Abstract
In dogs the most effective oral dose of FK 506 for prevention of renal homograft rejection was 1.5 mg/kg/day. With maximum credit allowed at 90 days, survival was increased to 61.0 ± 33.6 (SD) days compared with 13.0 ± 4.1 in untreated control animals. Higher doses were toxic. The smallest dose that was used (0.5 mg/kg/day) prolonged survival after renal transplantation to 33.7 ± 23.9 (SD) days. When the small dose of FK 506 was combined with 5 mg/kg/day of cyclosporine and 5 mg of prednisone, five of six canine kidney recipients lived for 90 days. These results were degraded by omission of any of the constituent drugs or reduction by half of the triple drug doses. Thirteen of the dogs treated with various drug regimens lived for 90 days, after which time treatment was stopped; 10 of the dogs eventually rejected the grafts, but three had continued graft function for 6 months or longer and may be permanently tolerant. Moreover, in dogs when 1 mg/kg of intramuscular FK was given to 19 kidney and seven liver recipients for 3 days on postoperative days 1 to 3, 4 to 6, or 7 to 9, the animals survived subsequently for 11 to more than 160 days. All but four of the grafts were eventually rejected, but the prolonged effect of a short course of delayed therapy suggests the possibility of tolerance induction. In cynomolgus monkeys and baboons, FK as a single drug was found to be immunosuppressive after kidney transplantation. Correlation in the dogs and primates between immunosuppression, toxicity, and FK blood levels was not possible because of presently imperfect standardization of assay and monitoring techniques. FK had serious side effects in dogs, but not so obviously in monkeys and not at all in baboons.
FK 506, a powerful new immunosuppressive agent, was discovered in Japan less than 4 years ago and reported in the literature for the first time in 1987.1–11 Within a few months of the first report, an international symposium was held in Sweden, at which time everything known about FK 506 as of June 1987 was discussed.12 Despite the intensity of these efforts, important issues remain unresolved. The questions concern the therapeutic efficacy of the agent when used alone or in combination with other drugs, its toxicity, and its pharmacologic monitoring.
An effort is made here to clarify these issues in dogs and subhuman primates. The principal inquiries in the canine experiments concerned the use of small doses of FK 506 in combination with subtherapeutic doses of other conventional agents or the use of large doses for tolerance induction. In cynomolgus monkeys and baboons, the efficacy and toxicity of long-term FK alone were studied. In all three species, an effort was made to correlate the results with blood levels of the drug with the use of newly developed assay techniques.
METHODS
Animal and operative procedures
Dogs
With a few exceptions that will be specifically noted, fasted mongrel dogs were used for donors and beagles were used as recipients. Weights were 10 to 13 kg. Anesthesia was induced with 25 mg/kg of intravenous pentobarbital, adding supplementary pentobarbital, 2 mg/kg of ketamine, or both when needed. One gram per day of cephalosporin was given for 3 days to the kidney recipients and for 5 days to the liver recipients. All animals were started on a diet ad libitum on the first postoperative day.
Most of the experiments were with kidney transplantation (Table I). The donor kidney from a mongrel donor was placed in the right iliac fossa of the recipient, anastomosing the renal artery to the proximal end of the transected common iliac artery, the renal vein to the side of the common iliac vein, and the ureter to the bladder. Bilateral recipient nephrectomy was performed.
Table I.
Canine experimental groups
| Group | n | Oral FK dose (mg/kg/day) |
Oral CyA (mg/kg/day) |
Prednisone (mg/day) |
IM FK, 1/mg/kg (on postop days shown) |
Donor recipient |
|---|---|---|---|---|---|---|
| Renal transplantation in dogs with oral FK 506 | ||||||
| 1 | 6 | — | — | — | — | M-B |
| 2 | 6 | 0.5 | — | — | — | M-B |
| 3 | 6 | 1.0 | — | — | — | M-B |
| 4 | 6 | 1.5 | — | — | — | M-B |
| 5 | 6 | 2.0 | — | — | — | M-B |
| 6 | 6 | 0.5 | 5 | 5 | — | M-B |
| 7 | 6 | 0.25 | 2.5 | 2.5 | — | M-B |
| 8 | 6 | 0.5 | 5 | — | — | M-B |
| 9 | 5 | 0.5 | — | 5 | — | M-B |
| 10 | 6 | — | 5 | 5 | — | M-B |
| 11 | 6 | — | 5 | — | — | M-B |
| 12 | 5 | — | — | 5 | — | M-B |
| Renal transplantation in dogs with FK 506 IM | ||||||
| 13 | 6 | — | — | — | 1,2,3 | M-B |
| 14 | 4 | — | — | — | 4,5,6 | M-B |
| 15 | 6 | — | — | — | 7,8,9 | M-B |
| 16 | 3 | — | — | — | 4,5,6;31,32 | M-B |
| Liver transplantation in dogs with FK 506 IM | ||||||
| 17 | 3 | — | — | — | 4,5,6 | B-B |
| 18 | 4 | — | — | — | 4,5,6 | M-B |
Legend: M, Mongrel; B, beagle; CyA, cyclosporine; IM, intramuscularly.
In the dogs receiving FK only (groups 2 to 5), the doses listed in Table I were reduced after 2 months to 75%. In all dogs all therapy was stopped after 90 days.
Orthotopic liver transplantation was performed in dogs weighing 10 to 15 kg with the aid of a heparin-free venovenous bypass.13 The liver transfer was from mongrel donor to beagle recipient in four experiments and from beagle to beagle in three (Table I).
Subhuman primates
All experiments were with renal transplantation (Table II). The cynomolgus monkeys (Macaca fascicularis) weighed 2.3 to 4.1 kg. The baboons weighed 8 to 12 kg. Blood types were determined, and donor and recipient combinations were made to ensure blood type compatibility. Cytotoxic crossmatches between donors and recipients were determined with conventional crossmatch techniques. Anesthesia was induced by an intramuscular injection of 10 mg/kg of ketamine. Animals were intubated and ventilated with a mixture of oxygen, nitrous oxide, and halothane. Renal allografts were placed intra-abdominally, and end-to-side anastomoses were performed between the renal artery and the abdominal aorta and between the renal vein and the inferior vena cava. The native kidneys of the recipients were removed. Ureteroneocystostomy was performed. One gram of cephalosporin was given daily for 3 days. Diet was resumed on the first postoperative day.
Table II.
Primate experimental groups*
| Group | n | Oral FK† dose (mg/kg/day) |
Postop days of oral FK dose |
IM FK dose (mg/kg/day) |
Postop days of IM FK dose |
|---|---|---|---|---|---|
| Renal transplantation in cynomolgus monkeys | |||||
| A | 3 | — | — | — | — |
| B | 3 | 0.3 | 1 onward | — | — |
| C | 2 | 0.3 | 1 onward | 1.0 | 14,15,16 |
| Renal transplantation in baboons | |||||
| D | 4 | — | — | — | — |
| E | 4 | 2.0 | 4 onward | 0.5 | 1–3 |
| F | 3 | 6.0 | 4 onward | 1.0 | 1–3 |
| Toxicology in baboons (nonop) | |||||
| G | 4 | — | — | — | — |
| H | 6 | — | — | 1.0 | 30 days (no operation) |
Legend: IM, Intramuscular.
Therapy stopped after 90 days in survivors.
There were four technical failures (artery thromboses) in the tiny cynomolgus monkeys and two technical failures in the baboons. These animals are not included in the data.
Drug administration
FK 506 for oral or intramuscular use was supplied as powder by the Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan. The oral doses were placed in commercial gelatin capsules and the intramuscular doses were suspended in normal saline solution. In the experiments involving transplantation, the extent of intramuscular administration varied from exclusive in the canine liver experiments, through temporary with baboons, to never in most of the series (Tables I and II).
Assurance of swallowing in dogs was by digital insertion of the capsule at the pharyngoesophageal junction. For the cynomolgus monkeys, FK was mixed with fruit paste during the first several days after the operation or dispersed onto pieces of fruit thereafter. Complete acceptance of the FK was carefully monitored face to face. The baboons readily ate fruit that was impregnated with FK. When the baboons were weak or hesitated to take diet, FK was suspended in 20 ml of normal saline solution and administered through the gastric tube under anesthesia with 5 mg/kg of intramuscular ketamine.
Cyclosporine was given in the commercial oil carrier that is used clinically. Prednisone was given orally to dogs in 5 mg tablets. To prevent vomiting in the dog caused by FK or other postoperative factors, 2 mg of atropine sulfate was given intramuscularly twice a day for the first postoperative week and once a day for the second postoperative week. The dose of atropine was reduced to 1 mg once a day during the third postoperative week. Monkeys and baboons did not vomit; thus atropine administration was unnecessary.
Biochemical and pathologic studies
Blood samples were taken every 3 mornings in the dog and once a week in the subhuman primates for the measurement of blood urea nitrogen, creatinine, aspartate aminotransferase, total bilirubin, glucose, and white blood cells, red blood cells, and platelets. Heparinized blood samples for plasma FK and whole blood cyclosporine measurements were collected, centrifuged, and stored frozen until the analyses. FK plasma levels were measured by enzyme immunoassay14 with monoclonal or polyclonal antibody to FK. Cyclosporine level was measured with whole blood by high-performance liquid chromatography using cyclosporine D as an internal standard.15
When an animal died or was killed, complete postmortem examination was performed immediately. Tissues were fixed with formalin, paraffin embedded, and stained with hematoxylin-eosin or with special techniques as indicated. Histopathologic changes were scored blindly according to subjective scales from 0 to 3. All tissues were examined blindly without knowledge of the postoperative course, type, or dosage of immunosuppressive therapy or length of survival.
Statistics
The values were described as the mean ± SD. The Mann-Whitney U test, χ2 test, and Student t test were applied for the statistical analyses, with which the difference of group means was considered significant if the probability of error was less than 0.05.
RESULTS
Canine experiments
Chronic FK alone for kidney transplantation
SURVIVAL AND REJECTION
Compared with that of untreated controls in group 1, survival was significantly improved with all oral daily doses of FK from 0.5 to 2 mg/kg (Table III). The best results were with 1.5 mg (p < 0.01); three of six dogs (group 4) lived for 90 days, and the mean survival was 61 ± 33.6 (SD) days. With daily doses lower than this, biochemical and histopathologic evidence of rejection became common (Table III). At the higher dose of 2 mg/kg/day, rejection was completely avoided, but four of six animals died of intussusception after 8, 8, 10, and 20 days. One of the other dogs lived for 57 days and the sixth dog is alive after 250 days, 5½ months after discontinuation of treatment.
Table III.
Summary of dose-effectiveness study in canine renal transplantation
| Latest biochemistry‡ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Treatment | Survival days* |
Histologic severity of graft rejection † |
Creatinine (mg/dl) |
AST (IU/L) |
Total bilirubin (mg/dl) |
Glucose (mg/dl) |
FK blood level§ (ng/ml) |
|||
| 0 | 1 | 2 | 3 | ||||||||
| 1 | Untreated control |
13.0 ± 4.1 | 0 | 0 | 0 | 6/6 | 11.3 ± 3.1 |
35 ± 15 | 0.2 ± 0.2 | 105 ± 66 | — |
| 2 | FK, 0.5 mg/kg/day |
33.7 ± 23.9 | 0 | 1/5 | 2/5 | 2/5 | 7.7 ± 5.3 | 79 ± 47 | 0.4 ± 0.3 | 143 ± 32 | 0.44 ± 0.57 |
| 3 | FK, 1.0 mg/kg/day |
31.0 ± 18.4 | 1/6 | 0 | 3/6 | 2/6 | 2.8 ± 1.8 | 26 ± 6 | 0.4 ± 0.4 | 128 ± 30 | 0.68 ± 0.25 |
| 4 | FK, 1.5 mg/kg/day |
61.0 ± 33.6 | 1/3 | 1/3 | 0 | 1/3 | 3.0 ± 3.0 | 37 ± 17 | 0.9 ± 0.7 | 98 ± 29 | 0.40 ± 0.41 |
| 5 | FK, 2.0 mg/kg/day |
32.1 ± 33.9 | 5/5 | 0 | 0 | 0 | 1.4 ± 0.5 | 55 ± 28 | 1.2 ± 1.4 | 79 ± 37 | 3.92 ± 4.01 |
Legend: AST, Aspartate aminotransferase.
Used 90 days as a ceiling of calculation.
Grafts from animals that lived for more than 90 days are excluded, 0 (none), 1 (mild), 2 (moderate), and 3 (severe).
Values from the latest measurement or at the ninetieth day.
By enzyme immunoassay with monoclonal antibody.
PLASMA FK LEVELS
With the monoclonal assay, the trough plasma levels from the last specimen collected were not different in groups 2, 3, and 4, but were elevated to an average of nearly 4 ng/ml in group 5 (p < 0.05). The highest levels were 7.6 ng/ml in a dog that died with jaundice after 57 days and 10.1 ng/ml in a dog that died of intussusception after 10 days.
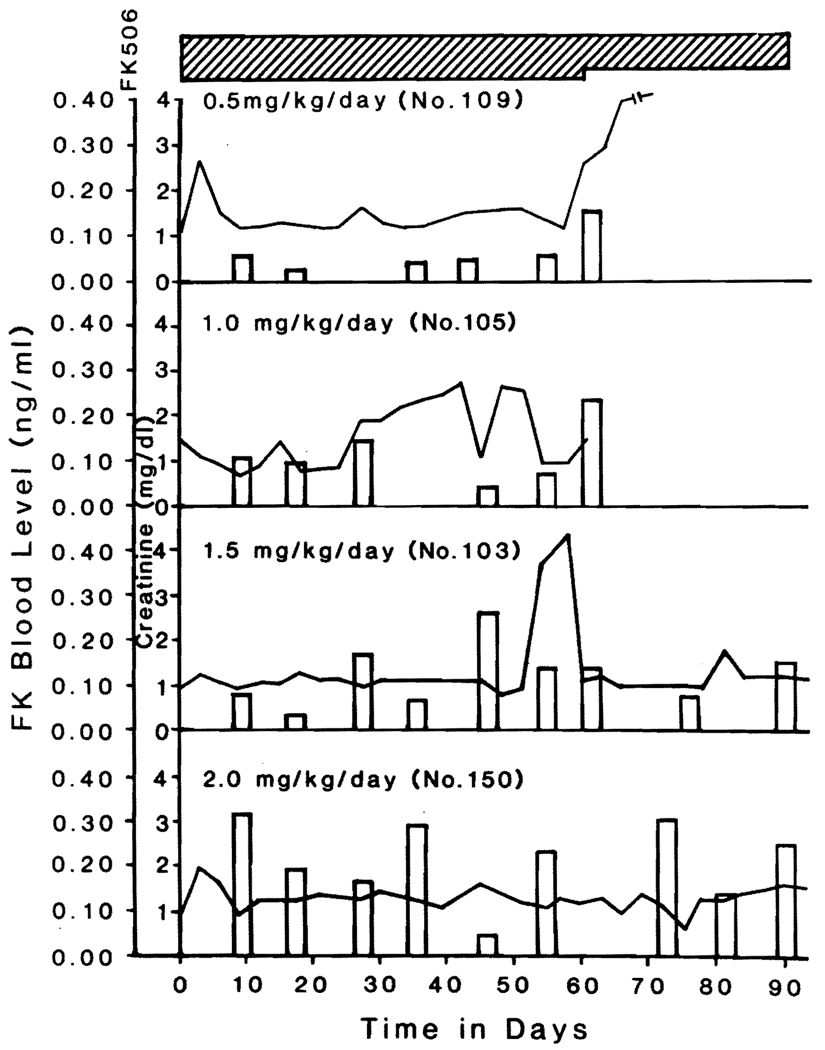
The possibility that much lower levels could be therapeutic was suggested by more complete data in the longest surviving dogs in each of groups 2 through 5. In these animals trough levels in samples stored throughout the course of therapy reflected, although inexactly, the oral doses and were never higher than 0.5 ng/ml (Fig. 1). All of the foregoing analyses were performed in Japan by those who had invented the technique and were experts in the monoclonal technique.14
Fig. 1.
Serum creatinine concentration and FK plasma levels in dogs that lived the longest in the dogs of groups 2 through 5 treated with different daily doses of FK 506.
There was no correlation between plasma levels and liver functions or blood glucose concentrations (Table III) with the possible exception of the single dog in group 5 that had terminal jaundice. There was no evidence whatever of bone marrow depression as judged by peripheral white cell, platelet, or red cell measures.
TOLERANCE INDUCTION
Three of six animals in group 4 lived after the discontinuation of FK at 90 days, but died at 98, 130, and 133 days after transplantation. In contrast, the single survivor from high-dose group 5 seemed tolerant after drug stoppage and still has perfect renal function 250 days after transplantation.
Drug combinations for kidney transplantation
SURVIVAL AND REJECTION
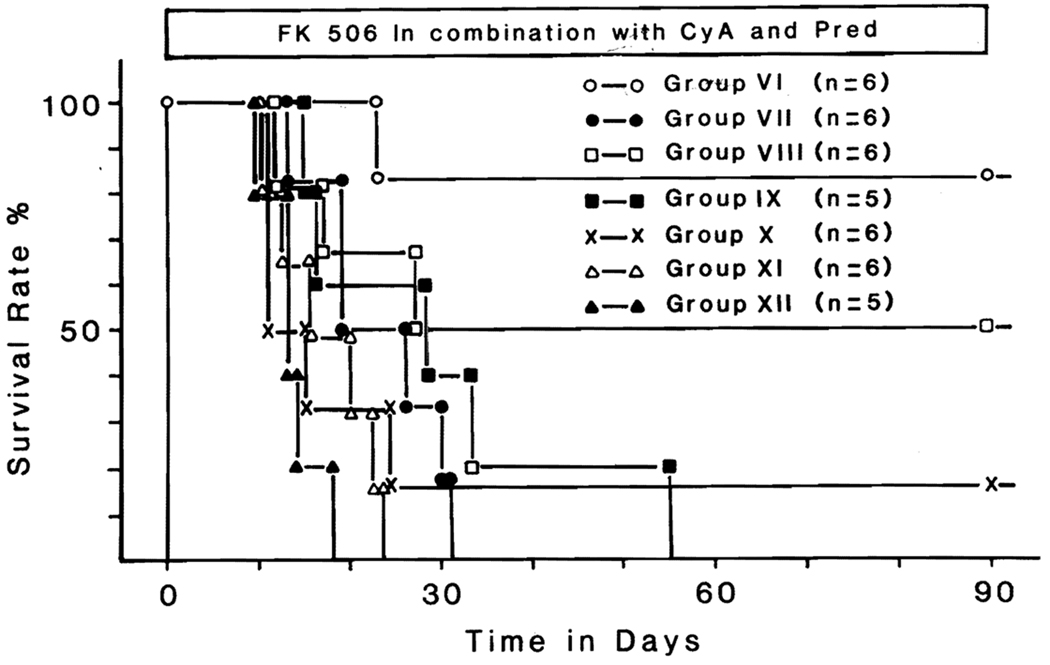
With daily doses of 0.5 mg/kg of FK, 5 mg/kg of cyclosporine, and 5 mg of prednisone (group 6), five (83%) of six recipients lived until the treatment was stopped at 90 days (Fig. 2). The only death was after 23 postoperative days from emaciation and pancreatitis. Although the creatinine level was 3.2 mg/dl, the kidney showed no histopathologic evidence of rejection. Omission of the prednisone (group 8), cyclosporine (group 9), or FK (group 10) from the triple drug formula or reduction of the triple drug doses by half (group 7) reduced survival (Fig. 2).
Fig. 2.
Survival in dogs receiving multiple drug treatment with FK 506, cyclosporine, and prednisone, along with control experiments. (See text for details.)
If only two drugs were used, the best combination was FK and cyclosporine, with a mean survival of 54.3 ± 39.4 (SD) days. One animal from this group died of intussusception after 12 days, one other died of rejection at day 17, and the third died of unknown causes at 27 days. The other three animals in group 8 lived for 90 days.
Individually, FK, cyclosporine, and prednisone provided mean survival of 33.7, 18, and 13.8 days, respectively (Fig. 2).
Except in groups 6 and 8, uncontrolled rejection was the cause of death in almost all of the dogs.
PLASMA FK AND BLOOD CYCLOSPORINE LEVELS
A 24-hour trough level of plasma FK was measured in each of the 24 animals in groups 6 through 9 from 12 to 90 days after surgery. The blood samples were the last ones collected from each dog and thus were obtained from a week to 3 months after surgery. The FK concentrations were 0.51 ± 0.38 (SD) ng/ml (range, 0.02 to 1.39). There were no significant differences in plasma levels between groups, nor was there any correlation with survival.
The 24-hour trough levels of blood cyclosporine measured with the high-performance liquid chromatography technique in the animals of groups 6, 7, 8, 10, and 11 were less than 50 ng/ml in every case and usually undetectable. From other work in our laboratory such levels have been thought to be suboptimal or even homeopathic.16
TOLERANCE INDUCTION
Nine dogs lived for 90 days until discontinuation of medication, five from group 6, three from group 8, and one from group 10. However, all five survivors from group 6 died soon after 90 days. Rejection was the cause of death after a total posttransplant survival time of 98, 100, 103, 114, and 120 days. Two of the three dogs from group 8 that survived for 3 months died of rejection after 129 and 162 days, but the third is well after 237 days. One dog treated with cyclosporine and prednisone (group 10) is in good health after 258 days.
FK bolus therapy intramuscularly
RENAL TRANSPLANTATION
Survival of mongrel grafts in beagle recipients was remarkably prolonged with three doses only of 1 mg/kg of intramuscular FK on days 1 to 3, or days 4 to 6, or even on days 7 to 9 when rejection should have been well established (Table IV). One of the animals treated on days 4 through 6 (group 14) is now 202 days postoperative but all others rejected their kidneys.
Table IV.
Prolongation of graft survival by FK bolus therapy
| Transplantation | Group | FK1, mg/kg/day, IM (on postop days) |
Survival days | p Value† |
|---|---|---|---|---|
| Kidney | 1 | None | 8, 9, 12, 14, 17, 18 | — |
| 13 | 1–3 | 21, 19, 32, 35, 42, 69 | <0.003 | |
| 14 | 4–6 | 42, 49, 51, >198 | <0.01 | |
| 15 | 7–9 | 11, 27, 36, 39,42, 49 | <0.02 | |
| 16 | 4, 5, 6, 31, and 32 | 46, 66, 67 | <0.02 | |
| Liver | —* | None | 6, 6, 7, 7, 7, 19, 35 | — |
| 17 | 4–6 | 58, 72, >160 | <0.02 | |
| 18 | 4–6 | 24, 28, >51, ±52 | <0.02 |
Three beagle dogs treated on days 4 to 6 developed signs of rejection of their mongrel grafts at 1 month and were rescued for 2 to 5 additional weeks with two additional single injections (group 16).
LIVER TRANSPLANTATION
Seven dogs had intramuscular FK on postoperative days 4, 5, and 6. Three of these beagle recipients were given livers from beagle donors (group 17); two lived for 58 and 72 days before dying of rejection and the third is well after 5½ months. The other four beagle were given mongrel livers (group 18). Two dogs died at 24 and 28 days whereas the other two are living after 51 and 52 days (Table IV).
Subhuman primate experiments
Renal transplantation
CYNOMOLGUS MONKEYS
Three untreated animals died of rejection after 7, 8, and 10 days (Table V). Oral treatment of five recipients with 0.3 mg/kg/day of FK (groups B and C) did not dramatically modify the initial course. Three of the monkeys without augmented treatment (group B) died, but the other two (group C) that were given an additional 1 mg/kg/day of intramuscular FK for 3 days on days 14, 15, and 16 lived for 3 months. These monkeys died after 94 and 96 days, a few days after discontinuation of maintenance FK.
Table V.
Summary of renal transplantation in cynomolgus monkeys
| Latest biochemistry* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Treatment | No. | Survival (days) |
Severity of graft rejection (histopathology) |
Creatinine (mg/ml) |
AST (IU/L) |
Total bilirubin (mg/dl) |
Glucose (mg/dl) |
FK (ng/ml) |
| A | Untreated controls |
757 | 8 | Not done | 16.7 | 696 | 0.5 | 156 | — |
| 852 | 10 | Mild to moderate |
12.9 | 102 | 0.2 | 63 | — | ||
| 844 | 7 | Mild to moderate |
9.9 | 342 | 0.7 | 227 | — | ||
| B | FK, 0.3 mg/kg/day |
719 | 8 | Severe | 35.5 | 600 | 0.6 | 187 | — |
| 750 | 9 | Mild to moderate |
34.9 | 66 | 0.3 | 77 | — | ||
| 753 | 10 | Severe | 18.9 | 145 | 0.6 | 194 | — | ||
| C | FK, 0.3 mg/kg/ day, po |
724 | 94 | Severe | 3.0 | 21 | 0.2 | 50 | 0.4 |
| FK, 1 mg/kg/ day, IM, at days 14–16 |
755 | 96 | Severe | 4.5 | 19 | 0.2 | 61 | 3.0 | |
Legend: AST, Aspartate aminotransferase; po, by mouth; IM, intramuscular.
Values from the latest blood samples or at the ninetieth day.
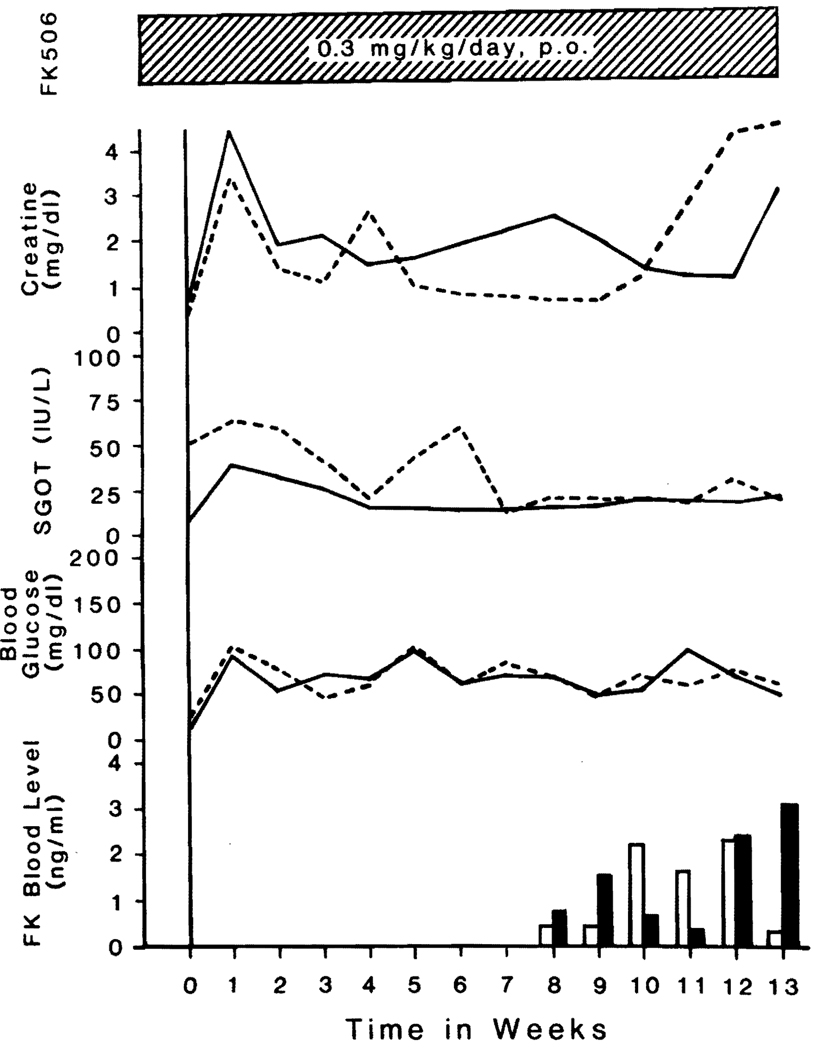
The course of the latter two animals is summarized in Fig. 3. During the third postoperative month, FK plasma levels ranged from 0.4 to 3 ng/ml as measured in the Pittsburgh laboratories with a polyclonal anti-FK antibody.14
Fig. 3.
Clinical course of two cynomolgus monkeys that lived for more than 90 days under FK treatment. One monkey (No. 724) is shown by solid lines and open column, and the other (No. 755) is shown by dotted lines and black column. The FK plasma levels were not measured during the first 2 months.
All of the kidney losses, early or late, were from rejection (Table V).
BABOONS
Four untreated baboons (group D) rejected their kidneys after 5 to 14 days (Table VI). Two baboons treated with what were considered large doses of intramuscular and oral FK every 12 hours also rejected their kidneys after 14 and 18 days (group E). When the intramuscular dose was doubled and the oral dose was tripled (group F), one of three baboons survived a bout of early renal failure and has a creatinine level of 1.8 mg/dl after 46 days. The other two developed renal failure and died (Table VI), but the histopathologic findings of rejection were rather mild.
Table VI.
Summary of renal transplantation in baboons
| Latest biochemistry* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Treatment | No. | Survival (Days) |
Severity of graft rejection (histopathology) |
Creatinine (mg/ml) |
AST (IU/L) |
Total bilirubin (mg/dl) |
Glucose (mg/dl) |
FK (ng/ml) |
| D | Untreated controls | 87 | 11 | Severe | 24.3 | 42 | 0.5 | 198 | — |
| 90 | 7 | Moderate to severe |
16.5 | 193 | 1.0 | 95 | — | ||
| 106 | 5 | Mild to moderate |
14.2 | 45 | 1.5 | 82 | — | ||
| 112 | 14 | Severe | 13.0 | 51 | 0.8 | 93 | — | ||
| E | FK, 0.5 mg/kg/day IM, for 3 days + FK, 2.0 mg/kg/day, po, from days 4 onward |
95 | 14 | Moderate | 19.3 | 52 | 1.4 | 122 | 206 |
| 97 | 18 | Severe | 17.5 | 48 | 1.7 | 256 | 202 | ||
| F | FK, 1.0 mg/day IM, for 3 days, + FK, 6 mg/kg po, from day 4 onward |
104 | 23 | Severe | 23.3 | 68 | 0.5 | 93 | 4.90 |
| 107 | >45 | — | 1.8 | 27 | 0.5 | 97 | 2.10 | ||
| 110 | 7 | Mild | 14.4 | 63 | 0.5 | 109 | 3.83 | ||
Legend: AST, Aspartate aminotransferase; IM, intramuscular; po, by mouth.
Values from the latest blood samples or at the ninetieth day.
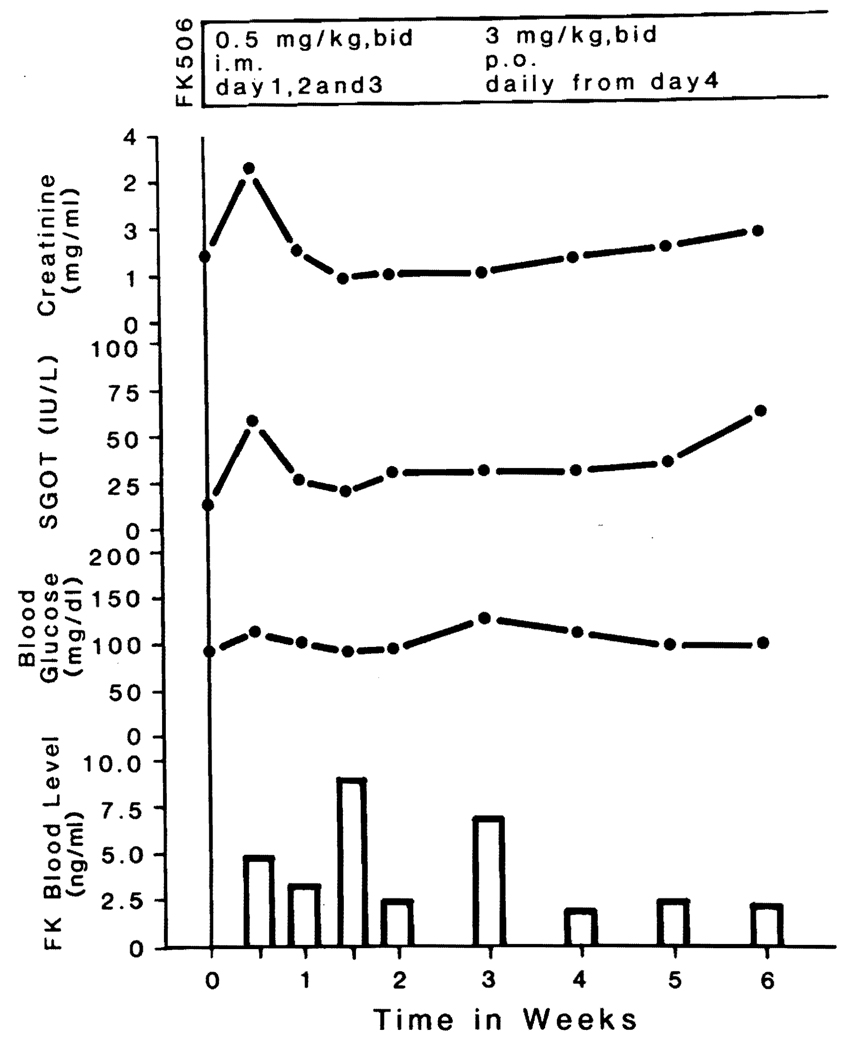
With the polyclonal enzyme immunoassay, the 12-hour trough levels of plasma FK in the treated baboons were 2 to 5 ng/ml at the time of the last blood samples (Table VI). The 12-hour trough levels in the surviving baboon are shown in Fig. 4.
Fig. 4.
Clinical course of a baboon that is alive more than 50 days after transplantation under FK treatment.
Unoperated baboons
Four baboons were killed after a month for tissues, and the other six were given 1 mg/kg/day of FK for 30 days before death. One of the latter six animals lost weight, three maintained their weight, and two actually gained weight. This performance was similar to that of the four control baboons that were given placebos. The treated animals were healthy throughout despite the fact that three were found to be malaria carriers. Major changes in blood sugars, liver chemistries, and hematologic studies were not seen during treatment. Major toxic effects were not found with histopathologic study. These toxicology studies will be reported separately, as well as the pharmacokinetic data obtained.
Host tissue pathology
The clear and dose-dependent immunosuppressive effect of FK was described under the individual groups and is partially summarized in Table III. Host tissues were also examined blindly with the same technique without knowledge of the experiment.
Dogs
As the doses of FK were increased, pathologic changes in the non-transplanted organs began to appear. These changes included pancreatic acinar cell degeneration and degranulation, fine isometric vacuolization of the proximal tubules of the kidney (in liver transplant experiments), and prominent centrilobular hepatocellular swelling and cholestasis (in kidney transplant experiments) similar to that observed with erythromycin toxicity in humans. These presumed toxic effects were present to some degree in all dogs receiving 2.0 mg/kg of FK 506 orally, had a variable presence in intermediate doses, but were not present in any of the control animals. Similar histologic findings have been detected (unpublished observations) in straight toxicologic studies in dogs.
Severe fibrinoid medial necrosis of medium-sized arteries was detected in virtually all organs, but was particularly noticeable in the gastrointestinal tract and heart. Nearby focal parenchymal ischemic necrosis was seen. Initially, we assumed that the arteritis represented a toxic side effect of FK in the dogs as reported by Thiru et al.17 and Collier.10 However, after breaking the blinded code analysis of the slides, we discovered that the arteritis was found in control animals with equal frequency and severity.
Monkeys
No hepatocellular swelling such as that observed in dogs was detected. After kidney transplantation, a severe necrotizing arteritis was detected in the extrarenal organs of monkeys given FK, but this was present with equal frequency and severity in the untreated control animals.
Baboons
In the baboons undergoing transplantation, hepatocellular swelling was not seen. Arteritis was not observed in any of the animals contributing to the definitive experiments. Necrotizing arteritis in the intestine was seen in one baboon that died 4 days after surgery of a hemorrhage from the ligated renal pedicle and was omitted from data analysis.
The untransplanted baboons of the toxicology study did not have arteritis or hepatocellular swelling. There was exocrine degranulation in the pancreas and increased cell turnover. The hyperglycemia described in rats by Nalesnik et al.18 and in baboons by Calne et al.11 was not found in our baboons. The lack of side effects in the baboons suggested that the toxicity of FK differs along species lines (Table VII).
Table VII.
Histopathologic abnormality of extrarenal organs from dogs, monkeys, and baboons*
| Animal | Treatment | Groups† | Heart: Necrotizing arteritis micromyocardial infarction |
Liver: Hepatocellular swelling, cholestasis |
Pancreas: Acinar cell degeneration |
Gastrointestinal tract: necrotizing arteritis |
|---|---|---|---|---|---|---|
| Dog | Control | 1 | +++ | − | − | ++ |
| FK | 2–5 | +++ | + | ++ | +++ | |
| FK in combination |
6–9 | + | + | + | + | |
| Monkey | Control | A | ++ | − | − | + |
| FK | B,C | ++ | − | − | + | |
| Baboons | Control | D | − | − | − | − |
| FK | E,F | − | − | − | + | |
| Toxicology | G | − | − | − | − | |
| H | − | − | + | − |
Abnormalities are classified as follows: (−) none, (+) mild, (++) moderate, and (+++) severe.
For details, see text.
DISCUSSION
A much clearer picture has emerged from these studies of the potential clinical value of FK 506 and the conditions that will permit its most effective and safe use. The practicality of combining FK with other conventional agents was evident in the canine experiments. The results obtained with subtherapeutic doses of FK, cyclosporine, and steroids were as good within a 90-day time frame as have ever been reported in dogs with any drug regimen.
The concept of drug synergism for immunosuppression is an old one,19 but difficult to prove until recently. Now, the interaction of drugs can be studied with great precision by measuring their effect on mixed lymphocyte culture systems. Using such in vitro “mini-transplant” models, Sawada et al.20 and Zeevi et al.21 showed striking synergism of FK and cyclosporine. The same has been seen equally clearly with heterotopic heart transplantation in rats.9 The synergism of FK and cyclosporine is of special interest, since the two drugs have similar, if not identical, actions4, 22 and may even compete for the same binding sites.23 Our previous belief that synergism would depend on differing mechanisms of the individual components of a drug cocktail was probably erroneous.
It is fortunate that immunosuppression can be enhanced with drug combinations without a parallel enhancement of toxicity. The dog was chosen for the drug combination studies because of the reported inability of this species to tolerate FK.7, 8, 10, 17
There is little doubt that dogs can be made violently ill with large doses of oral or intramuscular FK 506. The histopathologic changes in the liver and pancreas might be a clue to the pathogenesis of these events. Ironically, the most publicized side effect of FK 506, namely, widespread arteritis, may not actually be attributable to this agent. The carefully controlled histopathologic analyses in our investigation, in which the readings were decoded after the analyses were completed, revealed the same distribution and severity of arteritis in untreated control dogs as in those given FK, cyclosporine, or steroids, or any combination of these agents. The same applied to the cynomolgus monkeys.
In contrast, arteritis was not seen in the baboons that were also singularly free of the hepatocyte swelling and pancreatic lesions characteristic of the dog. Although Calne et al.11 have reported alarming side effects of FK in baboons, we have concluded that the safety of FK in baboons is equivalent to that in rats.9, 18
However, final judgment must be deferred pending definitive studies of FK pharmacokinetics. Preliminary results (unpublished observations) indicate that the half-life of FK 506 is about 6.3 hours in baboons compared with 6.1 hours in dogs, as measured with polyclonal antibodies. Since an intravenous dosage of FK 506 is currently unavailable, it is not possible to determine directly whether the extent of absorption of FK 506 is different between dogs and baboons. So far, decisive information has not been obtained regarding the correlation in any species of plasma levels of FK with either toxicity or immunosuppression. Interpretation of the results was made difficult by mixing the use of monoclonal and polyclonal assay techniques and with the further disadvantage that these assays that are still being perfected were done in Pittsburgh and Japanese laboratories half a world apart. Seemingly effective blood levels in dogs, monkeys, and baboons were as low as 0.2 ng/ml or higher than this by a factor of 40. It could not even be determined from the data so far obtained if the pharmacokinetics of FK in dogs and subhuman primates were fundamentally different.
In our monkey and baboon transplant experiments, mitigation of rejection was unequivocally demonstrated, but the effect was limited. The explanation could include underdosing with FK, poor alimentary absorption in the oral administration experiments, or species-specific peculiarities in the metabolism of the drug. All of these ambiguities will be clarified promptly once the monoclonal assay technique is standardized and stored samples are analyzed from experiments already completed.
For the past 25 years, clinical organ transplantation has depended on the daily or twice daily administration of immunosuppressive drugs, presumably for a lifetime. Discontinuation of treatment is usually followed by rejection. This was seen in 10 of the 13 canine and both of the cynomolgus renal recipients whose treatment was stopped after 90 days. In three dogs, tolerance, or something resembling this condition, was accidentally produced but so unreliably and unpredictably that it was of no practical value.
Because further work with FK is planned, thought should be given to the alternative of providing a short course of high-dose treatment. The striking effect of a short course of moderately high-dose FK therapy intramuscularly was particularly striking with both canine renal and hepatic transplantation. Three days of treatment, even as late as 7, 8, and 9 days after transplantation, far outlasted the intramuscular injections. Although the grafts were not accepted permanently, the results were as good or better than with almost all of the variations of long-term multiple drug therapy. The only other agent that has ever allowed extended homograft survival in dogs with such a short period of perioperative treatment has been antilymphocyte serum or its globulin derivative (antilymphocyte globulin).24
Supplementary Material
Acknowledgments
Supported by Research Project Grant No. AM-29961 from the National Institutes of Health and partially from the Fujisawa Pharmaceutical Company, Ltd.
We are grateful to the exploratory research laboratories, Fujisawa Pharmaceutical Co., Ltd., Dr. A. Sanghvi, Dr. V. Warty, and Dr. Evan Cadoff, for measuring FK blood level, and Dr. A. Zeevi, Dr. R. Duquesnoy, and Dr. J. Falk for performing cytotoxic crossmatches in subhuman primates. All of these colleagues also provided advice and encouragement throughout the study, as did numerous other members of the surgery, pathology, and medical departments at the University of Pittsburgh.
Footnotes
Presented at the Forty-ninth Annual Meeting of the Society of University Surgeons, San Antonio, Texas, Feb. 11–13, 1988.
This article includes pertinent material from Dr. Starzl’s SUS Invited Commentary, which he presented at the 1988 meeting.
REFERENCES
- 1.Goto T, Kino T, Hatanaka H, et al. Discovery of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant Proc. 1987;19 suppl 6:4–8. [PubMed] [Google Scholar]
- 2.Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiotics. 1987;40:1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- 3.Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiotics. 1987;40:1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 4.Ochiai T, Nakajima K, Nagata M, et al. Effect of a new immunosuppressive agent, FK 506, on heterotopic cardiac allotransplantation in the rat. Transplant Proc. 1987;19:1284–1286. [PubMed] [Google Scholar]
- 5.Ochiai T, Nagata M, Nakajima K, et al. Studies of the effects of FK 506 on renal allografting in the beagle dog. Transplantation. 1987;44:729–733. doi: 10.1097/00007890-198712000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ochiai T, Nakajima K, Nagata M, Hori S, Asano T, Isono K. Studies of the induction and maintenance of long-term graft acceptance by treatment with FK506 in heterotopic cardiac allotransplantation in rats. Transplantation. 1987;44:734–738. doi: 10.1097/00007890-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Todo S, Demetris AJ, Ueda Y, et al. Canine kidney transplantation with FK-506 alone or in combination with cyclosporine and steroids. Transplant Proc. 1987;19 suppl 6:57–61. [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Podesta L, ChapChap P, et al. Orthotopic liver transplantation in dogs receiving FK-506. Transplant Proc. 1987;19 suppl 6:64–67. [PMC free article] [PubMed] [Google Scholar]
- 9.Murase N, Todo S, Lee PH, et al. Heterotopic heart transplantation in the rat receiving FK-506 alone or with cyclosporine. Transplant Proc. 1987;19 suppl 6:71–75. [PMC free article] [PubMed] [Google Scholar]
- 10.Collier DSTJ, Thiru S, Calne R. Kidney transplantation in the dog receiving FK-506. Transplant Proc. 1987;19 suppl 6:62. [PubMed] [Google Scholar]
- 11.Calne R, Collier DSTJ, Thiru S. Observations about FK-506 in primates. Transplant Proc. 1987;19 suppl 6:63. [PubMed] [Google Scholar]
- 12.Starzl TE, Makowka L, Todo S, editors. FK 506: a potential breakthrough in immunosuppression. (satellite meeting of the European Society of Organ Transplantation) Transplant Proc. 1987;19 suppl 6:3–104. [Google Scholar]
- 13.Todo S, Kam I, Lynch S, Starzl TE. Animal research in liver transplantation with special reference to the dog. Semin Liver Dis. 1985;5:309–317. doi: 10.1055/s-2008-1040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Kobayashi M, Hashimoto K, et al. A highly sensitive method to assay FK-506 levels in plasma. Transplant Proc. 1987;19 suppl 6:23–29. [PubMed] [Google Scholar]
- 15.Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala T. Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther. 1985;38:296–300. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]
- 16.Todo S, Porter KA, Kam I, et al. Canine liver transplantation under Nva2—cyclosporine versus cyclosporine. Transplantation. 1986;41:296–300. doi: 10.1097/00007890-198603000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiru S, Collier DSTJ, Calne R. Pathological studies in canine and baboon renal allograft recipients immunosuppressed with FK-506. Transplant Proc. 1987;19 suppl 6:98–99. [PubMed] [Google Scholar]
- 18.Nalesnik MA, Todo S, Murase N. Toxicology of FK-506 in the Lewis rat. Transplant Proc. 1987;19 suppl 6:89–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 20.Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK 506 in vitro effects on the cloned T cell activation. J Immunol. 1987;139:1797–1803. [PubMed] [Google Scholar]
- 21.Zeevi A, Duquesnoy R, Eiras G, et al. Immunosuppressive effect of FK-506 on in vitro lymphocyte alloactivation: synergism with cyclosporine. A. Transplant Proc. 1987;19 suppl 6:40–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Zeevi A, Duquesnoy R, Eiras G, Todo S, Makowka L, Starzl TE. In vitro immunosuppressive effects of FR 900506 on human T lymphocyte alloactivation. Surg Res Comm. 1987;1:315–323. [PMC free article] [PubMed] [Google Scholar]
- 23.Sanghvi A, Warty V, Zeevi A, et al. FK-506 enhances cyclosporine uptake by peripheral blood lymphocytes. Transplant Proc. 1987;19 suppl 6:45–49. [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.