Abstract
Mature human erythrocytes were incubated with 14C-labeled palmitic acid bound to crystalline human albumin. Energy-dependent incorporation of the labeled palmitic acid into cell membrane phospholipids occurred, and various stages in this incorporation were defined.
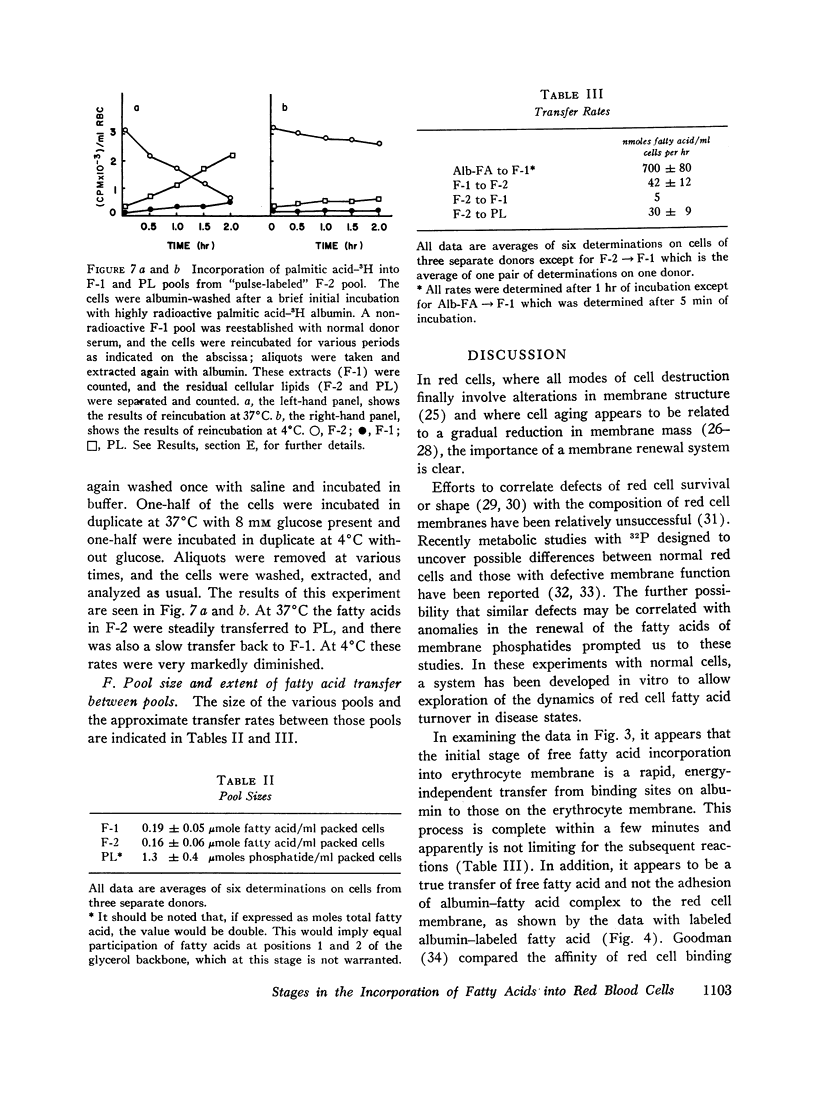
Initially the palmitic acid was rapidy transferred from the albumin to a “superficial” membrane pool of free fatty acid (F-1), which was removable when the cells were washed with defatted albumin. This process was independent of red cell metabolism.
The labeled fatty acid then passed into a second “deeper” membrane pool of free fatty acids (F-2), which was not extractable with albumin. This process was energy-dependent and proceeded at a slower rate than the initial transfer from albumin to F-1.
Ultimately the labeled fatty acid was incorporated into phosphatides (PL). This process also was dependent upon cellular metabolism.
The kinetics of pulse label studies suggest that the processes observed were sequential and that precursor-product relationships exist between the F-1 and F-2 pools and the F-2 and PL pools. [Formula: see text] From the size and specific activities of these pools, calculations of the extent of phospholipid turnover were made. An approximate figure of 2% /hr or 30 nmoles/ml of packed red blood cells per hr was obtained. The figure was further calculated to represent an energy cost to the red blood cell of approximately 5% of the energy available from glycolysis.
Full text
PDF


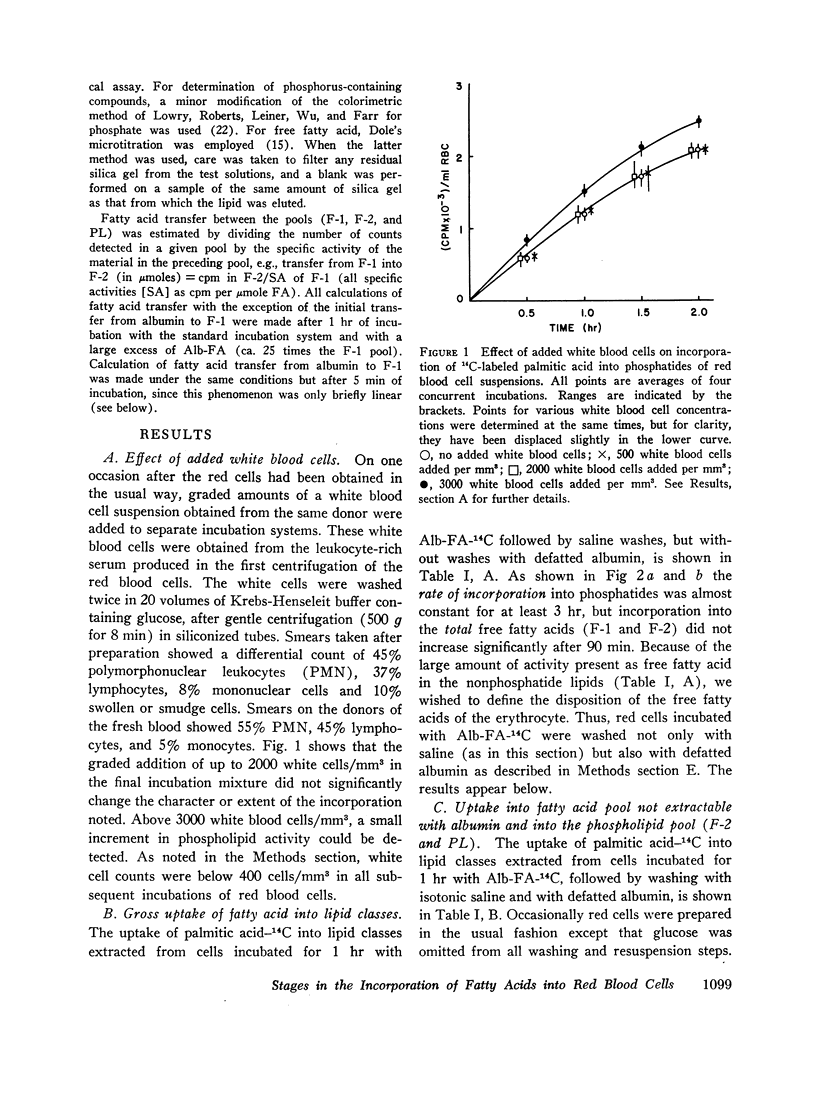
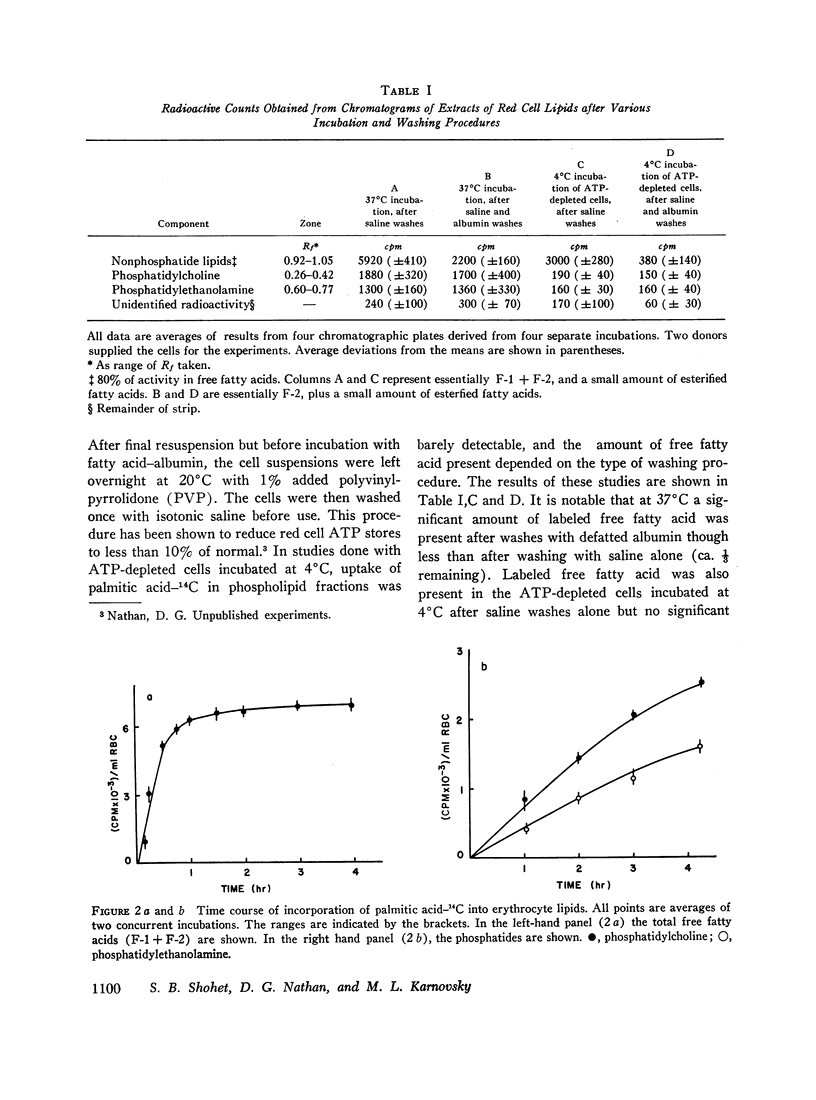
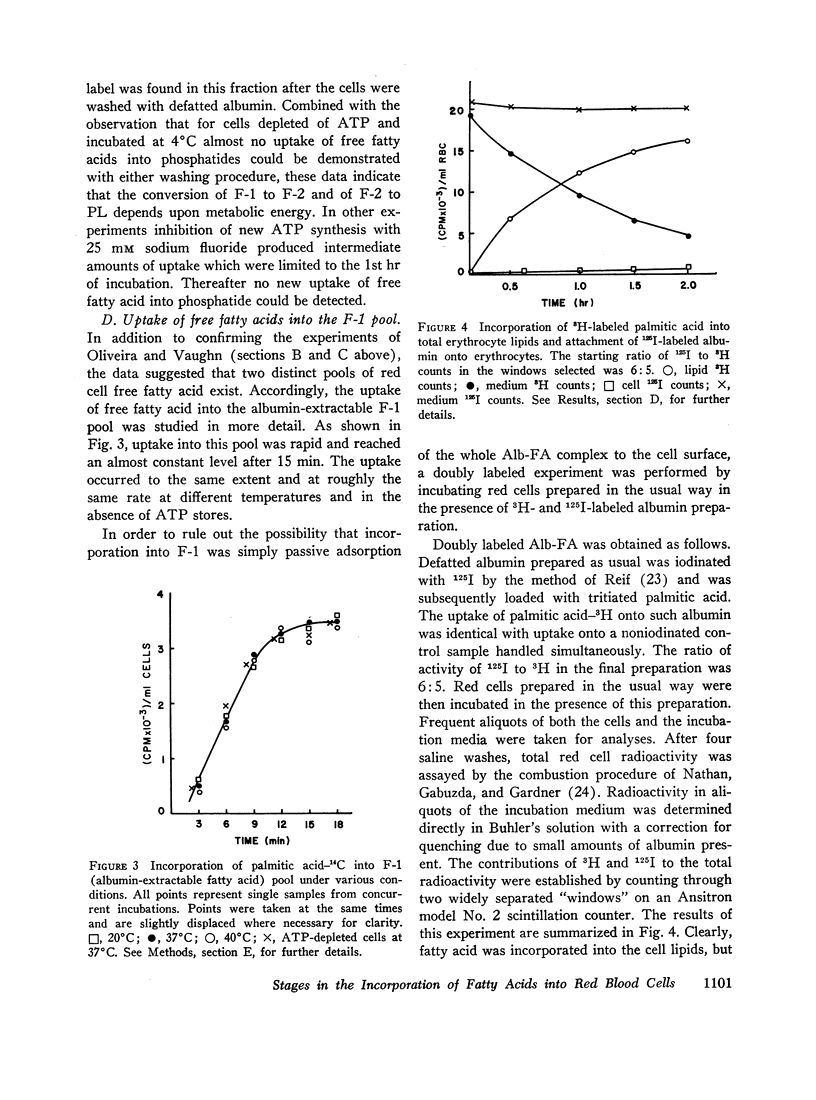
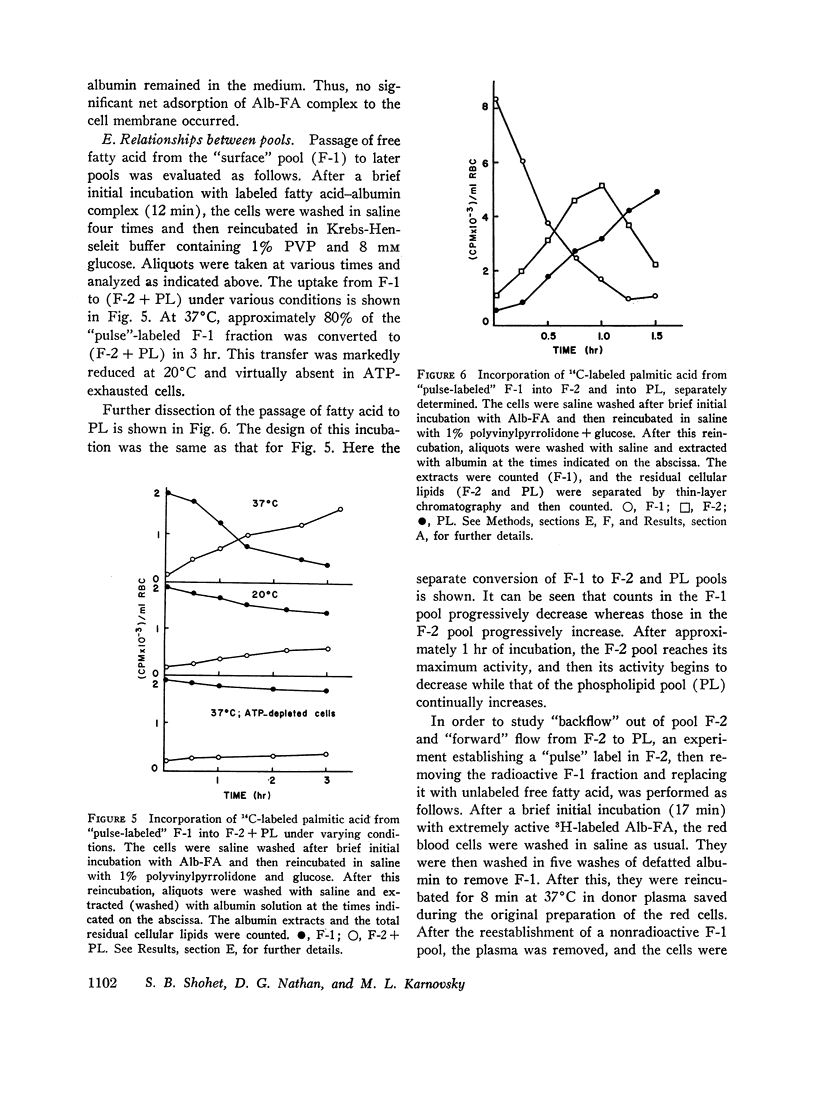





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLOW B. A., RUBENSTEIN R., LEE J. ERYTHROCYTE PHOSPHOLIPIDS: QUANTITATIVE THIN-LAYER CHROMATOGRAPHY IN PAROXYSMAL NOCTURNAL HAEMOGLOBINURIA AND HEREDITARY SPHEROCYTOSIS. Br J Haematol. 1965 May;11:315–322. doi: 10.1111/j.1365-2141.1965.tb06591.x. [DOI] [PubMed] [Google Scholar]
- Coleman R., Michell R. H., Finean J. B., Hawthorne J. N. A purified plasma membrane fraction isolated from rat liver under isotonic conditions. Biochim Biophys Acta. 1967 Sep 9;135(4):573–579. doi: 10.1016/0005-2736(67)90089-2. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Donabedian R. K., Karmen A. Fatty acid transport and incorporation into human erythrocytes in vitro. J Clin Invest. 1967 Jun;46(6):1017–1027. doi: 10.1172/JCI105591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Metabolism of lysophosphatidyl ethanolamine and lysophosphatidyl choline by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. J Lipid Res. 1967 Jul;8(4):359–365. [PubMed] [Google Scholar]
- Elsbach P. Phospholipid metabolism by phagocytic cells. I. A comparison of conversion of [32P]lysolecithin to lecithin and glycerylphosphorylcholine by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. Biochim Biophys Acta. 1966 Dec 7;125(3):510–524. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GILL T. J., 3rd, GOLD G. L., SOLOMON A. K. The kinetics of cardiac glycoside inhibition of potassium transport in human erythrocytes. J Gen Physiol. 1956 Nov 20;40(2):327–350. doi: 10.1085/jgp.40.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. The interaction of human erythrocytes with sodium palmitate. J Clin Invest. 1958 Dec;37(12):1729–1735. doi: 10.1172/JCI103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAHAN D. J., WATTS R. M., PAPAJOHN D. Some chemical characteristics of the lipids of human and bovine erythrocytes and plasma. J Lipid Res. 1960 Oct;1:421–432. [PubMed] [Google Scholar]
- HARRIS I. M., PRANKERD T. A., WESTERMAN M. P. Abnormality of phospholipids in red cells of patients with paroxysmal nocturnal haemoglobinuria. Br Med J. 1957 Nov 30;2(5056):1276–1277. doi: 10.1136/bmj.2.5056.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Karnovsky M. L. Concomitant alterations of sodium flux and membrane phospholipid metabolism in red blood cells: studies in hereditary spherocytosis. J Clin Invest. 1967 Feb;46(2):173–185. doi: 10.1172/JCI105520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M., ALLISON A. C., JAMES A. T. Phosphatides of human blood cells and their role in spherocytosis. Biochim Biophys Acta. 1961 Apr 15;48:571–582. doi: 10.1016/0006-3002(61)90055-5. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953 Sep;204(1):329–343. [PubMed] [Google Scholar]
- Keitt A. S. Pyruvate kinase deficiency and related disorders of red cell glycolysis. Am J Med. 1966 Nov;41(5):762–785. doi: 10.1016/0002-9343(66)90036-2. [DOI] [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- MARKS P. A., GELLHORN A., KIDSON C. Lipid synthesis in human leukocytes, platelets, and erythrocytes. J Biol Chem. 1960 Sep;235:2579–2583. [PubMed] [Google Scholar]
- MUNN J. I. Studies of lipids in human red cells. Br J Haematol. 1958 Jul;4(3):344–349. doi: 10.1111/j.1365-2141.1958.tb06036.x. [DOI] [PubMed] [Google Scholar]
- Mulder E., van Deenen L. L. Metabolism of red-cell lipids. I. Incorporation in vitro of fatty acids into phospholipids from mature erythrocytes. Biochim Biophys Acta. 1965 Jul 7;106(1):106–117. doi: 10.1016/0005-2760(65)90099-8. [DOI] [PubMed] [Google Scholar]
- Mulder E., van den Berg J. W., van Deenen L. L. Metabolism of red-cell lipids. II. Conversions of lysophosphoglycerides. Biochim Biophys Acta. 1965 Jul 7;106(1):118–127. doi: 10.1016/0005-2760(65)90100-1. [DOI] [PubMed] [Google Scholar]
- NATHAN D. G., GABUZDA T. G., GARDNER F. H. LIQUID SCINTILLATION COUNTING OF C14-LABELED HEMOGLOBIN AND HEMIN BY A MODIFIED SCHOENIGER TECHNIQUE. J Lab Clin Med. 1963 Sep;62:511–516. [PubMed] [Google Scholar]
- OLIVEIRA M. M., VAUGHAN M. INCORPORATION OF FATTY ACIDS INTO PHOSPHOLIPIDS OF ERYTHROCYTE MEMBRANES. J Lipid Res. 1964 Apr;5:156–162. [PubMed] [Google Scholar]
- Pittman J. G., Martin D. B. Fatty acid biosynthesis in human erythrocytes: evidence in mature erythrocytes for an incomplete long chain fatty acid synthesizing system. J Clin Invest. 1966 Feb;45(2):165–172. doi: 10.1172/JCI105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Reed C. F., Young L. E. Erythrocyte energy metabolism in hereditary spherocytosis. J Clin Invest. 1967 Jul;46(7):1196–1204. doi: 10.1172/JCI105613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Steinberg D. Relationship between fatty acid and glucose utilization in Ehrlich ascites tumor cells. J Lipid Res. 1966 Sep;7(5):657–663. [PubMed] [Google Scholar]
- Spector A. A., Steinberg D. The utilization of unesterified palmitate by Ehrlich ascites tumor cells. J Biol Chem. 1965 Oct;240(10):3747–3753. [PubMed] [Google Scholar]
- Spector A. A., Steinberg D. Turnover and utilization of esterified fatty acids in Ehrlich ascites tumor cells. J Biol Chem. 1967 Jul 10;242(13):3057–3062. [PubMed] [Google Scholar]
- Switzer S., Eder H. A. Transport of lysolecithin by albumin in human and rat plasma. J Lipid Res. 1965 Oct;6(4):506–511. [PubMed] [Google Scholar]
- VAN GASTEL, VAN DEN BERG D., DE GIER J., VAN DEENEN L. SOME LIPID CHARACTERISTICS OF NORMAL RED BLOOD CELLS OF DIFFERENT AGE. Br J Haematol. 1965 Mar;11:193–199. doi: 10.1111/j.1365-2141.1965.tb06577.x. [DOI] [PubMed] [Google Scholar]
- Waite M., van Deenen L. L. Hydrolysis of phospholipids and glycerides by rat-liver preparations. Biochim Biophys Acta. 1967 Jun 6;137(3):498–517. doi: 10.1016/0005-2760(67)90131-2. [DOI] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- Wittels B., Hochstein P. The effect of primaquine on lecithin metabolism in human erythrocytes. Biochim Biophys Acta. 1966 Dec 7;125(3):594–597. doi: 10.1016/0005-2760(66)90047-6. [DOI] [PubMed] [Google Scholar]
- Wittels B., Hochstein P. The identification of carnitine palmityltransferase in erythrocyte membranes. J Biol Chem. 1967 Jan 10;242(1):126–130. [PubMed] [Google Scholar]



