Abstract
INTRODUCTION:
We investigated the antianxiety and sedative effects of the essential oil of Ducrosia anethifolia. Boiss. (Apiaceae).
METHODS:
We used elevated plus maze, spontaneous motor activity and ketamine‐induced sleep tests in mice. In addition, the essential oil was analyzed by GC/MS. Twenty compounds were identified, and n‐decanal (70.1%) and alpha‐pinene (12.4%) constituted the major components.
RESULTS:
In elevated plus maze, Ducrosia anethifolia essential oil at doses of 25–200 mg/kg increased the percentage of open arm time and entries. Unlike diazepam, ducrosia anethifolia essential oil could not suppress spontaneous motor activity and did not alter ketamine‐induced sleep parameters. These results are indicative of antianxiety effect of Ducrosia anethifolia essential oil without sedative effect.
Keywords: Apiaceae, diazepam, elevated plus‐maze, ketamine, spontaneous motor activity
INTRODUCTION
There are many medicinal plants that have stimulating or calmative effects on the central nervous system,1 and the plant kingdom provides hundreds of CNS active substances covering the whole spectrum of activity such as psychoanaleptic, psycholeptic, and psychodysleptic (hallucinogenic) effects.2
Ducrosia anethifolia (DC.) Boiss. is one of these medicinal plants that belongs to the Apiaceae family. It is one of the three species of Iranian Ducrosia species growing wild in several areas of the country.3-5 This aromatic herb is distributed in Afghanistan, Pakistan, Syria, Lebanon, Iraq, and some other Arab states and countries along the Persian Gulf.5 D. anethifolia is commonly known in Iran as Moshgak, Roshgak, and Moshkbu.3-5 The whole herb – especially its aerial parts – has been used in Iranian folklore medicine as an analgesic and pain reliever for headache, backache, colic, and colds. In some regions of Iran, it is claimed to be especially effective against anxiety and insomnia. This herb is added to a variety of Persian foods for flavoring.3,6
In pharmacological and biological tests, extracts and fractions of D. anethifolia and some other species of Ducrosia are reported to have antimicrobial, antimycobacterial, antifungal, and CNS depressant effects.7-9 Phytochemical studies on D. anethifolia reveal that aliphatic aldehydes and other monoterpene hydrocarbons in its essential oil, and coumarins such as pangelin are the main components of the aerial parts.6,8-10 A few reports on the analysis of the essential oil of other Ducrosia species have been published, and these species contain some similar biologically active compounds.7,11-12
The present study was carried out in an attempt to investigate the potential anxiolytic and sedative effects of D. anethifolia essential oil, as an outstanding fraction of the plant, in mice using elevated plus maze (EPM), spontaneous motor activity, and ketamine‐induced sleep tests. In addition, we describe the essential oil constituents identified by GC/MS analysis, because knowledge of the chemical composition of a given plant is required in order to extrapolate the proposed mechanism of actions to its possible in vivo efficacy or safety.13
MATERIALS AND METHODS
Plant material and preparation of essential oil
Fresh aerial parts (leaves and flowers) of D. anethifolia, growing wild in Lalehzar mountainous area in Kerman province, Iran, were collected at an altitude of ca. 2800 m. The plant's identity as D. anethifolia was confirmed by the herbarium department of Iranian Research Institute of Forests and Rangelands, Karaj, Iran. A voucher specimen of the plant was deposited in the herbarium of School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran, for future evidence.
The essential oil was isolated by hydrodistillation of the plant's fresh‐cut aerial parts for 4 h, and then was dried over anhydrous sodium sulfate14 and stored in a refrigerator (4°C).
Essential oil analysis
The oil was analyzed by GC/MS using a Hewlett Packard 6890 mass selective detector coupled with a Hewlett Packard 6890 gas chromatograph, equipped with a cross‐linked 5% PH ME siloxane HP‐5MS capillary column (30 m × 0.25 mm, film thickness 0.25 µm). Operating conditions were as follows: carrier gas, helium with a flow rate of 2 ml/min; column temperature, 60–275°C at 4°C/min; injector and detector temperatures, 280°C; volume injected, 0.1 µL of the oil; split ratio, 1∶50. The MS operating parameters were as follows: ionization potential, 70 ev; ionization current, 2 A; ion source temperature, 200°C; resolution, 1000.
Identification of the components in the oil was based on retention indices relative to n‐alkanes and computer matching with the WILEY 275.L library, as well as by comparison of the fragmentation patterns of the mass spectra with those reported in the literature.15,16
Elevated plus‐maze
The EPM test is described in detail elsewhere.17,18 Briefly, the apparatus was comprised of two open arms (35 cm × 5 cm) and two closed arms (30 cm× 5 cm× 15 cm) that extended from a common central platform (5 cm × 5 cm). The floor and the walls of each arm were wooden and painted black. The entire maze was elevated to a height of 60cm above floor level as validated and described by Lister.19 Experiments were conducted in a quiet room that was illuminated by only a dim light. Male Swiss mice were orally pretreated with vehicle or different doses of the essential oil 30 min before their placement on the EPM. A group of animals received diazepam (3 mg/kg, p.o.) as reference drug. To begin a test session, mice were placed in the center of the maze facing one of the open arms. An entry into an arm was defined as the animal placing all four paws over the line marking that area. The number of entries and the time spent in the open and closed arms were recorded during a 5 min test period. The percentage of open arm entries (100× open/total entries) was calculated for each animal. Between each trial, the maze was wiped clean with a damp sponge and dried with paper towels.
Locomotor activity
Spontaneous locomotor activity was measured using the locomotor activity apparatus, which consisted of a black painted wooden cage (60 cm× 20 cm× 30 cm) equipped with infrared photobeams placed above the cage.20 The units of the activity counts were arbitrary and based on the beam breaks by movement of the mice. Vehicle, DAEO (200 and 400 mg/kg) or diazepam (3 mg/kg), was orally administered 30 min prior to placing the mice in the center of the apparatus, and the locomotor activity was measured at 5 min interval for a period of 15 min. Six mice were used for each treatment group. The treatments were randomized throughout the day, between 08:00 and 13:00 h, to control for diurnal variations in activity.
Ketamine‐induced sleeping time
The effect of plant essential oil on ketamine‐induced sleeping time was measured as described previously.21 Four groups of mice (n = 6) were orally pretreated with vehicle, DAEO (200 and 400 mg/kg) or diazepam (3 mg/kg) one hour prior to ketamine injection (100 mg/kg, i.p.). The interval between the administrations of ketamine until the loss of the righting reflex was recorded as onset of sleep, while the time from the loss to regaining of the righting reflex was recorded as the duration of sleep.22
Acute toxicity test
To obtain an estimate of lethal dose, two groups of normal healthy male mice, each containing 6 animals, were given DAEO at doses of 1600 and 2400 mg/kg. After the administration of the DAEO, the mice were observed for any signs of toxicity and mortality for 48 h.
Statistical analysis
Statistical analysis was performed using one‐way analysis of variance (ANOVA) with post hoc Tukey test. P < 0.05 was considered significant. All data are expressed as mean ± S.E.M.
RESULTS
Analysis of the essential oil
The fresh aerial parts of the plant yielded 1.4% (v/w) of a yellowish essential oil with a fresh‐scented odor and a predominant aromatic taste. Twenty components were characterized, representing 98.1% of the total oil components detected, which are listed in Table 1 with their percentage composition and retention indices. Among them, n‐decanal (70.1%), alpha‐pinene (12.4%), and dodecanal (5.4%) were the major components. Other natural constituents formed less than 2% of the total or were present only in trace amounts.
Table 1.
Composition of the essential oil of ducrosia anethifolia fresh aerial parts.
| No. | Compound | Retention Index | Percentage |
| 1 | n‐nonane | 890 | 0.2 |
| 2 | alpha‐ pinene | 930 | 12.4 |
| 3 | sabinene | 968 | 0.1 |
| 4 | beta‐ pinene | 972 | 0.2 |
| 5 | myrcene | 986 | 0.5 |
| 6 | n‐octanal | 997 | 0.2 |
| 7 | para‐cymene | 1021 | 0.1 |
| 8 | limonene | 1026 | 0.5 |
| 9 | trans‐beta‐ocimene | 1032 | 0.9 |
| 10 | gamma‐terpinene | 1056 | 0.3 |
| 11 | alpha‐terpinolene | 1081 | 1.7 |
| 12 | citronellal | 1145 | 0.3 |
| 13 | n‐decanal | 1199 | 70.1 |
| 14 | beta‐citronellol | 1223 | 0.2 |
| 15 | trans‐chrysanthenyl acetate | 1231 | 1.9 |
| 16 | undecanal | 1298 | 0.5 |
| 17 | n‐undecanol | 1371 | 1.1 |
| 18 | dodecanal | 1405 | 5.4 |
| 19 | beta‐caryophyllene | 1416 | 0.9 |
| 20 | pentadecane | 1503 | 0.6 |
Effects of Ducrosia anethifolia essential oil in EPM test
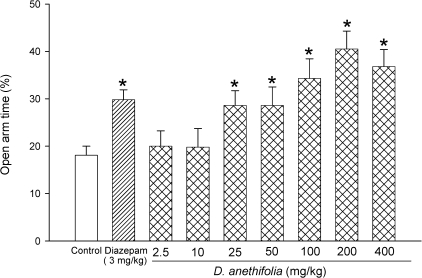
Various doses of DAEO (2.5, 10, 25, 50, 100, 200 and 400 mg/kg) were tested on the EPM. As shown in Figure 1, in control animals, the time spent in the open arms was 18%. DAEO at doses of 2.5 and 5 mg/kg did not produce a significant change of time spent in the open arms, while other doses (25, 50, 100, 200, and 400 mg/kg) significantly (p < 0.05) increased this parameter. Diazepam (3 mg/kg) also significantly (p < 0.05) increased the time spent in the open arms by 104% in comparison with the control group (Fig. 1).
Figure 1.
Effects of diazepam, saline, and different doses of D. anethifolia on the percentage of time spent in the open arms during a 5‐minute test in mice. Test compounds were injected 30 minutes prior to the EPM test. Data are presented as mean values (± S.E.M.). *P < 0.05 compared with vehicle‐treated control.
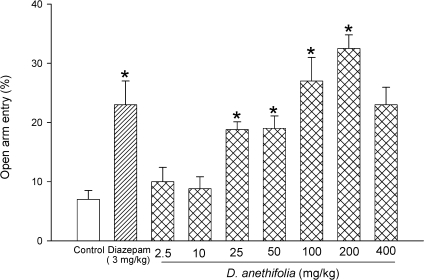
Effects of DAEO on the percent of open arm entries can be seen in Figure 2. Again, DAEO at doses of 2.5 and 10 mg/kg did not produce a significant change of this parameter. Compared with control group, DAEO at doses of 25, 50, 100, 200, and 400 mg/kg increased the time spent in open arm entries by 169%, 171%, 286%, 364%, and 229%, respectively. These changes were significant (p < 0.05) for doses of 25 to 200 mg/kg. Diazepam also significantly (p < 0.05) increased this parameter by 229%.
Figure 2.
Effects of diazepam, saline, and different doses of D. anethifolia extract on percentage entries in the open arms during a 5‐minute test in mice. Test compounds were injected 30 minutes prior to the EPM test. Data are presented as mean values (± S.E.M.). *P < 0.05 compared with vehicle‐treated control.
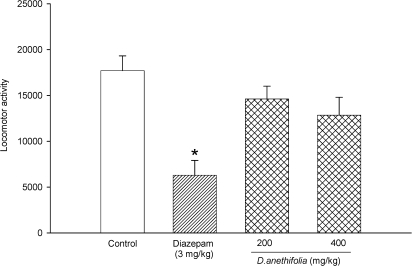
Effect of Ducrosia anethifolia essential oil on spontaneous locomotor activity
The effect of treatments on locomotor activity was measured at 5 min intervasl and during a 15 min period using locomotor activity apparatus. Locomotor activity was decreased in animals orally pretreated with DAEO at doses of 200 and 400 mg/kg compared with saline treated controls; however, these changes were not statistically significant (Fig. 3). Administration of diazepam at a dose of 3 mg/kg significantly (p < 0.01) suppressed the locomotor activity (Fig. 3).
Figure 3.
Effects of diazepam, saline, and different doses of D. anethifolia on spontaneous locomotor activity during a total of 15 minutes. The locomotor activity counts (mean ± S.E.M.) were measured over a 15‐minute period, beginning 30 minutes after the administration of saline, diazepam, or different doses of D. anethifolia. *P < 0.01 compared with vehicle‐treated control.
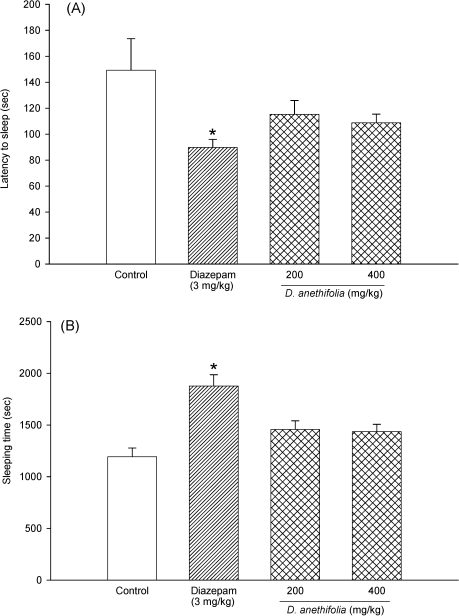
Effect of Ducrosia anethifolia essential oil on latency to sleep
Vehicle, DAEO or diazepam, was given one hour prior to ketamine injection, and the latency to loss of righting reflex was measured. In control animals, the righting reflex was lost after 149 s of ketamine injection. DAEO at doses of 200 and 400 mg/kg suppressed the latency to sleep by 23% and 27%, respectively, but these changes were not significant. Diazepam (3 mg/kg) as the reference drug significantly (p< 0.01) reduced the latency to sleep by 40% (Fig. 4A).
Figure 4.
Effects of diazepam and D. anethifolia extract on (A) the latency to loss of righting reflex and (B) total sleep time. The interval between the administration of ketamin until the loss of the righting reflex was recorded as onset of sleep. The time from loss of righting reflex to regaining of the righting reflex was recorded as duration of sleep. Results represent mean (± S.E.M.) from six mice. *P < 0.01 compared with vehicle‐treated control.
Effect of Ducrosia anethifolia essential oil on sleeping time
Vehicle, DAEO or diazepam, was given one hour prior to ketamine injection and the total sleep time was measured. The mean of total sleep time in control animals was 1192 s. DAEO at doses of 200 and 400 mg/kg increased the ketamine‐induced sleep time by 22% and 20%, respectively. Diazepam produced a significant (p < 0.01) increase of sleep time by 57% (Fig. 4B).
Acute toxicity test
DAEO at doses of 1600 and 2400 mg/kg did not affect the vital signs of animals and during a 48‐hour observation period did not cause any mortality.
DISCUSSION
A search for medicinal plants is still important and might provide a useful source for therapy and prevention of diseases or, alternatively, as simple dietary adjuncts to existing therapies.23 The aim of the present study was to evaluate the antianxiety effects of D. anethifolia essential oil in mice. Different doses of the essential oil were tested on the EPM. Doses of 2.5 and 10 mg/kg were ineffective. The anxiolytic effect started at a dose of 25 mg/kg and increased in the dose range of 50–200 mg/kg. As expected, diazepam as a benzodiazepine drug produced significant increases in the percentage of open arm time and entries into the open arms. These data are consistent with the results of numerous previous studies, which have shown that diazepam and other benzodiazepines produce significant anxiolytic effects in a variety of anxiolytic screening procedures, including elevated plus‐maze test procedures.24-26 Benzodiazepines mainly exert their antianxiety effects via interaction with gamma‐amino‐butyric acid (GABA) receptors.27 Since DAEO, unlike diazepam, could not affect ketamine‐induced sleep time and spontaneous motor activity, it seems that DAEO has a different mechanism of action. In addition to benzodiazepines, buspirone, and antidepressant drugs are commonly used for treating anxiety.27-28 Buspirone has partial agonistic activity at 5HT1A receptors, and tricyclic antidepressants and selective serotonine reuptake inhibitors inhibit synaptic reuptake of norepinephrine and serotonine to different extents.27 It is not clear which of the above‐mentioned mechanisms explains the antianxiety effect of DAEO; further studies are needed to clarify the exact mechanism.
This is the first study that shows an effect of DAEO on the central nervous system. The pharmacological studies on another species of Ducrosia – namely D. ismaelis – show it to be a highly significant dose‐dependent central nervous system depressant with marked neuromuscular blocking actions for its essential oil. Other experiments on D. ismaelis essential oil show a parasympatholytic activity on smooth muscles and heart.7
According to the results of GC/MS analysis, DAEO consists of about 20 compounds and n‐decanal (70.1%) and alpha‐pinene (12.4%) were the major components, and these two compounds may have the greatest role in these pharmacological effects. A complete survey of the literature shows that the antianxiety effects of these two compounds has yet to be studied. Satou et al. (2010) have recently described the anxiolytic effect for Alpinia zerumbet essential oil, which also contains alpha‐pinene.29 Since this compound is commonly found in essential oils of both plants, it may be responsible for a part of the observed effect, although further detailed study is needed to confirm this theory.
In conclusion, DAEO shows antianxiety effect and does not cause sedation – as confirmed by ketamine‐induced sleep time and spontaneous motor activity tests – and it may have some advantages when compared with available chemical antianxiety drugs. However, further studies are needed to determine the possible mechanism of actions of DAEO, and their potential for clinical use needs to be demonstrated in clinical trials.
REFERENCE
- 1.Schulz V, Hansel R, Blumenthal M, Tyler VE. Rational phytotherapy: A reference guide for physicians and pharmacists. Berlin: Springer; 2004. [Google Scholar]
- 2. Carlini EA. Plants and central nervous system. Pharmacol Biochem Behav. 2003;75:501–12. doi: 10.1016/s0091-3057(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 3.Aynehchi Y. Materia Medica and Iranian medicinal plants. Tehran: Tehran University Publications; 1991. [Google Scholar]
- 4.Ghahreman A. Flore De L'Iran. Teheran: Institut de Recherches des Forets et des Paturages; 1993. [Google Scholar]
- 5.Mozaffarian V. A dictionary of Iranian plant names. Tehran: Farhang Moaser; 1996. [Google Scholar]
- 6. Haghi G, Safaei A, Safari J. Extraction and determination of the main components of the essential oil of Ducrosia anethifolia by GC and GC/MS. Iranian J Pharm Res. 2004;3(Suppl. 2):275. [Google Scholar]
- 7. Al‐Meshal IA. Isolation and characterization of a bioactive volatile oil from Ducrosia ismaelis Asch. Res Commun Chem Pathol Pharmacol. 1986;54:129–32. [PubMed] [Google Scholar]
- 8. Janssen AM, Scheffer JJ, Baerheim Svendsen A, Aynehchi Y. The essential oil of Ducrosia anethifolia (DC.) Boiss. chemical composition and antimicrobial activity. Pharm Weekbl Sci. 1984;6:157–60. doi: 10.1007/BF01954043. [DOI] [PubMed] [Google Scholar]
- 9. Stavri M, Mathew KT, Bucar F, Gibbons S. Pangelin, an antimycobacterial coumarin from Ducrosia anethifolia. Planta Med. 2003;69:956–9. doi: 10.1055/s-2003-45109. [DOI] [PubMed] [Google Scholar]
- 10. Sefidkon F, Javidtash I. Essential oil composition of Ducrosia anethifolia (DC.) Boiss. from Iran. J Essent Oil Res. 2002;14:278–9. [Google Scholar]
- 11. Al‐Meshal IA, Khalifa TI, Hassan MMA. Physico‐chemical characteristics and spectroscopy of Ducrosia ismaelis oil. Spectrosc Lett. 1985;18:495–6. [Google Scholar]
- 12. Rustaiyan A, Mazloomifar H, Masoudi SH, Aghjani Z. Volatile oil of Ducrosia assadii Alava. and Prangos acaulis (DC.) Bornm. from Iran. J Essent Oil Res. 2006;18:682–4. [Google Scholar]
- 13. Minaiyan M, Ghannadi A, Karimzadeh A. Antiulcerogenic effect of ginger (Zingiber officinale Roscoe) on cysteamine induced duodenal ulcer in rats. Daru. 2006;14:97–101. [Google Scholar]
- 14.European Pharmacopoeia Commission, Council of Europe, European pharmacopoeia. Sainte Ruffine: Maisonneuve SA; 1975. [Google Scholar]
- 15.Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream, IL: Allured Publishing Co.; 1995. [Google Scholar]
- 16.Sandra P, Bicchi C. Capillary gas chromatography in essential oil analysis. Heidelberg: Dr. A. Huethig; 1987. [Google Scholar]
- 17. Hogg S. A review of the validity and variability of the elevated plus‐maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 18. Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: An ethological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- 19. Lister RG. The use of a plus‐maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 20. Rabbani M, Wright EJ, Little HJ. Tolerance to competitive NMDA antagonists, but no cross tolerance with barbiturates. Pharmacol Biochem Behav. 1995;50:9–15. doi: 10.1016/0091-3057(94)00215-5. [DOI] [PubMed] [Google Scholar]
- 21. Figallo EM, Wingard LB., Jr Effects of physostigmine, scopolamine, and mecamylamine on the sleeping time induced by ketamine in the rat. Psychopharmacology (Berl) 1979;61:59–62. doi: 10.1007/BF00426811. [DOI] [PubMed] [Google Scholar]
- 22. Rabbani M, Sajjadi SE, Zarei HR. Anxiolytic effects of Stachys lavandulifolia Vahl on the elevated plus‐maze model of anxiety in mice. J Ethnopharmacol. 2003;89:271–6. doi: 10.1016/j.jep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23. Minaiyan M, Ghannadi A, Salehi E. Antiulcerogenic effect of Zataria multiflora Boiss. on cysteamine induced duodenal ulcer in rats. Iranian J Pharm Sci. 2005;1:223–39. [Google Scholar]
- 24. Andrews JS, Stephens DN. Drug discrimination models in anxiety and depression. Pharmacol Ther. 1990;47:267–80. doi: 10.1016/0163-7258(90)90090-o. [DOI] [PubMed] [Google Scholar]
- 25. Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus‐maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 26. Winslow JT, Insel TR. Infant rat separation is a sensitive test for novel anxiolytics. Prog Neuropsychopharmacol Biol Psych. 1991;15:745–57. doi: 10.1016/0278-5846(91)90003-j. [DOI] [PubMed] [Google Scholar]
- 27.Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's the pharmacological basis of therapeutics. New York: McGraw‐Hill; 2006. [Google Scholar]
- 28. Schmitt R, Gazalle FK, Lima MS, Cunha A, Souza J, Kapczinski F. The efficacy of antidepressants for generalized anxiety disorder: A systematic review and meta‐analysis. Rev Bras Psiquiatr. 2005;27:18–24. doi: 10.1590/s1516-44462005000100007. [DOI] [PubMed] [Google Scholar]
- 29. Satou T, Murakami S, Matsuura M, Hayashi S, Koike K. Anxiolytic effect and tissue distribution of inhaled Alpinia zerumbet essential oil in mice. Nat Prod Commun. 2010;5:143–6. [PubMed] [Google Scholar]