (×4). Inset: higher power view of the muscularis inflammation, which was comprised primarily of mononuclear cells and eosinophils (×10). (C) Group 5 (POD 203) with adjunct BMC: well-developed PP were more frequently observed in this group (upper intestinal strip) than in the non-BMC group 4 (lower intestinal strip, POD 70) (×5). Inset: higher power view of the marked PP (×10). (D) Group 4 (POD 92), without adjunct BMC: arteriopathy of a mesenteric artery branch including smooth muscle vacuolization and arteritis, which begins as mononuclear inflammation within the intimal layer (endothelialitis) (×20).
In the nonirradiated allografts of the tacrolimus-treated recipients dying of GVHD (groups 2 and 3), the intact PP and MLN were well populated. Loss of lymphoid cells and an increase of fibrotic changes were prominent in the PP and MLN of the irradiated grafts of group 4, findings attributable to chronic rejection (13). These abnormalities were not present or were not severe in the intestinal allografts of group 5 that had been transplanted in conjunction with donor BMC (Table 2 and Fig. 3C). Severity of arteritis was significantly increased in the MLN of the irradiated allografts of group 4 animals compared with that of group 5 with adjunct donor BMC (Table 2 and Fig. 3D). Smooth muscle vacuolization was not increased over that seen in nonirradiated isografts at comparable times, and no evidence of other arterial injury such as atherosclerosis or smooth muscle fibrosis was seen in any of the irradiated grafts.
Comparison in the tacrolimus-treated groups 2–5 of the histopathologic findings in the allografts, and particularly of vascular abnormalities that might be attributed to the ex vivo irradiation in groups 4 and 5, was made difficult by the different posttransplantation times of specimen procurement at death or sacrifice: about 2 months in group 2, 3 months in group 4, and 5 months or beyond in group 5. Consequently, nine additional experiments were performed with the group 5 protocol, except that the weekly doses of tacrolimus were continued instead of being discontinued after posttransplantation day 27. Four animals were sacrificed at 60 days, and the other 5 at 150 days. There were no arterial changes in any of the allografts (Table 2), which had nearly normal PP and MLN that were well populated with leukocytes. The protection by immunosuppression suggested that the vascular lesions were largely due to rejection.
Recipient chimerism
High percentages of donor cells in recipient blood were associated with rampant GVHD and the death of all tacrolimus-treated BN recipients of unaltered LEW intestine grafts whether or not adjunct BMC was given (Groups 2 and 3, Table 3). Although the BMC-augmented animals of group 3 lived longer and had marginally greater chimerism, the clinical courses were much the same.
TABLE 3.
Percentages of donor phenotype cells in the peripheral blood after LEW → BN transplantation under short course of tacrolimusa
| Group | Graft | Posttransplantation days |
|||||
|---|---|---|---|---|---|---|---|
| day 7 | day 14 | day 28 | day 42 | day 70 | day 120 | ||
| 2 | Intestine | 3.5±1.5 | 2.3±1.3 | 3.9±2.3 | 3.8±2.6 | NA | NA |
| 3 | Intestine+BMC | 8.6±3.7b | 6.6±2.2c | 3.9±2.9 | 8.3±6.0 | NA | NA |
| 4 | Intestine (10˙Gy) | 0.7±1.2c | 0.4±0.3c | 1.2±0.6b | 1.2±1.0 | 0.5±0.4 | 0.2 |
| 5 | Intestine (10 Gy)+BMC | 2.1±1.3 | 1.4±1.4 | 2.2±2.0 | 2.2±1.2 | 1.2±1.2 | 1.6±1.4 |
1.0 mg/kg/day on days 0–13, 20, and 27.
P<0.05 vs. group 2.
P<0.01 vs. group 2.
NA, not applicable.
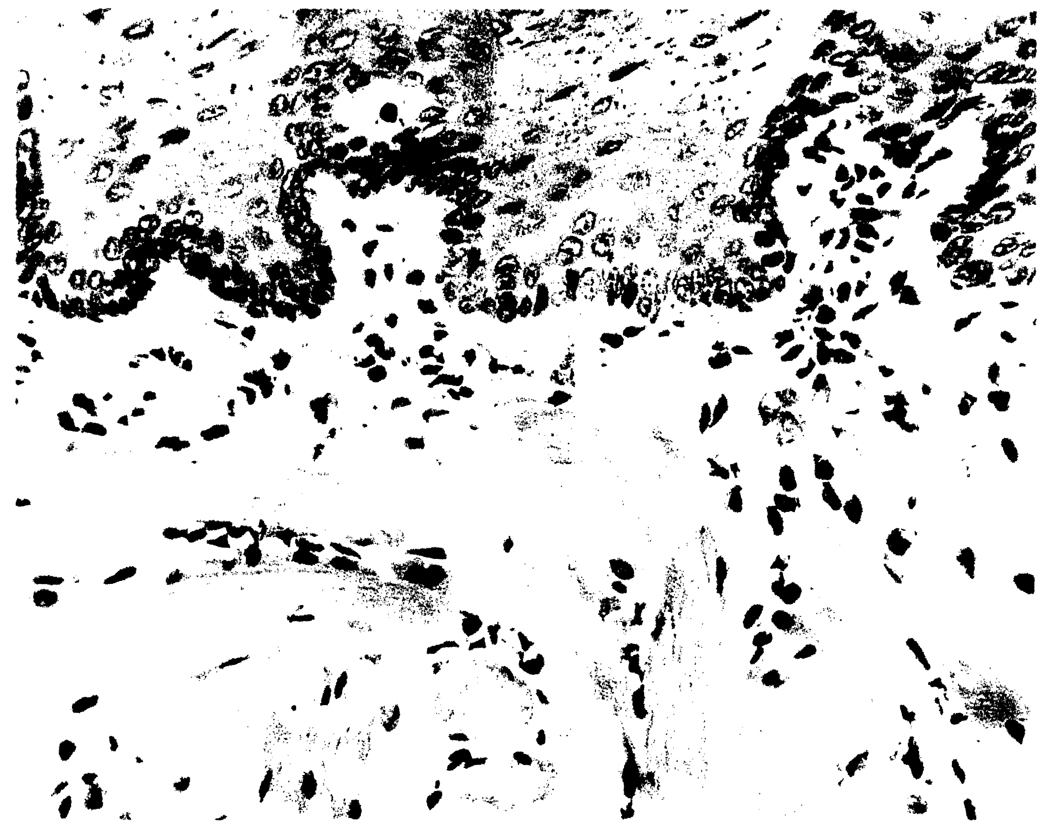
Donor phenotype cells remained at low but stable levels after transplantation under tacrolimus of grafts irradiated with 10 Gy (group 4). When the recipients of irradiated intestine also were administered adjunct donor BMC (group 5), stable chimerism was maintained in those GVHD-free animals at about twice the level as in group 4 (Table 3). Chimerism in the tissues of the group 5 recipients was confirmed by immunohistochemical stain with the L21-6 mAb that identified donor (LEW) MHC class II-positive cells (Fig. 4)
FIGURE 4.
Microchimerism at POD 157 in the tongue of a BN recipient of an irradiated LEW intestine and adjunct BMC (group 5). Donor cells (red) are scattered among other recipient cells. No evidence of epithelial injury indicative of GVHD was observed (L21-6 immunoperoxidase with aminoethylcarbazole, ×20).
BN-to-LEW transplantation
Survival
Although the BN intestinal allografts invariably were rejected by untreated LEW recipients (group 6), none of the recipients treated with the 27-day course of tacrolimus developed GVHD, and all survived for 150 days after SITx (Table 1, groups 7–9) without regard for graft irradiation alone or in combination with donor BMC infusion.
Histopathology
The BN intestine allografts were intact in groups 7–9, and apoptosis was seen in less than 2 cells per 10 crypts (Table 2). The mural inflammation found in LEW grafts transplanted into BN recipients was not seen in this combination. The most normal allografts were those of group 9 that had been irradiated and were transplanted in conjunction with adjunct BMC. In addition to well-preserved overall architecture, the MLN and PP in the allografts of group 9 were filled with lymphocytes and had a better score for lymphoid contents than either the unaltered grafts of group 7 or the irradiated grafts of group 8. Irradiated intestinal allografts without adjunct BMC (group 8) showed the most severe histopathological abnormalities including increased incidence of MLN fibrosis and arterial inflammation. Arteritis in group 8 was more severe than in group 7 (P<0.001). Arteritis was slightly enhanced in group 9 relative to the control allografts of group 7; however, the difference did not reach statistical significance (P=0.123).
Cell Constituency of Graft MLN and PP
In both the LEW → BN and BN → LEW models, the rapidity and completeness of leukocyte replacement in the MLN and PP were increased by intestinal irradiation alone or with adjunct BMC (Fig. 5). With both strain combinations, the repopulating recipient leukocytes in graft MLN and PP included CD4+, CD8+, B, and natural killer cells, in proportions similar to those in normal animals of the donor and recipient strains, except for a higher percentage of B cells. This is shown in Figure 6 15 days after LEW → BN transplantation under the experimental conditions of group 2 (unaltered intestine and no BMC) and group 5 (intestinal irradiation plus adjunct BMC).
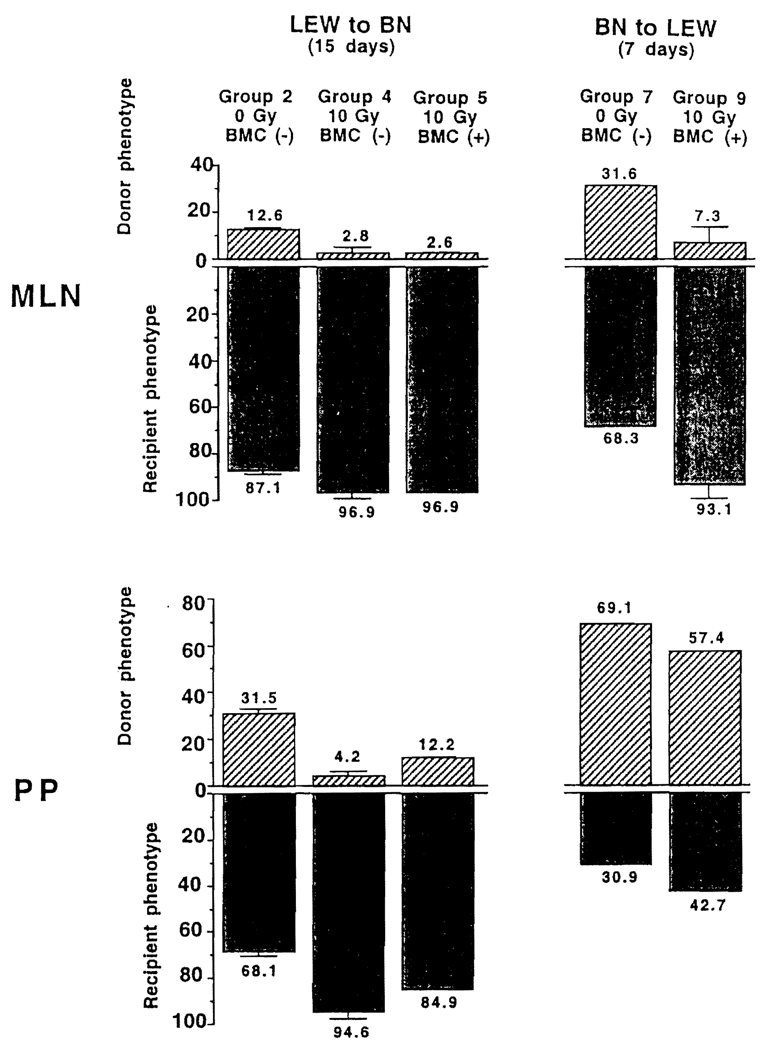
FIGURE 5.
Percentages of donor and recipient cells in graft MLN and PP: (Left panel) 15 days after LEW → BN intestinal transplantation; (Right panel) 7 days after BN → LEW transplantation. Note that the replacement was more rapid in the irradiated grafts without or with adjunct BMC than in the nonirradiated grafts and that it occurred more slowly in PP than in MLN under all the experimental conditions of groups 2, 4, 5, 7 and 9. n=3 for all experiments.
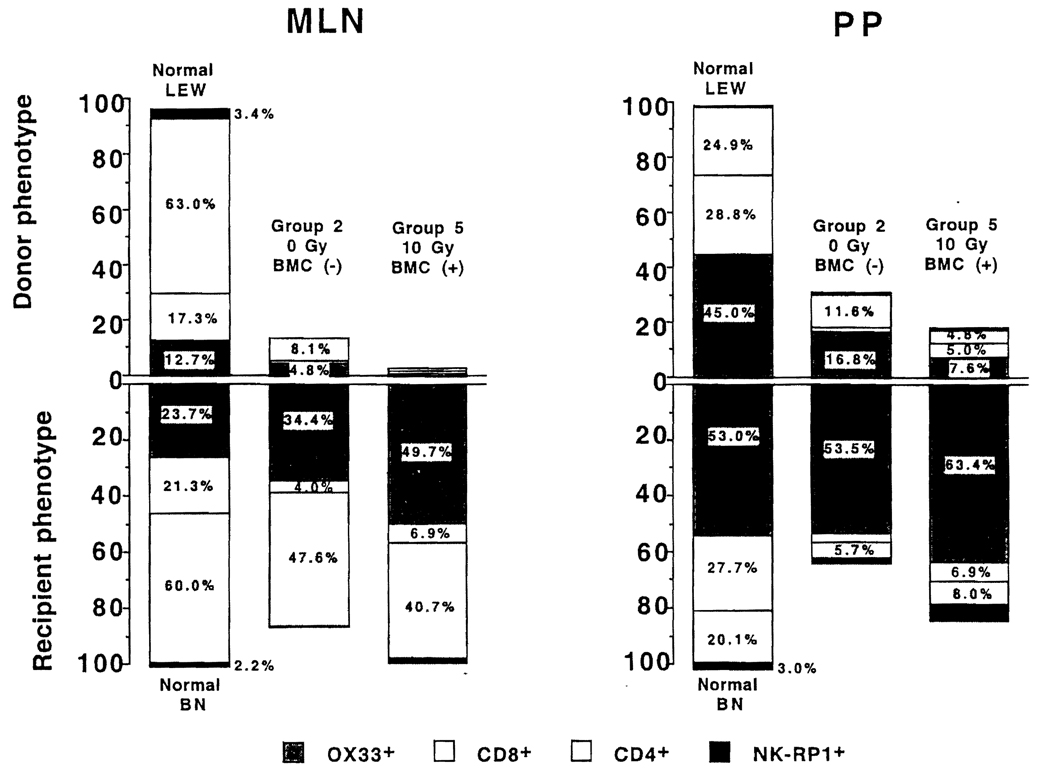
FIGURE 6.
(Left panel) Donor and recipient leukocyte lineages in graft MLN 15 days after LEW → BN transplantation of nonirradiated intestine only (group 2, second bar) and of irradiated intestine plus adjunct bone marrow (group 5, third bar). The upright portion of the first bar shows the lineages in the MLN of normal nonoperated LEW rats, and the inverted portion of the first bar depicts this information for the MLN of normal BN rats. (Right panel) Findings in the PP of the same allografts as the left panel.
LEW → BN transplantation
After 15 days of tacrolimus treatment, more donor leukocytes in groups 4 and 5 had been replaced by recipient cells than in group 2 (Fig. 5, left panel). Compared with the MLN, the replacement was somewhat retarded in the PP (Fig. 5, left panel, bottom).
BN → LEW transplantation
Because the cell exchange previously had been demonstrated by immunohistochemistry studies to be complete by 15 days (13), flow cytometry analysis of the BN allografts was done at 7 days. Even at this early time, the majority of leukocytes in the MLN of the BN grafts already had become recipient (LEW) (Fig. 5, right panel, upper) but not in the PP (right panel, lower).
DISCUSSION
Attempts to modify intestinal allografts have been driven mainly by the historically rooted conviction that GVHD would plague clinical trials. As had been predicted by Billingham (14), the lethal consequences of GVHD were proved by Monchik and Russell (15) in genetically controlled rat experiments in which semiallogeneic small bowel was transplanted from parent to F1 hybrid offspring recipients who were incapable of rejecting the grafts but were subject to their attack. Although the F1 hybrid rat models emphasized the peril of a host-graft immunologic imbalance if this favored the intestinal graft, such laboratory experiments overstated the threat of GVHD to noncytoablated humans. GVHD has been shown to be a trivial problem in an extensive clinical intestinal transplant experience (16–21) and for that matter in outbred large animal models (22–26) unless the recipient is cytoablated (27).
A similarly distorted impression of the risk from GVHD is obtained from the LEW → BN intestinal transplantation model used for many of the experiments reported here, our previous studies (5, 6, 28), and those of Foster et al. (29). The fully allogeneic LEW/BN rat strain combination used in our experiments had unusual advantages for examination of the HVG and GVH components of the double immune reaction that follows intestinal and all other kinds of transplantation (5). The distinction of donor from recipient leukocytes in tissues and blood can be made precisely because of the availability of the L26–6 mAb that densely stains class II+ cells of almost all rat strains including LEW, but not those of BN (11).
The failure of BN cells to stain apparently is due to an abnormality of the invariant chain. Inefficient antigen presentation due to the abnormality has been postulated to be the explanation for the universal donor status (i.e., low response inducer) of this strain (6, 11). Possibly related to the same abnormality, the BN rat is highly susceptible to GVHD (5–7) allowing this usually invisible limb of the two-way immune reaction to be exposed for investigation (5), as in parent to defenseless offspring F1 hybrid models. It has been established unequivocally (15, 30) and confirmed herein that the GVHD that develops after intestinal transplantation in immunologically “unbalanced” donor/recipient combinations can be readily circumvented by graft irradiation. This objective also can be met by pretreating the donor with antilymphocyte serum (31, 32), but only when donor animals were pretreated for 48 hr before harvesting intestinal grafts. Ex vivo graft infusion with antilymphocyte serum failed to prevent GVHD (31). Because this study was designed to fit a clinical setting in which pretreatment of donor with antilymphocyte serum or similar mAbs for 48 hr usually is not possible, ex vivo irradiation was chosen as the method to modify intestinal allografts.
The tacrolimus-treated BN rats in our experiments who received unaltered LEW intestine alone or in conjunction with donor bone marrow (experimental groups 2 and 3) all died of GVHD after 41–70 days. Interestingly, the addition of infused donor BMC to the passenger leukocyte load of the intestine did not demonstrably intensify the GVHD, a previously unpublished observation that justified the initiation in 1995 of clinical protocols of adjunct BMC for intestinal and multivisceral recipients (20, 21, 33). It is noteworthy that at the time of recipient death from GVHD, the transplanted intestinal grafts were intact with well-preserved MLN and PP (i.e., gut-associated lymphoid tissue [GALT]).
As expected, the GVHD in the LEW → BN model was completely avoided by irradiating the LEW intestine of the group 4 BN recipients. However, all of the animals died after 70–134 days of nutritional insufficiency caused by chronic bowel rejection that included involution and disappearance of the GALT. The persistence of very low level microchimerism in these animals was consistent with the survival of a subset of radiation-resistant stem cells that has been described many times over the last 40 years (reviewed by Hendry and Lord [34]) but demonstrated convincingly only since 1990 (35–37). However, the number of the passenger leukocytes in the recipients of irradiated bowel apparently was reduced below the threshold necessary to acutely induce the donor-specific clonal exhaustion-deletion that is the seminal basis of organ allograft acceptance and acquired tolerance (1, 2, 4). The same antitolerogenic effect is caused by lethal whole body irradiation of the donor (38). K0 et al. (39) have recently demonstrated the same principle, by administering a donor leukocyte-specific antibody to the recipient on the day of rat heart transplantation.
In contrast, a long period of immunosuppression-free survival of BN recipients of irradiated LEW allografts was regularly accomplished by infusing 2.5×108 adjunct donor BMC, analogous to restoration of the tolerogenicity of leukocyte-depleted liver allografts by repletion of hepatic passenger leukocytes (40, 41). Failure of the large dose of BMC to cause the GVHD regularly produced by the intestinal passenger leukocytes in the LEW → BN model was explained by the differences in the lineage constituency of the two leukocyte populations and the state of maturation of the lineages (5).
Such experiments have demonstrated both by positive and negative example that the penalty for passenger leukocyte depletion, without substitution of the alternative supply of mobile donor cells required for clonal exhaustion-deletion, is erosion of the seminal mechanisms of engraftment (1, 2, 4). Conversely, replacement of the intestinal leukocytes lost to ex vivo irradiation by immature donor BMC was able to alter both the GVH and HVG immune reactions in a favorable way. As described in detail elsewhere, the mechanisms by which this was accomplished involve an antigen-specific double immune reaction (HVG and GVH), no different in principle with the intestine than with transplanted solid organs (1–6). The distribution of the donor leukocytes between the recipient lymphoid and nonlymphoid tissues, blood, and the allograft itself rapidly changes in a time-dependent manner as described in detail (10, 42, 43) and summarized elsewhere (4).
The adverse effect of ex vivo irradiation on tolerogenesis was most unequivocally shown when the intestinal transplantation was done with the BN → LEW model in which the confounding factor of GVHD was not present. All of the tacrolimus-treated LEW recipients survived ≥150 days in good condition but, as previously reported (13), moderate chronic rejection including damage to the GALT structures (MLN and PP) was found despite tacrolimus in the nonirradiated allografts of group 7. These changes were far more advanced, however, in the irradiated allografts of the tacrolimus-treated animals of group 8. In contrast, there was essentially no evidence of rejection in the irradiated allografts that had been transplanted with adjunct BMC to tacrolimus-treated recipients (group 9).
Although the best results in both the LEW → BN and BN → LEW models were obtained with the combination of bowel irradiation and BMC infusion, the possibility of subtle damage to the intestine from ex vivo irradiation, taking months or years to develop, was not ruled out in these experiments, even at the seemingly conservative dose of 10 Gy that also is thought to be safe for dog (24), pig (44), and presumably human intestine. Direct damage to the endothelial cells has been proposed to be the chief mechanism of irradiation damage (45, 46) ultimately leading to destruction of the microcirculation. The findings of radiation vasculopathy are dose and time dependent. They include subendothelial connective tissue proliferation, disruption of the elastic lamina, accumulation of intimal and subintimal fibrinoid substances, degeneration of smooth muscle, dense fibrosis of the adventitia, aggregation of foamy histiocytes in the damaged wall, and obliteration of vasa vasorum (45–47).
Local or regional radiotherapy for malignant neoplasms, with maximum tolerated doses (usually exceeding 20 Gy), is the usual cause of the radiation vasculopathy. Since ex vivo irradiation is considered to be less harmful than irradiation of tissues with blood circulation, the 10 Gy dose used in our experiments cannot be considered comparable to the dosages in the oncology studies. In the context of transplantation, however, vasculopathy of small muscular arteries has been described in recipients of allogenic bone marrow transplantation conditioned by total body irradiation (48). In addition to the irradiation, the complication has been attributed to multiple factors (e.g., immune activation, immunosuppression, infection, GVHD) (48). Combined with these alloimmunity-associated factors, all of which are operational in the organ recipient, it is theoretically possible that an otherwise inconsequential dose of ex vivo irradiation could become significant.
Thus, although it was reassuring that 10 Gy irradiation of syngeneic bowel (isografts) caused no demonstrable impairment of weight gain or histopathologic changes, the finding of slight but consistent increases of arteritis in the irradiated intestine of both the LEW → BN and BN → LEW models was disquieting. Consequently, additional experiments were performed, using the same protocol as in group 5 (i.e., intestinal irradiation plus adjunct BMC) except that weekly doses of tacrolimus were continued throughout the 150 days of observation instead of stopping at day 27. The arterial changes were completely prevented, establishing an alloimmune etiology of the vasculitis. However, they did not conclusively eliminate the possibility of an irradiation cofactor.
Collectively, the studies reported herein illustrate how insight into the mechanisms of allograft acceptance and tolerance can help devise realistic clinical strategies to improve organ transplantation. The insight began to evolve a decade ago with observations of intestinal passenger leukocyte migration and replacement (6, 13, 28) that quickly matured to the definition of all transplantation outcomes in terms of a double immune reaction (HVG and GVH) (1–5). The role of intestinal transplantation in the evolution was an important one because the two immune responses could be so easily studied separately in models like those of the present report. At the beginning of this period, intestinal transplant conferences were dominated by studies of lymphoid depletion by a variety of methods (22, 24, 29–32, 44), even including mechanical removal of all accessible lymphoid tissue. At a practical level, it was soon learned that avoidance of the GVH reaction was not needed in most experimental intestinal transplantation models or clinically. The current experiments show how such depletion of the host-reactive passenger leukocytes is overtly inimical to long-term intestinal graft survival unless an alternative leukocyte source is provided.
With all organs, the key objective is provision of a large enough volume of optimally immunogenic/tolerogenic donor leukocytes to induce some level of tolerance, even though this requires an umbrella of immunosuppression and is incomplete (1, 2, 4). This objective can be partly accomplished by infusing adjunct BMC as has been done safely in current clinical trials (20, 21). This study demonstrates further active modification of the intestinal leukocyte composition is advantageous. A further search for methods to do this other than with ex vivo irradiation would be considered because of the difficulty of eliminating with absolute certainty the possibility of radiation-associated vasculopathy.
Footnotes
This work was supported by the National Institutes of Health grants DK 29961, R01 AI038899, and R01 DK54232.
REFERENCES
- 1.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS. The lost chord: microchimerism and allograft survival. Immunol Today. 1996;17:577. doi: 10.1016/s0167-5699(96)10070-0. discussion 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown Norway rats. Transplantation. 1995;60:158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murase N, Demetris AJ, Woo J, et al. Graft-versus-host disease after Brown Norway-to-Lewis and Lewis-to-Brown Norway rat intestinal transplantation under FK506. Transplantation. 1993;55:1. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanabe M, Murase N, Demetris AJ, et al. The influence of donor and recipient strains in isolated small bowel transplantation in rats. Transplant Proc. 1994;26:3733. [PMC free article] [PubMed] [Google Scholar]
- 8.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small-bowel and liver grafts. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 9.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVH reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 10.Terakura M, Murase N, Demetris AJ, Ye Q, Thomson A, Starzl TE. Lymphoid/non-lymphoid compartmentalization of donor leukocyte chimerism in rat recipients of heart allografts, with or without adjunct bone marrow. Transplantation. 1998;66:350. doi: 10.1097/00007890-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 11.Yagihashi A, Takahashi S, Murase N, Starzl TE, Iwaki Y. A monoclonal antibody (L21-6) recognizing an invariant chain expressed on the cell surface in rats with the exception of the BN (RT1n): a study of tissue and strain distributions. Transplant Proc. 1995;27:1519. [PMC free article] [PubMed] [Google Scholar]
- 12.Gill TJ, III, Kunz HW, Misra DN, Hassett AL. The major histocompatibility complex of the rat. Transplantation. 1987;43:773. [PubMed] [Google Scholar]
- 13.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 14.Billingham RE. Reactions of grafts against their hosts: transplantation immunity works both ways—hosts destroy grafts and grafts may harm hosts. Science. 1959;130:947. doi: 10.1126/science.130.3381.947. [DOI] [PubMed] [Google Scholar]
- 15.Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70(5):693. [PubMed] [Google Scholar]
- 16.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172(5):335. [PMC free article] [PubMed] [Google Scholar]
- 17.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 18.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Elmagd K, Reyes J, Todo S, et al. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg. 1998;186:512. doi: 10.1016/s1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen Z, MacGregor AB, Moore KT, Falk RE, Langer B, Cullen JB. Canine small bowel transplantation: a study of the immunological responses. Arch Surg. 1976;111:248. doi: 10.1001/archsurg.1976.01360210042008. [DOI] [PubMed] [Google Scholar]
- 23.Grant D, Duff J, Zhong R, et al. Successful intestinal transplantation in pigs treated with cyclosporine. Transplantation. 1988;45:279. doi: 10.1097/00007890-198802000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Williams JW, McClellan T, Peters TG, et al. Effect of pretransplant graft irradiation on canine intestinal transplantation. Surg Gynecol Obstet. 1988;167:197. [PubMed] [Google Scholar]
- 25.Diliz-Perez HS, McClure J, Bedetti C, et al. Successful small bowel allotransplantation in dogs with cyclosporine and prednisone. Transplantation. 1984;37:126. doi: 10.1097/00007890-198402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricour C, Revillion Y, Arnaud-Battandier F, et al. Successful small bowel allografts in piglet using cyclosporine. Transplant Proc. 1983;15 suppl 1:3019. [Google Scholar]
- 27.Gruessner RWG, Uckun FM, Pirenne J, et al. Recipient preconditioning and donor-specific bone marrow infusion in a pig model of total bowel transplantation. Transplantation. 1997;63:12. doi: 10.1097/00007890-199701150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Murase N, Demetris AJ, Woo J, et al. Lymphocyte traffic and graft-versus-host disease after fully allogeneic small bowel transplantation. Transplant Proc. 1991;23:3246. [PMC free article] [PubMed] [Google Scholar]
- 29.Foster PF, Sankary HN, Kociss K, Xiao F, Williams JW. The interaction of gamma irradiation of the allograft and recipient administration of cyclosporine in rat small bowel transplantation. Transplant Proc. 1992;24:1175. [PubMed] [Google Scholar]
- 30.Lee KK, Schraut WH. In vitro allograft irradiation prevents graft-versus-host disease in small-bowel transplantation. J Surg Res. 1985;38:364. doi: 10.1016/0022-4804(85)90050-2. [DOI] [PubMed] [Google Scholar]
- 31.Shaffer D, Maki T, DeMichele SJ, et al. Studies in small bowel transplantation: prevention of graft-versus-host disease with preservation of allograft function by donor pretreatment with antilymphocyte serum. Transplantation. 1988;45:262. [PubMed] [Google Scholar]
- 32.Shaffer D, Ubhl CS, Simpson MA, et al. Prevention of graft-versus-host disease following small bowel transplantation with polyclonal and monoclonal antilymphocyte serum: the effect of timing and route of administration. Transplantation. 1991;52:948. doi: 10.1097/00007890-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Elmagd K, Fung J, Reyes J, et al. Hepatic and intestinal transplantation at the University of Pittsburgh. In: Cecka JM, Terasaki PI, editors. Clinical Transplants. Los Angeles: UCLA Tissue Typing Laboratory; 1998. p. 263. [PMC free article] [PubMed] [Google Scholar]
- 34.Hendry JH, Lord BI. The analysis of the early and late response to cytokine insults in the haemopoietic cell hierarchy. In: Potten CS, Hendry JH, editors. Cytotoxic insult to tissues. Edinburgh: Churchill-Livingstone; 1983. p. 1. [Google Scholar]
- 35.van Bekkum DW. Radiation sensitivity of the hemopoietic stem cell. Radiat Res. 1991;128(1 suppl):S4. [PubMed] [Google Scholar]
- 36.Scott BR, Dillehay LE. A model for hematopoietic death in man from irradiation of bone marrow during radioimmunotherapy. Br J Radiol. 1990;63:862. doi: 10.1259/0007-1285-63-755-862. [DOI] [PubMed] [Google Scholar]
- 37.Inoue T, Hirabayashi Y, Mitsui H, et al. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction. Exp Hematol. 1995;23:1296. [PubMed] [Google Scholar]
- 38.Sun J, MeCaughan GW, Gallagher ND, Sheil AGR, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60:233. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Ko S, Deiwick A, Jager MD. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nature Med. 1999;5:1292. doi: 10.1038/15248. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu Y, Goto S, Lord R, et al. Liver passenger leukocytes can be reconstituted by splenic lymphocytes. Transplant Int. 1996;9:593. [Google Scholar]
- 41.Sun J, Sheil AGR, Wang C, et al. Tolerance to rat liver allografts. Transplantation. 1996;62:1725. doi: 10.1097/00007890-199612270-00005. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto T, Ye Q, Lu L, Demetris AJ, Starzl TE, Murase N. Donor hematopoietic progenitor cells in nonmyeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67:833. doi: 10.1097/00007890-199903270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichikawa N, Demetris AJ, Starzl TE, et al. Donor and recipient leukocytes in organ allografts of recipients with variable donor-specific tolerance: with particular reference to chronic rejection. Liver Trans. 2000;6:686. doi: 10.1053/jlts.2000.19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant D, Duff J, Zhong R, Mimeault R, Inch R, Stiller C. Effect of ex vivo allograft irradiation combined with cyclosporine therapy in a pig intestinal transplant model. Transplant Proc. 1989;21:2879. [PubMed] [Google Scholar]
- 45.Chuang VP. Radiation-induced arteritis. Semin Roentgenol. 1994;29:64. doi: 10.1016/s0037-198x(05)80072-0. [DOI] [PubMed] [Google Scholar]
- 46.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23:297. [PubMed] [Google Scholar]
- 47.Himmel PD, Hassett JM. Radiation-induced chronic arterial injury. Semin Surg Oncol. 1986;2:225. doi: 10.1002/ssu.2980020405. [DOI] [PubMed] [Google Scholar]
- 48.Selby DM, Rudzki JR, Bayeve ES, Chandra RS. Vasculopathy of small muscular arteries in pediatric patients after bone marrow transplantation. Hum Pathol. 1999;30:734. doi: 10.1016/s0046-8177(99)90132-6. [DOI] [PubMed] [Google Scholar]